Modification of Physiochemical and Techno-Functional Properties of Stink Bean (Parkia speciosa) by Germination and Hydrothermal Cooking Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Treatment Preparation
2.1.1. Raw Stink Bean (SBR)
2.1.2. Germinated Stink Bean (SBG)
2.1.3. Stink Bean Hydrothermally Cooked (SBHTC)
2.2. Physiochemical Characteristics of Raw (R), Germinated (G) and Hydrothermally Cooked (HTC) Stink Beans (SB)
2.2.1. Length, Width, and Thickness
2.2.2. Equivalent Diameter
2.2.3. Sphericity
2.2.4. Aspect ratio
2.2.5. Seed Volume
2.2.6. Surface Area
2.2.7. Weight of Seed
2.2.8. Seed Volume
2.3. Field Emission—Scanning Electron Microscopic (FE-SEM)
2.4. Proximate Composition
2.5. Techno-Functional Characteristics
2.5.1. Hunter Color Measurement
2.5.2. Least gelation Concentration (LGC)
2.5.3. Emulsion Properties
2.5.4. Swelling Capacity (SC)
2.5.5. Hydration Capacity
2.5.6. Pasting Properties
2.5.7. Water Absorption and Oil Absorption Capacity
2.6. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.7. Gelatinization Characterization
2.8. Antioxidant Properties
2.8.1. Total Polyphenol Content (TPC)
2.8.2. Assessment of Antioxidant Activity by DPPH Assay
2.8.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physiochemical Characteristics
3.2. Proximate Composition
3.3. Techno-Functional Properties
3.3.1. Hunter Color
3.3.2. Least Gelation Concentration
3.3.3. Emulsion, Swelling, Hydration, and Pasting Properties, Alongside Water and Oil Absorption Capacities
3.4. FTIR Analysis
3.5. Gelatinization Properties
3.6. FE-SEM Analysis
3.7. Antioxidant Activity and Total Phenolic Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medhe, S.V.; Kamble, M.T.; Kettawan, A.K.; Monboonpitak, N.; Kettawan, A. Effect of hydrothermal cooking and germination treatment on functional and physicochemical properties of Parkia timoriana bean flours: An underexplored legume Species of Parkia genera. Foods 2022, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.A.; Sogi, D.S.; Wani, A.A.; Gill, B.S. Physical and cooking characteristics of some Indian kidney bean (Phaseolus vulgaris L.) cultivars. J. Saudi Soc. Agric. Sci. 2017, 16, 7–15. [Google Scholar] [CrossRef]
- Bhat, R.; Karim, A. Exploring the nutritional potential of wild and underutilized legumes. Compr. Rev. Food Sci. Food Saf. 2009, 8, 305–331. [Google Scholar] [CrossRef]
- Lawal, O.; Adebowale, K.; Ogunsanwo, B.; Sosanwo, O.; Bankole, S. On the functional properties of globulin and albumin protein fractions and flours of African locust bean (Parkia biglobossa). Food Chem. 2005, 92, 681–691. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physicochemical and functional properties of flours from three Black gram (Phaseolus mungo L.) cultivars. Int. J. Food Sci. Technol. 2013, 48, 771–777. [Google Scholar] [CrossRef]
- Prosper, E.O.; Uguru, H. Influence of maturation on engineering properties of three bean (Phaseolus vulgaris L.) varieties, related to machine design. Int. J. Res.-Granthaalayah 2018, 6, 93–113. [Google Scholar] [CrossRef]
- Khattab, R.; Arntfield, S.; Nyachoti, C. Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. LWT-Food Sci. Technol. 2009, 42, 1107–1112. [Google Scholar] [CrossRef]
- Sridaran, A.; Karim, A.A.; Bhat, R. Pithecellobium jiringa legume flour for potential food applications: Studies on their physico-chemical and functional properties. Food Chem. 2012, 130, 528–535. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Rani, N.F.A.; Hussin, A.S.M. Identification of antioxidant and antibacterial activities for the bioactive peptides generated from bitter beans (Parkia speciosa) via boiling and fermentation processes. LWT 2020, 131, 109776. [Google Scholar] [CrossRef]
- Asikin, Y.; Taira, E.; Wada, K. Alterations in the morphological, sugar composition, and volatile flavor properties of petai (Parkia speciosa Hassk.) seed during ripening. Food Res. Int. 2018, 106, 647–653. [Google Scholar] [CrossRef]
- Jamaluddin, F.; Mohamed, S.; Lajis, M.N. Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of β-sitosterol and stigmasterol. Food Chem. 1994, 49, 339–345. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Pasuk, S.; Ritthiruangdej, P. Relationship between antioxidant properties and chemical composition of some Thai plants. J. Food Compos. Anal. 2008, 21, 229–240. [Google Scholar] [CrossRef]
- Siow, H.-L.; Gan, C.-Y. Extraction of antioxidative and antihypertensive bioactive peptides from Parkia speciosa seeds. Food Chem. 2013, 141, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-J.; Ang, L.-H.; Ng, L.-T. Antioxidant activities and polyphenolic constituents of bitter bean Parkia speciosa. Int. J. Food Prop. 2014, 17, 1977–1986. [Google Scholar] [CrossRef]
- Saelim, K.; Jampaphaeng, K.; Maneerat, S. Functional properties of Lactobacillus plantarum S0/7 isolated fermented stinky bean (Sa Taw Dong) and its use as a starter culture. J. Funct. Foods 2017, 38, 370–377. [Google Scholar] [CrossRef]
- Murakami, A.; Ali, A.M.; Mat-Salleh, K.; Koshimizu, K.; Ohigashi, H. Screening for the in vitro anti-tumor-promoting activities of edible plants from Malaysia. Biosci. Biotechnol. Biochem. 2000, 64, 9–16. [Google Scholar] [CrossRef]
- Li, C.; Jeong, D.; Lee, J.H.; Chung, H.-J. Influence of germination on physicochemical properties of flours from brown rice, oat, sorghum, and millet. Food Sci. Biotechnol. 2020, 29, 1223–1231. [Google Scholar] [CrossRef]
- Medhe, S.; Jain, S.; Anal, A.K. Effects of sprouting and cooking processes on physicochemical and functional properties of moth bean (Vigna aconitifolia) seed and flour. J. Food Sci. Technol. 2019, 56, 2115–2125. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, N.; Sodhi, N.S.; Rana, J.C. Diversity in properties of seed and flour of kidney bean germplasm. Food Chem. 2009, 117, 282–289. [Google Scholar] [CrossRef]
- Kamble, M.T.; Rudtanatip, T.; Soowannayan, C.; Nambunruang, B.; Medhe, S.V.; Wongprasert, K. Depolymerized fractions of sulfated galactans extracted from Gracilaria fisheri and their antibacterial activity against Vibrio parahaemolyticus and Vibrio harveyi. Mar. Drugs 2022, 20, 469. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis of AOAC International; AOAC: Gaithersburg, MD, USA, 2019; Volume 21. [Google Scholar]
- FAO. Chapter 3: Calculation of the energy content of foods-Energy conversion factors. In Report of a Technical Workshop. Paper 77; FAO: Rome, Italy, 2003. [Google Scholar]
- Nasrin, T.A.A.; Noomhorm, A.; Anal, A.K. Physico-chemical characterization of culled plantain pulp starch, peel starch, and flour. Int. J. Food Prop. 2015, 18, 165–177. [Google Scholar] [CrossRef]
- Chinma, C.E.; Abu, J.O.; Asikwe, B.N.; Sunday, T.; Adebo, O.A. Effect of germination on the physicochemical, nutritional, functional, thermal properties and in vitro digestibility of Bambara groundnut flours. LWT 2021, 140, 110749. [Google Scholar] [CrossRef]
- Alberto, J.; Rivas, G.; Fang, R.; Elizabeth, N.; Francisco, R. Effect of the addition of common bean flour on the cooking quality and antioxidant characteristics of spaghetti. J. Microbiol. Biotechnol. Food Sci. 2021, 2, 730–744. [Google Scholar]
- Gupta, S.; Chhabra, G.S.; Liu, C.; Bakshi, J.S.; Sathe, S.K. Functional properties of select dry bean seeds and flours. J. Food Sci. 2018, 83, 2052–2061. [Google Scholar] [CrossRef]
- Zahir, M.; Fogliano, V.; Capuano, E. Soybean germination limits the role of cell wall integrity in controlling protein physicochemical changes during cooking and improves protein digestibility. Food Res. Int. 2021, 143, 110254. [Google Scholar] [CrossRef]
- Miceli, A.; Miceli, C. Effect of thermal treatments on vitality and physical characteristics of bean, chickpea and lentil. J. Stored Prod. Res. 2012, 51, 86–91. [Google Scholar] [CrossRef]
- Falade, K.O.; Akinrinde, I.M. Physical, chemical and adsorption isotherm characteristics of fermented soybean cultivars, and cracked and dehulled African locust bean using selected Bacillus spp. J. Food Sci. Technol. 2021, 58, 2749–2760. [Google Scholar] [CrossRef]
- Mubarak, A. Nutritional composition and antinutritional factors of mung bean seeds (Phaseolus aureus) as affected by some home traditional processes. Food Chem. 2005, 89, 489–495. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT-Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Xu, L.; Chen, L.; Ali, B.; Yang, N.; Chen, Y.; Wu, F.; Jin, Z.; Xu, X. Impact of germination on nutritional and physicochemical properties of adlay seed (Coixlachryma-jobi L.). Food Chem. 2017, 229, 312–318. [Google Scholar] [CrossRef]
- Atudorei, D.; Stroe, S.-G.; Codină, G.G. Impact of germination on the microstructural and physicochemical properties of different legume types. Plants 2021, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, L.Á.; Abhilasha, A.; Singh, J.; Elias, M.C.; Colussi, R. Rice germination and its impact on technological and nutritional properties: A review. Rice Sci. 2022, 29, 201–215. [Google Scholar] [CrossRef]
- Piecyk, M.; Wołosiak, R.; Drużynska, B.; Worobiej, E. Chemical composition and starch digestibility in flours from Polish processed legume seeds. Food Chem. 2012, 135, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, M.; Wu, H.; Jing, L.; Gong, B.; Gou, M.; Zhao, K.; Li, W. The compositional, physicochemical and functional properties of germinated mung bean flour and its addition on quality of wheat flour noodle. J. Food Sci. Technol. 2018, 55, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Childs, M.; Loos, M.; Taylor, A.; Smart, L.B.; Abbaspourrad, A. The effects of germination on the composition and functional properties of hemp seed protein isolate. Food Hydrocoll. 2023, 134, 108085. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.; Tyler, R.; Toews, R.; Gawalko, E. Effect of cooking on the composition of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L.). Food Res. Int. 2010, 43, 589–594. [Google Scholar] [CrossRef]
- Corzo-Ríos, L.J.; Sánchez-Chino, X.M.; Cardador-Martínez, A.; Martínez-Herrera, J.; Jiménez-Martínez, C. Effect of cooking on nutritional and non-nutritional compounds in two species of Phaseolus (P. vulgaris and P. coccineus) cultivated in Mexico. Int. J. Gastron. Food Sci. 2020, 20, 100206. [Google Scholar] [CrossRef]
- Kumari, M.; Urooj, A.; Prasad, N.N. Effect of storage on resistant starch and amylose content of cereal–pulse based ready-to-eat commercial products. Food Chem. 2007, 102, 1425–1430. [Google Scholar] [CrossRef]
- Kutoš, T.; Golob, T.; Kač, M.; Plestenjak, A. Dietary fibre content of dry and processed beans. Food Chem. 2003, 80, 231–235. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Bubolz, V.K.; da Silva, J.; Dittgen, C.L.; Ziegler, V.; de Oliveira Raphaelli, C.; de Oliveira, M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT 2019, 111, 363–369. [Google Scholar] [CrossRef]
- Khalil, A.W.; Zeb, A.; Mahmood, F.; Tariq, S.; Khattak, A.B.; Shah, H. Comparison of sprout quality characteristics of desi and kabuli type chickpea cultivars (Cicer arietinum L.). LWT-Food Sci. Technol. 2007, 40, 937–945. [Google Scholar] [CrossRef]
- Wu, X.; Tan, M.; Zhu, Y.; Duan, H.; Ramaswamy, H.S.; Bai, W.; Wang, C. The influence of high pressure processing and germination on anti-nutrients contents, in vitro amino acid release and mineral digestibility of soybeans. J. Food Compos. Anal. 2023, 115, 104953. [Google Scholar] [CrossRef]
- Siddiq, M.; Ravi, R.; Harte, J.; Dolan, K. Physical and functional characteristics of selected dry bean (Phaseolus vulgaris L.) flours. LWT-Food Sci. Technol. 2010, 43, 232–237. [Google Scholar] [CrossRef]
- Chinma, C.E.; Adewuyi, O.; Abu, J.O. Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus). Food Res. Int. 2009, 42, 1004–1009. [Google Scholar] [CrossRef]
- Aguilera, Y.; Benítez, V.; Mollá, E.; Esteban, R.M.; Martín-Cabrejas, M.A. Influence of dehydration process in castellano chickpea: Changes in bioactive carbohydrates and functional properties. Plant Foods Hum. Nutr. 2011, 66, 391–400. [Google Scholar] [CrossRef]
- Setia, R.; Dai, Z.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y. Impacts of short-term germination on the chemical compositions, technological characteristics and nutritional quality of yellow pea and faba bean flours. Food Res. Int. 2019, 122, 263–272. [Google Scholar] [CrossRef]
- Thakur, S.; Scanlon, M.; Tyler, R.; Milani, A.; Paliwal, J. Pulse flour characteristics from a wheat flour miller’s perspective: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 775–797. [Google Scholar] [CrossRef]
- Yadav, U.; Singh, N.; Kaur, A.; Thakur, S. Physico-chemical, hydration, cooking, textural and pasting properties of different adzuki bean (Vigna angularis) accessions. J. Food Sci. Technol. 2018, 55, 802–810. [Google Scholar] [CrossRef]
- Guldiken, B.; Franczyk, A.; Boyd, L.; Wang, N.; Choo, K.; Sopiwnyk, E.; House, J.; Paliwal, J.; Nickerson, M. Physicochemical, nutritional and functional properties of chickpea (Cicer arietinum) and navy bean (Phaseolus vulgaris) flours from different mills. Eur. Food Res. Technol. 2022, 248, 1847–1858. [Google Scholar] [CrossRef]
- Bekele, D.W.; Admassu, S. Pasting, thermal and structural properties of haricot beans flour (Phaseolus vulgaris, L.) as affected by variety and germination. Int. J. Food Prop. 2023, 26, 963–973. [Google Scholar] [CrossRef]
- Obinna-Echem, P.C.; Barber, L.I. Effect of germination and pre-gelatinization on the proximate composition and pasting properties of maize flour a base ingredient for cereal-based infant complementary food. Int. J. Biotechnol. Food Sci. 2019, 7, 30–37. [Google Scholar]
- Acevedo, B.A.; Thompson, C.M.; González Foutel, N.S.; Chaves, M.G.; Avanza, M.V. Effect of different treatments on the microstructure and functional and pasting properties of pigeon pea (Cajanus cajan L.), dolichos bean (Dolichos lablab L.) and jack bean (Canavalia ensiformis) flours from the north-east Argentina. Int. J. Food Sci. Technol. 2017, 52, 222–230. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Singh, B. Effect of germination time and temperature on the functionality and protein solubility of sorghum flour. J. Cereal Sci. 2017, 76, 131–139. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Singh, B. Influence of grain activation conditions on functional characteristics of brown rice flour. Food Sci. Technol. Int. 2017, 23, 500–512. [Google Scholar] [CrossRef]
- Ghumman, A.; Kaur, A.; Singh, N. Impact of germination on flour, protein and starch characteristics of lentil (Lens culinari) and horsegram (Macrotyloma uniflorum L.) lines. LWT-Food Sci. Technol. 2016, 65, 137–144. [Google Scholar] [CrossRef]
- El Darra, N.; Rajha, H.N.; Saleh, F.; Al-Oweini, R.; Maroun, R.G.; Louka, N. Food fraud detection in commercial pomegranate molasses syrups by UV–VIS spectroscopy, ATR-FTIR spectroscopy and HPLC methods. Food Control 2017, 78, 132–137. [Google Scholar] [CrossRef]
- Błaszczak, W.; Doblado, R.; Frias, J.; Vidal-Valverde, C.; Sadowska, J.; Fornal, J. Microstructural and biochemical changes in raw and germinated cowpea seeds upon high-pressure treatment. Food Res. Int. 2007, 40, 415–423. [Google Scholar] [CrossRef]
- Pal, R.S.; Bhartiya, A.; Yadav, P.; Kant, L.; Mishra, K.K.; Aditya, J.P.; Pattanayak, A. Effect of dehulling, germination andcooking on nutrients, anti-nutrients, fatty acid composition andantioxidant properties in lentil (Lens culinaris). J. Food Sci. Technol. 2017, 54, 909–920. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Hao, J.; Rao, H.; Zhao, D.; Liu, X. Antioxidant benefits and potential mechanisms of slightly acidic electrolyzed water germination in sesame. Foods 2023, 12, 4104. [Google Scholar] [CrossRef]
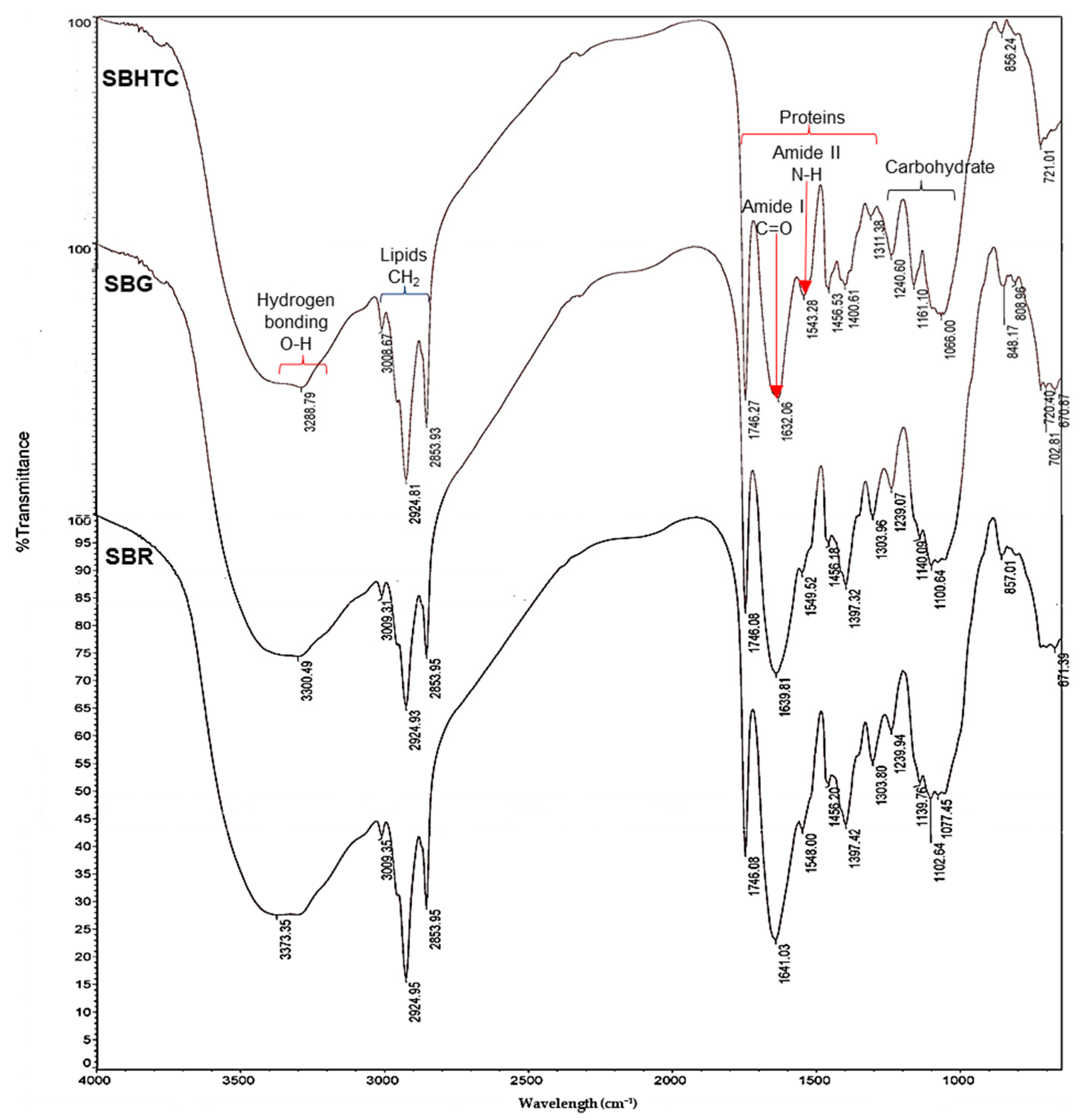
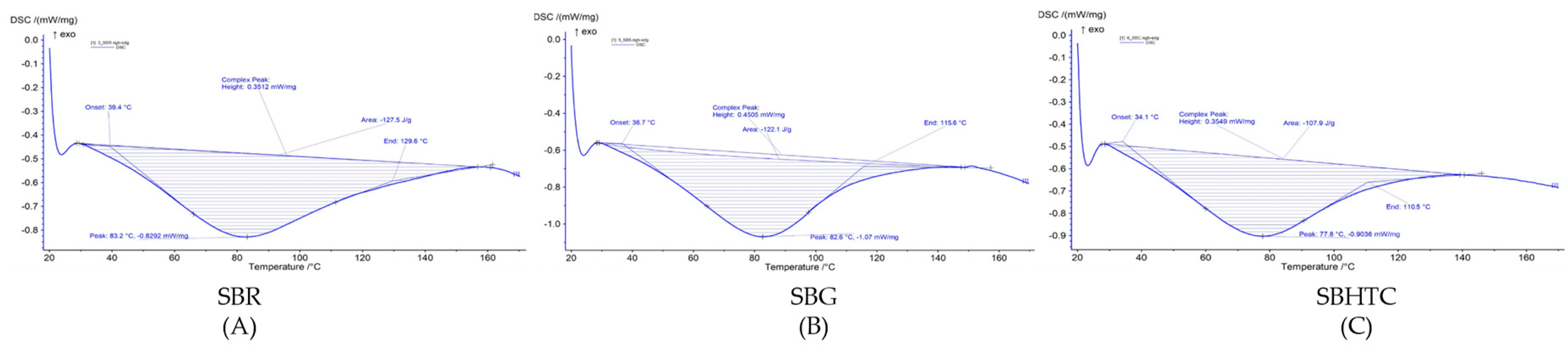
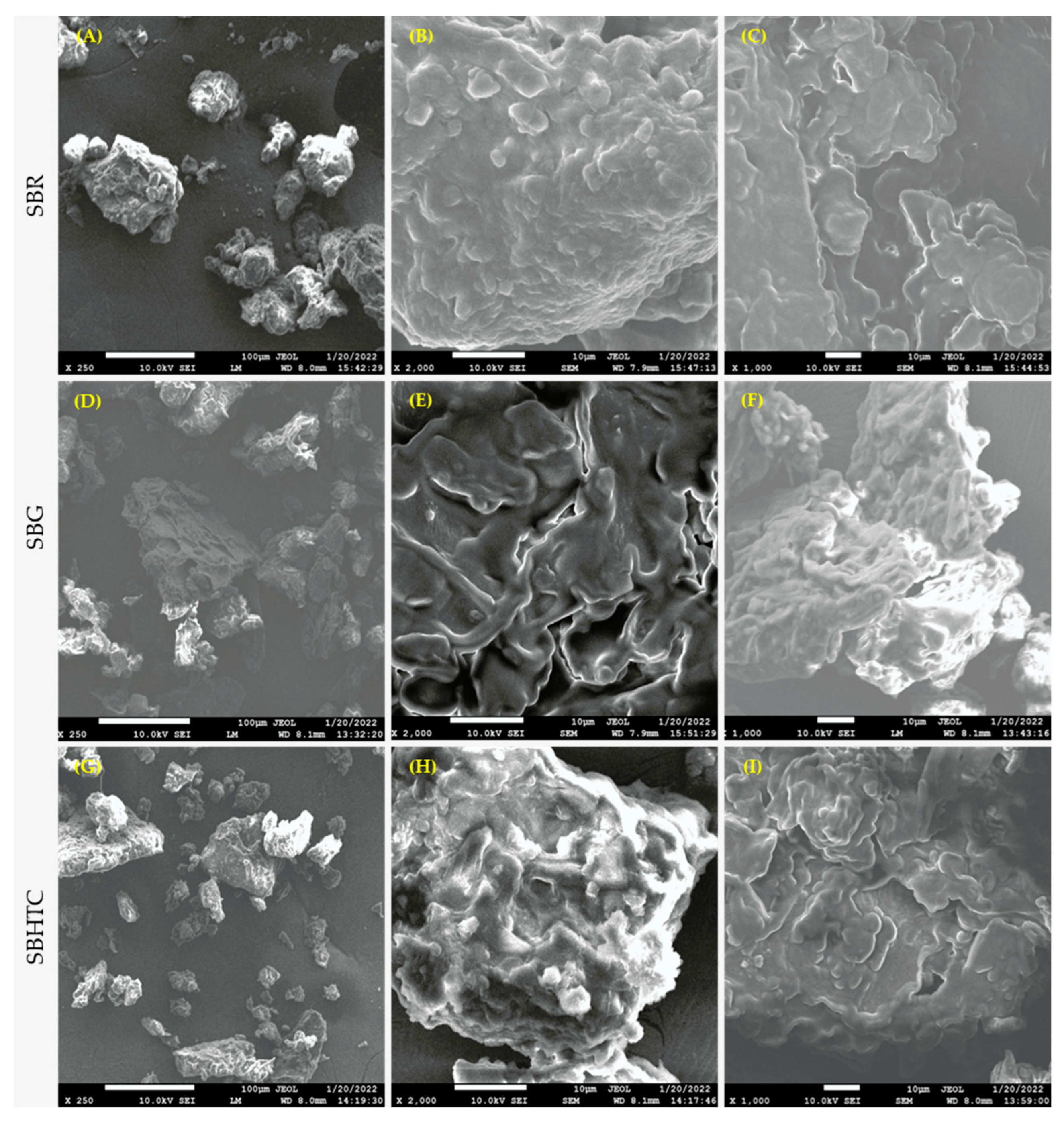
| Parameter | SBR | SBG | SBHTC |
|---|---|---|---|
| Length (mm) | 24.36 ± 0.22 b | 27.15 ± 0.24 c | 21.41 ± 0.21 a |
| Width (mm) | 17.97 ± 0.15 c | 17.95 ± 0.16 b | 15.45 ± 0.19 a |
| Thickness (mm) | 9.48 ± 0.10 b | 10.15 ± 0.16 c | 8.36 ± 0.11 a |
| Diameter (mm) | 16.05 ± 0.13 b | 16.68 ± 0.17 c | 14.00 ± 0.14 a |
| Sphericity (%) | 65.98 ± 0.28 b | 61.49 ± 0.37 a | 65.46 ± 0.30 b |
| Aspect ratio | 0.74 ± 0.00 b | 0.63 ± 0.01 a | 0.72 ± 0.01 b |
| Volume (mm3) | 1175.78 ± 27.98 b | 1323.20 ± 38.01 c | 787.56 ± 20.64 a |
| Surface area (mm2) | 685.74 ± 11.22 b | 742.97 ± 14.58 c | 523.97 ± 10.17 a |
| Weight (g/100 seed) | 197.33 ± 4.10 b | 271.40 ± 1.54 c | 154.28 ± 2.46 a |
| Volume (mL/100 seed) | 210.00 ± 2.89 b | 278.00 ± 3.06 c | 149.67 ± 0.33 a |
| Proximate Composition | SBR | SBG | SBHTC |
|---|---|---|---|
| Moisture | 4.49 ± 0.13 B | 4.38 ± 0.02 B | 3.78 ± 0.07 A |
| Total proteins | 31.40 ± 0.50 A | 35.61 ± 0.06 C | 33.68 ± 0.04 B |
| Total fats | 23.18 ± 0.06 B | 20.29 ± 0.05 A | 28.73 ± 0.21 C |
| Total carbohydrates * | 39.16 ± 0.47 B | 37.97 ± 0.05 B | 33.56 ± 0.25 A |
| Total dietary fibers | 24.96 ± 0.09 B | 23.02 ± 0.05 A | 29.90 ± 0.09 C |
| Soluble dietary fibers | 7.06 ± 0.32 B | 5.26 ± 0.16 A | 8.52 ± 0.04 C |
| Insoluble dietary fibers | 17.90 ± 0.49 A | 17.76 ± 0.11 A | 21.38 ± 0.13 B |
| Ash | 6.26 ± 0.01 C | 6.14 ± 0.03 B | 4.03 ± 0.01 A |
| Energy (kcal) | 490.86 ± 0.60 B | 476.93 ± 0.17 A | 527.53 ± 0.57 C |
| SBRF | SBGF | SBHTCF | SBR | SBG | SBHTC | ||
|---|---|---|---|---|---|---|---|
| Hunter color values | L | 41.33 ± 0.04 a | 44.48 ± 0.03 b | 44.36 ± 0.01 b | 49.33 ± 0.15 B | 45.08 ± 0.12 A | 49.59 ± 0.17 B |
| a* | −4.92 ± 0.01 a | −4.35 ± 0.01 b | 0.20 ± 0.01 c | −9.21 ± 0.03 A | −7.33 ± 0.03 B | 0.08 ± 0.03 C | |
| b* | 19.45 ± 0.05 a | 19.43 ± 0.02 a | 20.15 ± 0.00 b | 36.04 ± 0.07 C | 32.38 ± 0.02 B | 28.09 ± 0.15 A | |
| Croma | 20.07 ± 0.05 b | 19.91 ± 0.02 a | 20.15 ± 0.00 b | 37.20 ± 0.08 C | 33.20 ± 0.02 B | 28.09 ± 0.15 A | |
| Hue Angle | 104.20 ± 0.02 c | 102.62 ± 0.03 b | 89.44 ± 0.03 a | 104.34 ± 0.02 C | 102.76 ± 0.06 B | 89.84 ± 0.06 A | |
| Whitening Index | 62.01 ± 0.03 c | 58.98 ± 0.03 a | 59.17 ± 0.01 b | 62.86 ± 0.08 B | 64.18 ± 0.11 C | 57.70 ± 0.22 A | |
| Browning Index | 51.17 ± 0.13 b | 47.31 ± 0.08 a | 58.68 ± 0.03 c | 99.91 ± 0.21 C | 99.02 ± 0.49 B | 79.12 ± 0.97 A | |
| Concentration (g/100 mL) | SBRF | SBGF | SBHTCF |
|---|---|---|---|
| 2 | - | - | - |
| 4 | - | - | - |
| 6 | - | ± | - |
| 8 | - | ± | ± |
| 10 | ± | + | ± |
| 12 | ± | + | + |
| 14 | + | + | + |
| 16 | + | + | + |
| 18 | + | + | + |
| 20 | + | + | + |
| Parameter | SBR | SBG | SBHTC |
|---|---|---|---|
| Emulsion properties | |||
| Capacity (%) | 56.43 ± 2.49 a | 56.46 ± 1.19 a | 12.08 ± 1.13 b |
| Stability (%) | 56.10 ± 1.74 a | 55.49 ± 1.80 a | 3.80 ± 0.88 b |
| Swelling properties | |||
| Capacity (mL/seed) | 0.91 ± 0.00 a | 0.00 ± 0.00 | 0.11 ± 0.03 a |
| Index | 0.01± 0.0 a | 0.00 ± 0.0 | 0.01± 0.0 a |
| Hydration properties | |||
| Capacity (g/seed) | 0.51 ± 0.03 b | 0.48 ± 0.01 b | 0.11 ± 0.01 a |
| Index | 1.38 ± 0.03 a | 2.57 ± 0.03 b | 1.66 ± 0.11 a |
| Pasting properties | |||
| Temperature (°C) | 55.85 ± 0.4 a | 86.95 ± 0.6 c | 74.5 ± 0.8 b |
| Peak viscosity (cP) | 2.16 ± 0.01 a | 4.96 ± 0.17 b | 4.70 ± 0.07 b |
| Trough viscosity (cP) | 0.46 ± 0.02 a | 0.44 ± 0.04 a | 2.79 ± 0.07 b |
| Final viscosity (cP) | 0.25 ± 0.01 a | 1.62 ± 0.07 b | 2.3 ± 0.12 c |
| Peak time (min) | 2.28 ± 0.01 a | 4.68 ± 0.08 b | 5.27 ± 0.03 c |
| WAC (mL/g) | 0.86 ± 0.0 a | 0.72 ± 0.00 b | 0.69 ± 0.03 b |
| OAC (g/g) | 0.86 ± 0.02 a | 0.81 ± 0.03 a | 0.83 ± 0.03 a |
| Treatment | SBR | SBG | SBHTC |
|---|---|---|---|
| ORAC (mM TE/g) | 862.2 ± 10.0 a | 1115.4 ± 68.1 b | 665.4 ± 22.9 c |
| DPPH (mM TE/g) | 44.8 ± 0.3 a | 59.8 ± 3.1 b | 66.0 ± 3.2 b |
| TPC (mg GE/g) | 6.05 ± 0.49 a | 9.25 ± 0.59 b | 3.40 ± 0.30 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medhe, S.V.; Kettawan, A.K.; Kamble, M.T.; Monboonpitak, N.; Thompson, K.D.; Kettawan, A.; Pirarat, N. Modification of Physiochemical and Techno-Functional Properties of Stink Bean (Parkia speciosa) by Germination and Hydrothermal Cooking Treatment. Foods 2023, 12, 4480. https://doi.org/10.3390/foods12244480
Medhe SV, Kettawan AK, Kamble MT, Monboonpitak N, Thompson KD, Kettawan A, Pirarat N. Modification of Physiochemical and Techno-Functional Properties of Stink Bean (Parkia speciosa) by Germination and Hydrothermal Cooking Treatment. Foods. 2023; 12(24):4480. https://doi.org/10.3390/foods12244480
Chicago/Turabian StyleMedhe, Seema Vijay, Aurawan Kringkasemsee Kettawan, Manoj Tukaram Kamble, Nuntawat Monboonpitak, Kim D. Thompson, Aikkarach Kettawan, and Nopadon Pirarat. 2023. "Modification of Physiochemical and Techno-Functional Properties of Stink Bean (Parkia speciosa) by Germination and Hydrothermal Cooking Treatment" Foods 12, no. 24: 4480. https://doi.org/10.3390/foods12244480






