Effect of Fenugreek (Trigonella foenum-graecum L.) Seed Extracts on the Structure of Myofibrillar Protein Oxidation in Duck Meat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction of the Duck Meat MP
2.3. Extraction of FSE and Construction of the Fenton System
2.4. Hydroxyl Radicals Scavenging Capacity
2.5. Determining Carbonyl Content
2.6. Determining Total Sulfhydryl Content
2.7. SDS-PAGE Electrophoresis
2.8. Fourier Transform Infrared (FTIR) Spectroscopy
2.9. Fluorescence Spectroscopy of Endogenous Tryptophan
2.10. Particle Size
2.11. Zeta Potential
2.12. Determining Surface Hydrophobicity
2.13. Solubility
2.14. Scanning Electron Microscope Analysis
2.15. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Capacity of FSE
3.2. The Effect of FSE on MP Oxidation
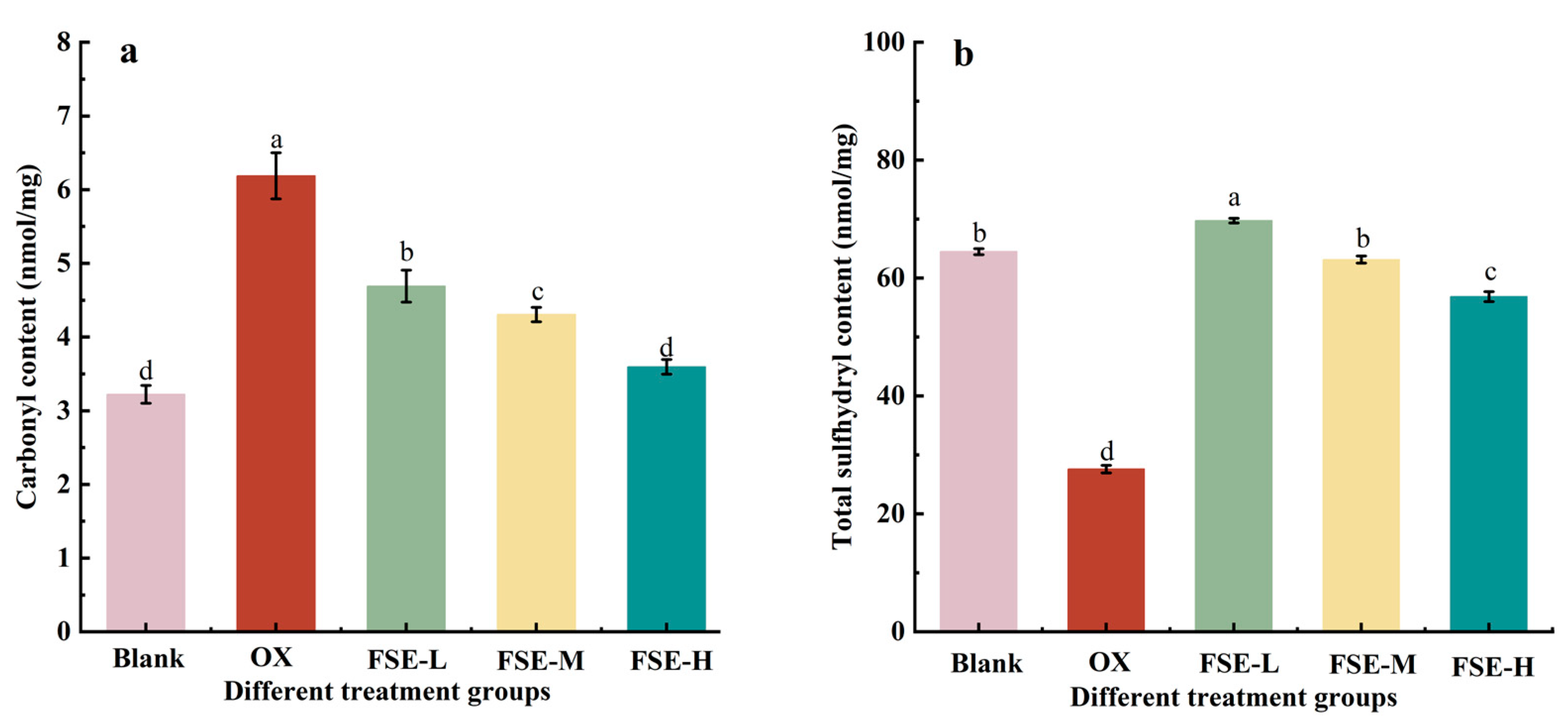
3.2.1. Carbonyl and Total Sulfhydryl Contents in MP
3.2.2. SDS-PAGE Electrophoresis of MP
3.3. The Effect of FSE on MP Structure
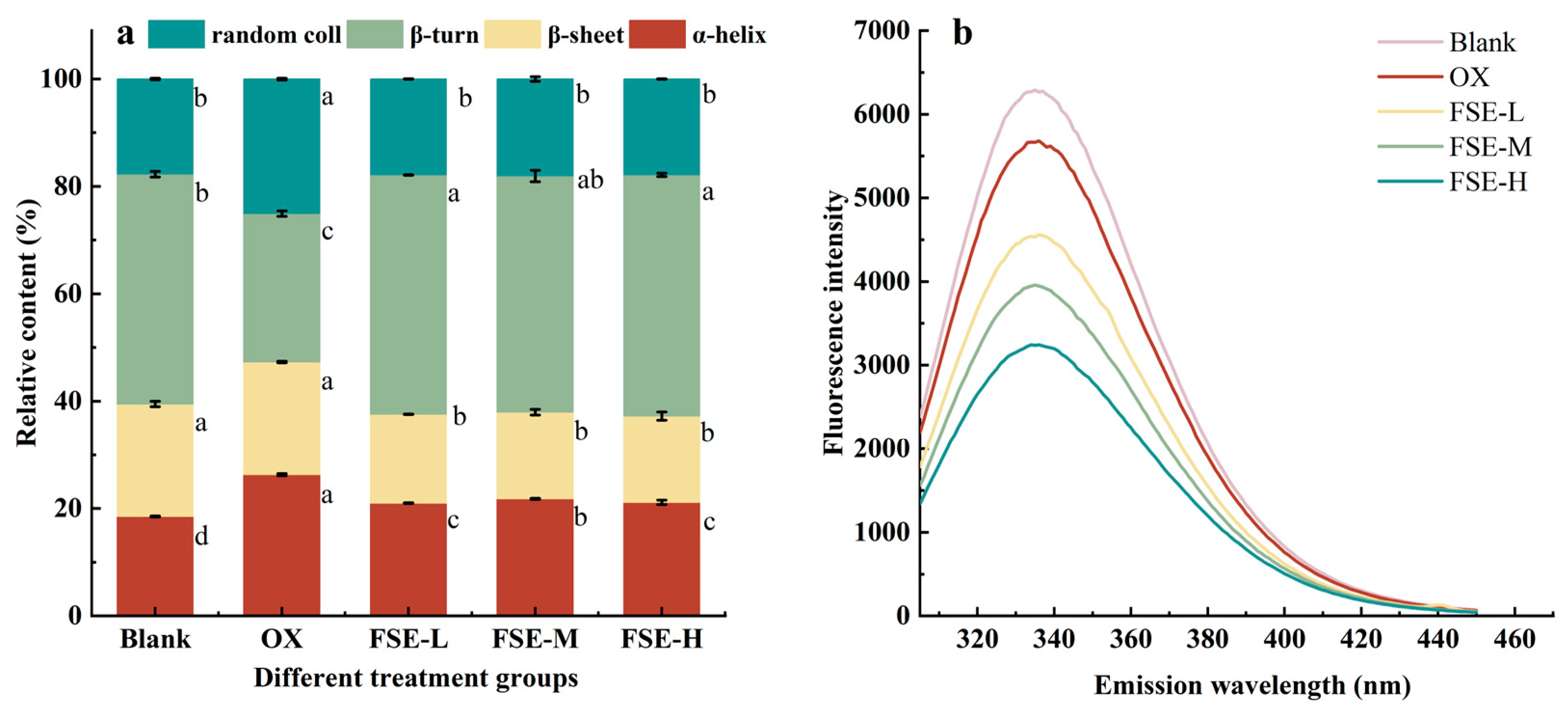
3.3.1. Secondary Structure
3.3.2. Tertiary Structure
3.3.3. Particle Size
3.3.4. Zeta Potential
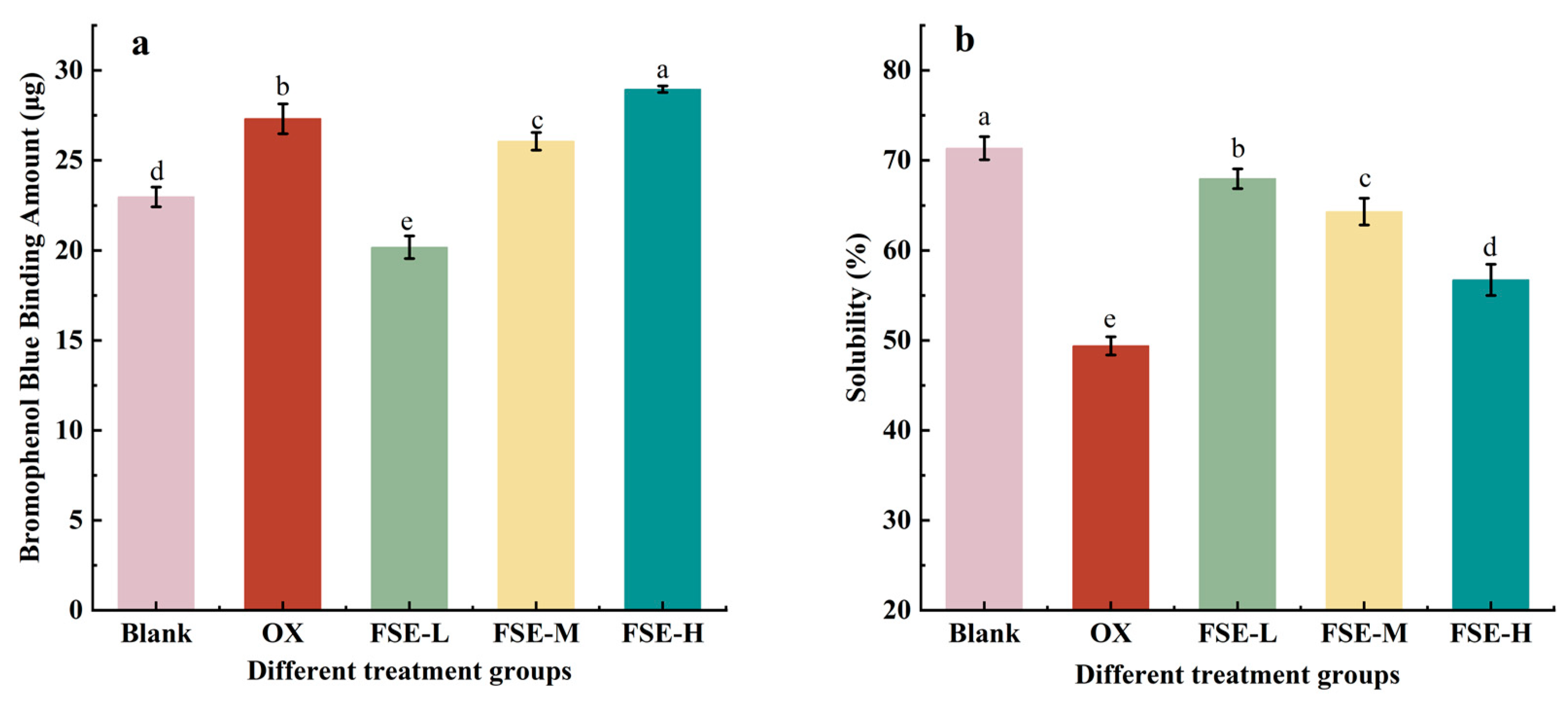
3.3.5. Hydrophobicity
3.3.6. Solubility
3.3.7. Microstructure
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Myofibrillar protein | MP |
| Fenugreek seed extracts | FSE |
| Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis | SDS-PAGE |
| Bromophenol blue | BPB |
| Myosin heavy chain | MHC |
References
- Biswas, S.; Banerjee, R.; Bhattacharyya, D.; Patra, G.; Das, A.K.; Das, S.K. Technological investigation into duck meat and its products—A potential alternative to chicken. Worlds Poult. Sci. J. 2019, 75, 609–620. [Google Scholar] [CrossRef]
- Moczkowska, M.; Poltorak, A.; Montowska, M.; Pospiech, E.; Wierzbicka, A. The effect of the packaging system and storage time on myofibrillar protein degradation and oxidation process in relation to beef tenderness. Meat Sci. 2017, 130, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Rysman, T.; Jongberg, S.; Van Royen, G.; Van Weyenberg, S.; De Smet, S.; Lund, M.N. Protein thiols undergo reversible and irreversible oxidation during chill storage of ground beef as detected by 4,4′-dithiodipyridine. J. Agric. Food Chem. 2014, 62, 12008–12014. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.L.; Blanchard, S.P.; Ooizumi, T.; Ma, Y. Hydroxyl radical and ferryl-generating systems promote gel network formation of myofibrillar protein. J. Food Sci. 2010, 75, C215–C221. [Google Scholar] [CrossRef] [PubMed]
- Yulong, B.; Per, E. Effects of protein oxidation on the texture and water-holding of meat: A review. Crit. Rev. Food Sci. Nutr. 2018, 59, 3564–3578. [Google Scholar] [CrossRef]
- Hellwig, M. The chemistry of protein oxidation in food. Angew. Chem. Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef]
- Huang, Y.; Hua, Y.; Qiu, A. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249. [Google Scholar] [CrossRef]
- Soladoye, O.; Juárez, M.; Aalhus, J.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Compr. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Bao, Y.; Boeren, S.; Ertbjerg, P. Myofibrillar protein oxidation affects filament charges, aggregation and water-holding. Meat Sci. 2017, 135, 102–108. [Google Scholar] [CrossRef]
- Cheng, S.; He, Y.; Zeng, T.; Wang, D.; He, J.; Xia, Q.; Zhou, C.; Pan, D.; Cao, J. Heat stress induces various oxidative damages to myofibrillar proteins in ducks. Food Chem. 2022, 390, 133209. [Google Scholar] [CrossRef]
- Zhao, J.; Xiong, Y.L.; Mcnear, D.H. Changes in Structural Characteristics of Antioxidative Soy Protein Hydrolysates Resulting from Scavenging of Hydroxyl Radicals. J. Food Sci. 2013, 78, C152–C159. [Google Scholar] [CrossRef] [PubMed]
- Utrera, M.; Parra, V.; Estévez, M. Protein oxidation during frozen storage and subsequent processing of different beef muscles. Meat Sci. 2014, 96, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Gokulakrishnan, P.; Giriprasad, R.; Yatoo, M. Fruit-based natural antioxidants in meat and meat products: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1503–1513. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martinez-Larranaga, M.R.; Wang, X.; Martinez, M.; Anadon, A.; Martinez, M.A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, P.; Fan, W.; Liu, Y. Antioxidant Mechanism of Yam Saponin, Hesperidin, Ginger Extract on Oxidative Chicken Myofibrillar Protein. J. Biobased Mater. Bioenergy 2021, 15, 428–434. [Google Scholar] [CrossRef]
- Bridi, R.; Giordano, A.; Peñailillo, M.F.; Montenegro, G. Antioxidant Effect of Extracts from Native Chilean Plants on the Lipoperoxidation and Protein Oxidation of Bovine Muscle. Molecules 2019, 24, 3264. [Google Scholar] [CrossRef]
- Turgut, S.S.; Işıkçı, F.; Soyer, A. Antioxidant activity of pomegranate peel extract on lipid and protein oxidation in beef meatballs during frozen storage—ScienceDirect. Meat Sci. 2017, 129, 111–119. [Google Scholar] [CrossRef]
- Jia, N.; Kong, B.; Liu, Q.; Diao, X.; Xia, X. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 2012, 91, 533–539. [Google Scholar] [CrossRef]
- Khole, S.; Chatterjee, S.; Variyar, P.; Sharma, A.; Devasagayam, T.P.A.; Ghaskadbi, S. Bioactive constituents of germinated fenugreek seeds with strong antioxidant potential. J. Funct. Foods 2014, 6, 270–279. [Google Scholar] [CrossRef]
- Ghosal, S.; Srivastava, R.S.; Chatterjee, D.C.; Dutta, S.K. Fenugreekine, a new steroidal sapogenin-peptide ester of Trigonella foenum-graecum. Phytochemistry 1974, 13, 2247–2251. [Google Scholar] [CrossRef]
- Khoja, K.K.; Howes, M.J.R.; Hider, R.; Sharp, P.A.; Farrell, I.W.; Latunde-Dada, G.O. Cytotoxicity of Fenugreek Sprout and Seed Extracts and Their Bioactive Constituents on MCF-7 Breast Cancer Cells. Nutrients 2022, 14, 784. [Google Scholar] [CrossRef] [PubMed]
- Wagh, R.V.; Chatli, M.K.; Ruusunen, M.; Puolanne, E.; Ertbjerg, P. Effect of Various Phyto-extracts on Physico-chemical, Colour, and Oxidative Stability of Pork Frankfurters. Asian-Australas. J Anim Sci. 2015, 28, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Sabow, A.B.; Ahmad, B.H.; Saleh, S.J. Role of dried fenugreek (Trigonella foenum-graecum L.) leaves as antioxidant and antimicrobial in quality preservation in burgers made of mutton and beef cattle meat during refrigerator storage. Tikrit J. Agric. Sci. 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Han, M.; Zhang, Y.; Fei, Y.; Xu, X.; Zhou, G. Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. Eur. Food Res. Technol. 2008, 228, 665–670. [Google Scholar] [CrossRef]
- Oueslati, K.; Promeyrat, A.; Gatellier, P.; Daudin, J.D.; Kondjoyan, A. Stoichio-kinetic modeling of fenton chemistry in a meat-mimetic aqueous-phase medium. J. Agric. Food. Chem. 2018, 66, 5892–5900. [Google Scholar] [CrossRef]
- Berardo, A.; Claeys, E.; Vossen, E.; Leroy, F.; De Smet, S. Protein oxidation affects proteolysis in a meat model system. Meat Sci. 2015, 106, 78–84. [Google Scholar] [CrossRef]
- Ozyurek, M.; Bektasoglu, B.; Guclu, K.; Apak, R. Hydroxyl radical scavenging assay of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method using catalase for hydrogen peroxide degradation. Anal. Chim. Acta 2008, 616, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carpena, J.G.; Morcuende, D.; Estevez, M. Avocado by-products as inhibitors of color deterioration and lipid and protein oxidation in raw porcine patties subjected to chilled storage. Meat Sci. 2011, 89, 166–173. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Park, D.; Ooizumi, T. Variation in the cross-Linking pattern of porcine myofibrillar protein exposed to three oxidative environments. J. Agric. Food. Chem. 2009, 57, 153–159. [Google Scholar] [CrossRef]
- Nguyen, T.; Kim, S.; Kim, J.G. Diffuse reflectance spectroscopy to quantify the met-myoglobin proportion and meat oxygenation inside of pork and beef. Food Chem. 2018, 275, 369–376. [Google Scholar] [CrossRef]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2017, 245, 871–878. [Google Scholar] [CrossRef]
- Xie, W.; Huang, Y.; Xiang, Y.; Xiong, S.; Manyande, A.; Du, H. Insights into the Binding Mechanism of Polyphenols and Fish Myofibrillar Proteins Explored Using Multi-spectroscopic Methods. Food Bioprocess Technol. 2020, 13, 797–806. [Google Scholar] [CrossRef]
- Hoac, T.; Daun, C.; Trafikowska, U.; Zackrisson, J.; Åkesson, B. Influence of heat treatment on lipid oxidation and glutathione peroxidase activity in chicken and duck meat. Innov. Food Sci. Emerg. Technol. 2006, 7, 88–93. [Google Scholar] [CrossRef]
- Crudden, A.; Afoufa-Bastien, D.; Fox, P.F.; Brisson, G.; Kelly, A.L. Effect of hydrolysis of casein by plasmin on the heat stability of milk. Int. Dairy J. 2005, 15, 1017–1025. [Google Scholar] [CrossRef]
- Farouk, M.M.; Swan, J.E. Effect of rigor temperature and frozen storage on functional properties of hot-boned manufacturing beef. Meat Sci. 1998, 49, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.S.; Yeh, W.T. Biochemical and morphological changes in grass shrimp (Penaeus monodon) muscle following freezing by air blast and liquid nitrogen methods. J. Food Biochem. 2010, 17, 147–160. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Alara, O.R.; Abayomi, O.O. Extraction, characterization and antioxidant activity of fenugreek (Trigonella-foenum graecum) seed oil. Mater. Sci. Energy. Technol. 2019, 2, 349–355. [Google Scholar] [CrossRef]
- Morzel, M.; Gatellier, P.; Sayd, T.; Renerre, M.; Laville, E. Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci. 2006, 73, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Hu, H.; Yang, C. Effect of oxidative modification by reactive oxygen species (ROS) on the aggregation of whey protein concentrate (WPC). Food Hydrocoll. 2022, 123, 107189. [Google Scholar] [CrossRef]
- Jongberg, S.; Torngren, M.A.; Gunvig, A.; Skibsted, L.H.; Lund, M.N. Effect of green tea or rosemary extract on protein oxidation in Bologna type sausages prepared from oxidatively stressed pork. Meat Sci. 2013, 93, 538–546. [Google Scholar] [CrossRef]
- Xiang, R.; Cheng, J.R.; Zhu, M.J.; Liu, X.M. Effect of mulberry (Morus alba) polyphenols as antioxidant on physiochemical properties, oxidation and bio-safety in Cantonese sausages. LWT-Food Sci. Technol. 2019, 116, 108504. [Google Scholar] [CrossRef]
- Prodpran, T.; Benjakul, S.; Phatcharat, S. Effect of phenolic compounds on protein cross-linking and properties of film from fish myofibrillar protein. Int. J. Biol. Macromol. 2012, 51, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Melody, J.L.; Lonergan, S.M.; Rowe, L.J.; Huiatt, T.W.; Mayes, M.S.; Huff-Lonergan, E. Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. J. Anim. Sci. 2004, 82, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhou, F.; Sun, D.-W.; Zhao, M. Effect of Oxidation on the Emulsifying Properties of Myofibrillar Proteins. Food Bioprocess Technol. 2012, 6, 1703–1712. [Google Scholar] [CrossRef]
- Pan, J.F.; Lian, H.L.; Jia, H.; Hao, R.Y.; Wang, Y.J.; Ju, H.P.; Li, S.J.; Dong, X.P. Dose affected the role of gallic acid on mediating gelling properties of oxidatively stressed Japanese seerfish myofibrillar protein. LWT-Food Sci. Technol. 2020, 118, 108849. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, Z.; Zeng, W. Structural and functional modifications of myofibrillar protein by natural phenolic compounds and their application in pork meatball. Food Res. Int. 2021, 148, 110593. [Google Scholar] [CrossRef]
- Ramachander, R.; Jiang, Y.; Li, C.; Eris, T.; Young, M.; Dimitrova, M.; Narhi, L. Solid state fluorescence of lyophilized proteins. Anal. Biochem. 2008, 376, 173–182. [Google Scholar] [CrossRef]
- Guo, X.; Qiu, H.; Deng, X.; Mao, X.; Guo, X.; Xu, C.; Zhang, J. Effect of chlorogenic acid on the physicochemical and functional properties of coregonus peled myofibrillar protein through hydroxyl radical oxidation. Molecules 2019, 24, 3205. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, S.; Alimi, H.; Bouzenna, H.; Elfeki, A.; Hfaiedh, N. Phytochemical study and protective effect of Trigonella foenum graecum (Fenugreek seeds) against carbon tetrachloride-induced toxicity in liver and kidney of male rat. Biomed. Pharmacother. 2017, 88, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, S.; Zhao, G.; Li, Y.; Liu, X.; Yang, L.; Zhu, L.; Liu, H. Fabrication and emulsifying properties of non-covalent complexes between soy protein isolate fibrils and soy soluble polysaccharides. Food Funct. 2022, 13, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Derman, S.; Akdeste, Z.M. Particle size and zeta potential investigation of synthetic peptide-protein conjugates/Sentetik peptid-protein konjugatlarnn parack boyutu ve zeta potensiyel analizi. Turk. J. Biochem. Turk Biyokim. Derg. 2015, 40, 282–289. [Google Scholar] [CrossRef]
- Mukherjee, D.; Chang, S.K.; Zhang, Y.; Mukherjee, S. Effects of Ultra-High Pressure Homogenization and Hydrocolloids on Physicochemical and Storage Properties of Soymilk. J. Food Sci. 2017, 82, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, X.; Zhou, G. Potential of high pressure homogenization to solubilize chicken breast myofibrillar proteins in water. Innov. Food Sci. Emerg. Technol. 2016, 33, 170–179. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Zhang, Z.; Regenstein, J.M.; Zhou, P.; Yang, Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochem. 2017, 34, 960–967. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Chen, L.; Xu, X.; Zhou, G.; Li, Z.; Feng, X. Dose-dependent effects of rosmarinic acid on formation of oxidatively stressed myofibrillar protein emulsion gel at different NaCl concentrations. Food Chem. 2017, 243, 50–57. [Google Scholar] [CrossRef]
- Kang, D.; Zhang, W.; Lorenzo, J.M.; Chen, X. Structural and functional modification of food proteins by high power ultrasound and its application in meat processing. Crit. Rev. Food Sci. Nutr. 2020, 61, 1914–1933. [Google Scholar] [CrossRef]
- Hafid, H.; Patriani, P. Physicochemical and microbiological quality of buffalo meat patty with the addition of fenugreek seed (Trigonella foenum graecum) during storage time. J. Ilmu Dan Teknol. Has. Ternak JITEK 2023, 18, 73–86. [Google Scholar] [CrossRef]
- Zuckerman, H.; Bowker, B.C.; Eastridge, J.S.; Solomon, M.B. Microstructure alterations in beef intramuscular connective tissue caused by hydrodynamic pressure processing. Meat Sci. 2013, 95, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Q.; Zhang, L.T.; Li, R.J.; Zheng, B.D.; Rea, M.C.; Miao, S. Effect of plant protein mixtures on the microstructure and rheological properties of myofibrillar protein gel derived from red sea bream (Pagrosomus major). Food Hydrocoll. 2019, 96, 537–545. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Pan, Q.; Wu, B.; Wang, H.; Yi, Y.; Xu, W.; Guo, D. Effect of Fenugreek (Trigonella foenum-graecum L.) Seed Extracts on the Structure of Myofibrillar Protein Oxidation in Duck Meat. Foods 2023, 12, 4482. https://doi.org/10.3390/foods12244482
Chen M, Pan Q, Wu B, Wang H, Yi Y, Xu W, Guo D. Effect of Fenugreek (Trigonella foenum-graecum L.) Seed Extracts on the Structure of Myofibrillar Protein Oxidation in Duck Meat. Foods. 2023; 12(24):4482. https://doi.org/10.3390/foods12244482
Chicago/Turabian StyleChen, Mingyue, Qingmei Pan, Binbin Wu, Hongxun Wang, Yang Yi, Wei Xu, and Danjun Guo. 2023. "Effect of Fenugreek (Trigonella foenum-graecum L.) Seed Extracts on the Structure of Myofibrillar Protein Oxidation in Duck Meat" Foods 12, no. 24: 4482. https://doi.org/10.3390/foods12244482
APA StyleChen, M., Pan, Q., Wu, B., Wang, H., Yi, Y., Xu, W., & Guo, D. (2023). Effect of Fenugreek (Trigonella foenum-graecum L.) Seed Extracts on the Structure of Myofibrillar Protein Oxidation in Duck Meat. Foods, 12(24), 4482. https://doi.org/10.3390/foods12244482





