Hyperspectral Imaging and Chemometrics for Authentication of Extra Virgin Olive Oil: A Comparative Approach with FTIR, UV-VIS, Raman, and GC-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals, Reagents, and Standards
2.3. Fatty Acid Analysis by Gas Chromatography-Mass Spectrometry (GC-MS)
2.3.1. Transesterification
2.3.2. Preparation of FAME Standard Calibration Curves
2.3.3. Data Acquisition on GC-MS
2.3.4. Identification and Quantification of FAMEs
2.4. Spectral Acquisition by Hyperspectral Imaging
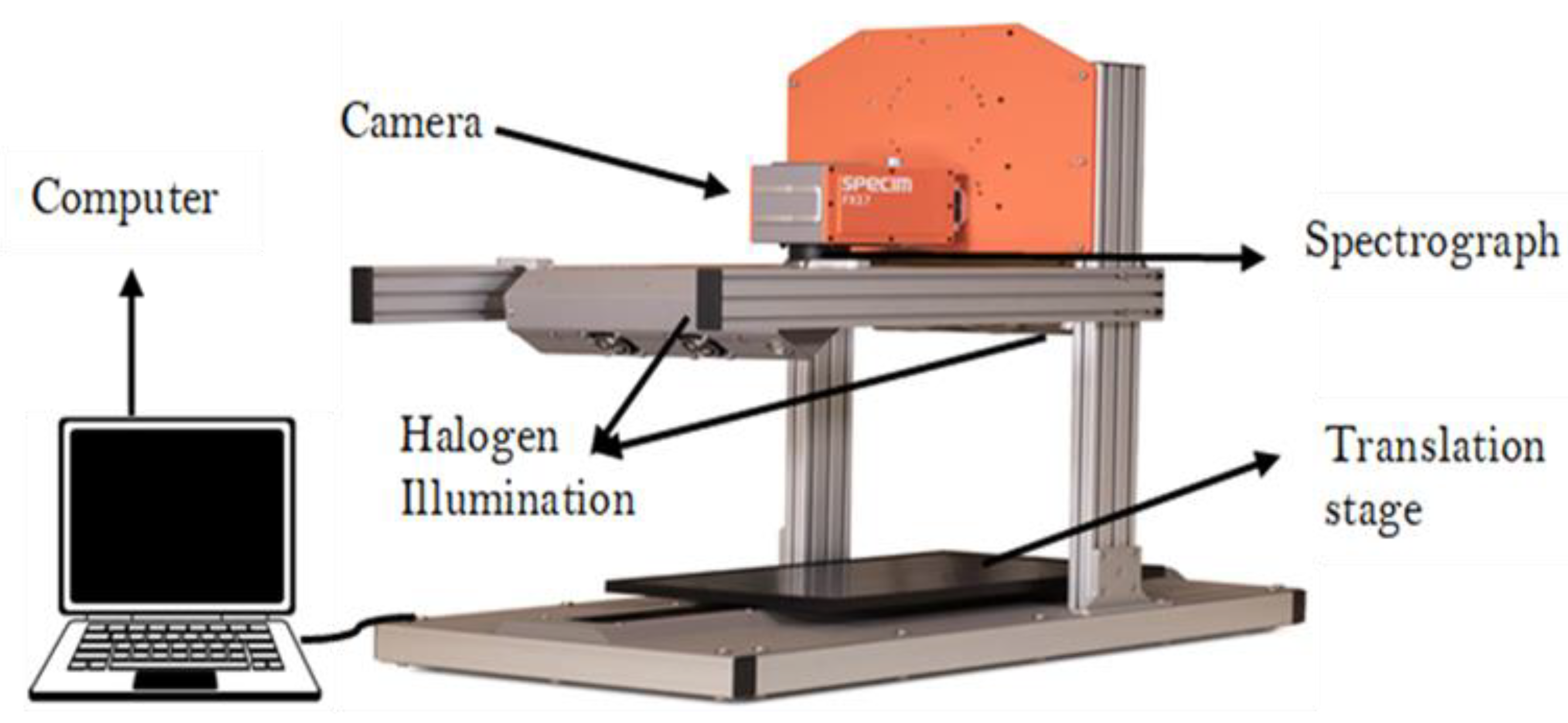
2.4.1. Hyperspectral Imaging System and Software
2.4.2. Sample Image Acquisition
2.4.3. Data Acquisition
2.5. Spectral Data Acquisition by Fourier Transform Infrared Spectroscopy (FTIR)
2.6. Raman Spectroscopy Analysis
2.7. Ultraviolet-Visible (UV-Vis) Spectra Acquisition
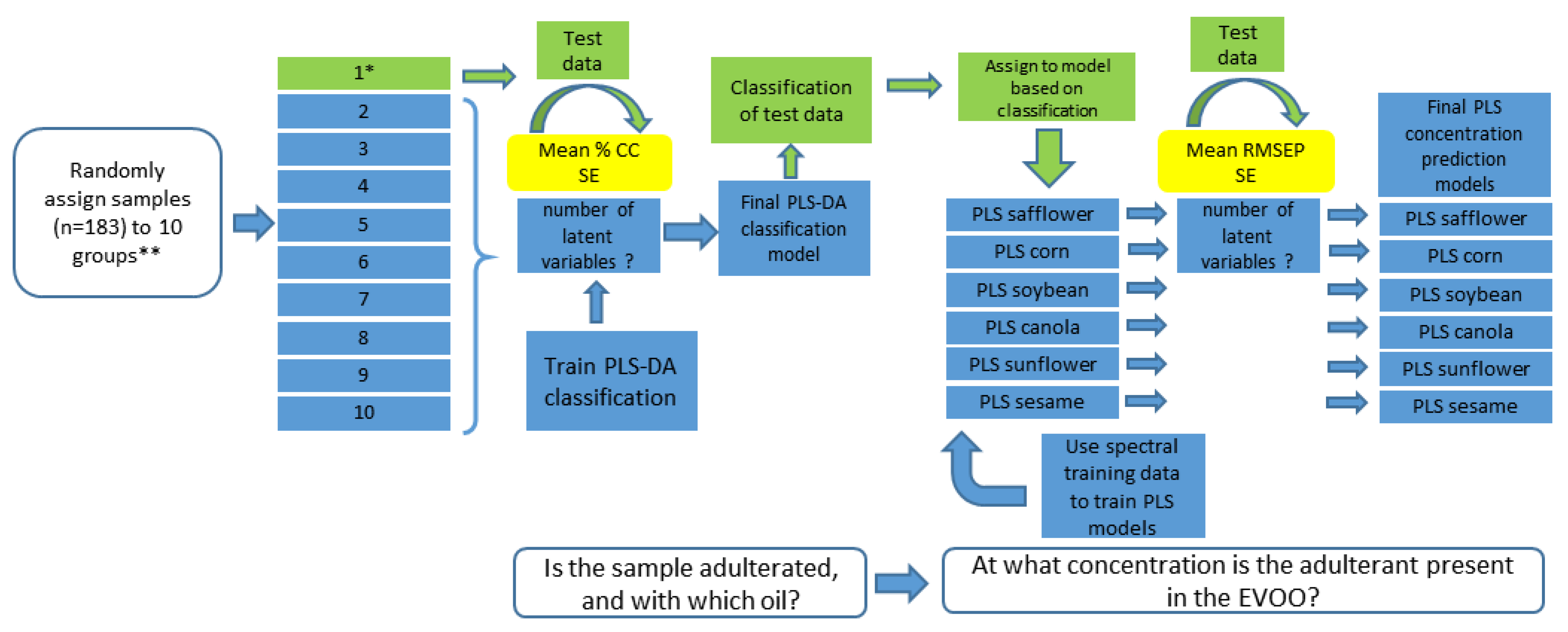
2.8. Chemometrics and Statistical Analysis: Model Construction
3. Results and Discussion
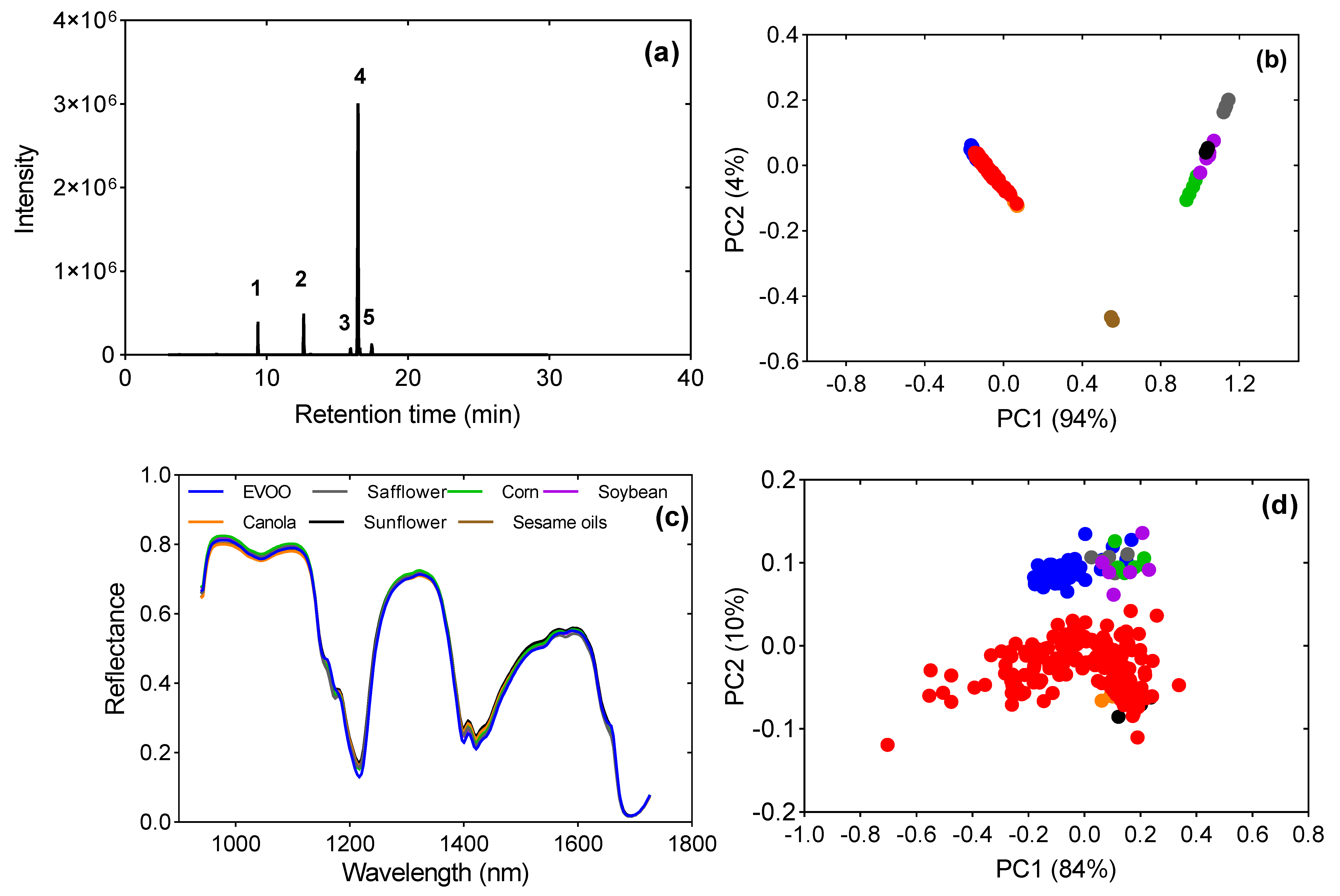
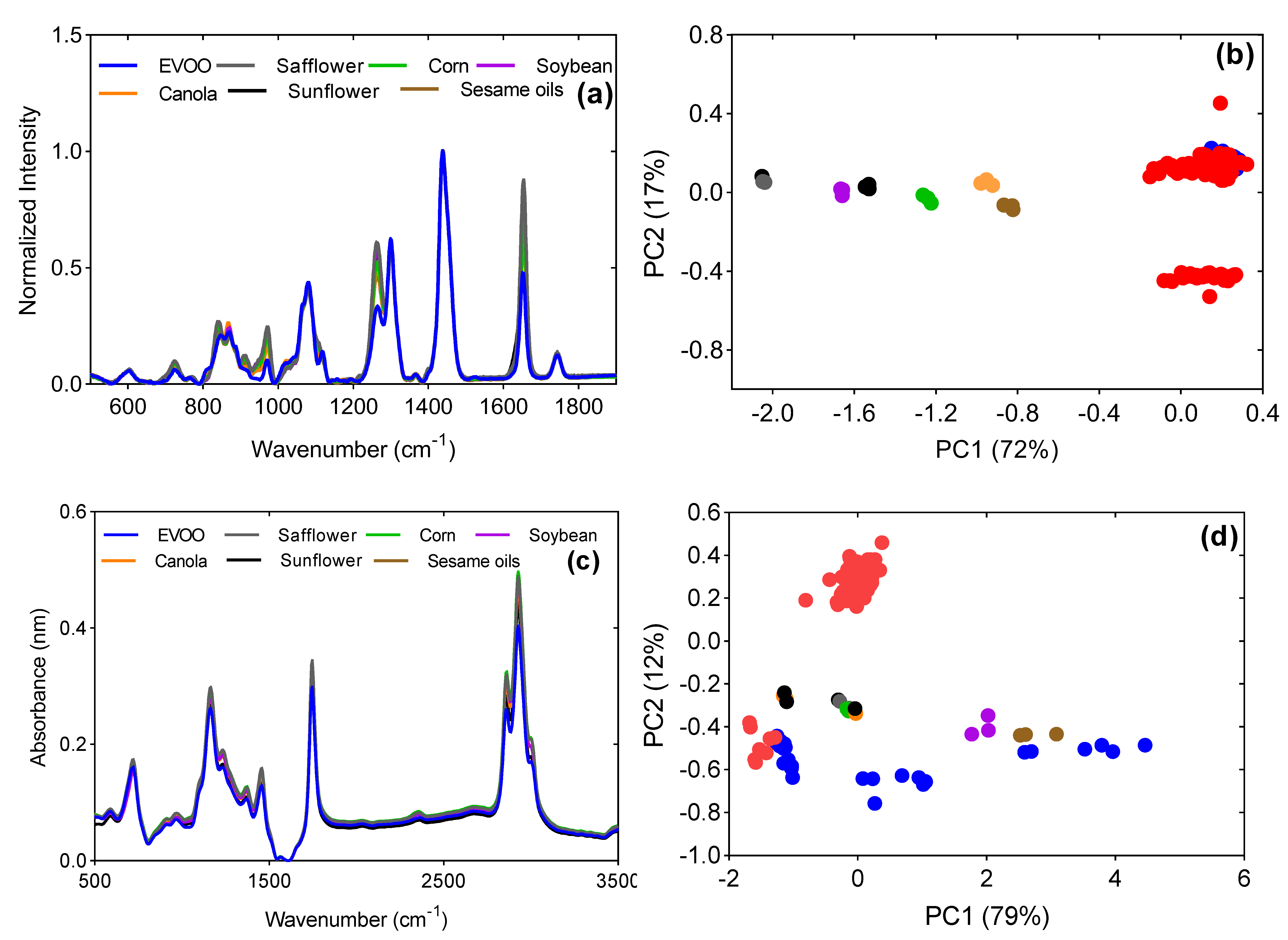
3.1. Chemical Interpretation and PCA Unsupervised Classification
3.2. Discrimination of Oil Types and Determining Adulteration by PLS-DA
3.3. Prediction of Adulterant Concentration in Olive Oil by PLS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Olive Council (IOC). Trade Standard Applying to Olive Oils and Olive-Pomace Oils. COI/T.15/NC No 3/Rev. 14. 2019. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/12/trade-standard-REV-14-Eng.pdf (accessed on 5 September 2022).
- Kakouri, E.; Revelou, P.K.; Kanakis, C.; Daferera, D.; Pappas, C.S.; Tarantilis, P.A. Authentication of the Botanical and Geographical Origin and Detection of Adulteration of Olive Oil Using Gas Chromatography, Infrared and Raman Spectroscopy Techniques: A Review. Foods 2021, 10, 1565. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zou, M.-Q.; Qi, X.-H.; Liu, F.; Zhang, C.; Yin, F. Quantitative Detection of Adulterated Olive Oil by Raman Spectroscopy and Chemometrics. J. Raman Spectrosc. 2011, 42, 1784–1788. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Aparicio-Ruiz, R.; Tena, N.; García-González, D.L. Authenticity of Olive Oil: Mapping and Comparing Official Methods and Promising Alternatives. Food Res. Int. 2013, 54, 2025–2038. [Google Scholar] [CrossRef]
- Zou, M.Q.; Zhang, X.F.; Xiao-Hua, Q.I.; Han-Lu, M.; Dong, Y.; Chun-Wei, L.I.U.; Guo, X.U.N.; Wang, H. Rapid Authentication of Olive Oil Adulteration by Raman Spectrometry. J. Agric. Food Chem. 2009, 57, 6001–6006. [Google Scholar] [CrossRef]
- Moore, J.C.; Spink, J.; Lipp, M. Development and Application of a Database of Food Ingredient Fraud and Economically Motivated Adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef] [PubMed]
- Jabeur, H.; Zribi, A.; Bouaziz, M. Extra-Virgin Olive Oil and Cheap Vegetable Oils: Distinction and Detection of Adulteration as Determined by GC and Chemometrics. Food Anal. Methods 2016, 9, 712–723. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Saftić, L.; Mašek, T.; Kraljević Pavelić, S. Comparison of Triacylglycerol Analysis by MALDI-TOF/MS, Fatty Acid Analysis by GC-MS and Non-Selective Analysis by NIRS in Combination with Chemometrics for Determination of Extra Virgin Olive Oil Geographical Origin. A Case Study. LWT 2018, 95, 326–332. [Google Scholar] [CrossRef]
- Jiménez-Carvelo, A.M.; Osorio, M.T.; Koidis, A.; González-Casado, A.; Cuadros-Rodríguez, L. Chemometric Classification and Quantification of Olive Oil in Blends with Any Edible Vegetable Oils Using FTIR-ATR and Raman Spectroscopy. LWT Food Sci. Technol. 2017, 86, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Rohman, A.; Che Man, Y.b.; Ismail, A.; Hashim, P. FTIR Spectroscopy Coupled with Chemometrics of Multivariate Calibration and Discriminant Analysis for Authentication of Extra Virgin Olive Oil. Int. J. Food Prop. 2017, 20, S1173–S1181. [Google Scholar] [CrossRef]
- de Lima, T.K.; Musso, M.; Bertoldo Menezes, D. Using Raman Spectroscopy and an Exponential Equation Approach to Detect Adulteration of Olive Oil with Rapeseed and Corn Oil. Food Chem. 2020, 333, 127454. [Google Scholar] [CrossRef]
- El-Abassy, R.M.; Donfack, P.; Materny, A. Visible Raman Spectroscopy for the Discrimination of Olive Oils from Different Vegetable Oils and the Detection of Adulteration. J. Raman Spectrosc. 2009, 40, 1284–1289. [Google Scholar] [CrossRef]
- Mendes, T.O.; da Rocha, R.A.; Porto, B.L.S.; de Oliveira, M.A.L.; dos Anjos, V.d.C.; Bell, M.J.V. Quantification of Extra-Virgin Olive Oil Adulteration with Soybean Oil: A Comparative Study of NIR, MIR, and Raman Spectroscopy Associated with Chemometric Approaches. Food Anal. Methods 2015, 8, 2339–2346. [Google Scholar] [CrossRef]
- Uncu, O.; Ozen, B. A Comparative Study of Mid-Infrared, UV–Visible and Fluorescence Spectroscopy in Combination with Chemometrics for the Detection of Adulteration of Fresh Olive Oils with Old Olive Oils. Food Control 2019, 105, 209–218. [Google Scholar] [CrossRef]
- Didham, M.; Truong, V.K.; Chapman, J.; Cozzolino, D. Sensing the Addition of Vegetable Oils to Olive Oil: The Ability of UV–VIS and MIR Spectroscopy Coupled with Chemometric Analysis. Food Anal. Methods 2020, 13, 601–607. [Google Scholar] [CrossRef]
- Milanez, K.D.T.M.; Nóbrega, T.C.A.; Nascimento, D.S.; Insausti, M.; Band, B.S.F.; Pontes, M.J.C. Multivariate Modeling for Detecting Adulteration of Extra Virgin Olive Oil with Soybean Oil Using Fluorescence and UV–Vis Spectroscopies: A Preliminary Approach. LWT 2017, 85, 9–15. [Google Scholar] [CrossRef]
- Ok, S. Detection of Olive Oil Adulteration by Low-Field NMR Relaxometry and UV-Vis Spectroscopy upon Mixing Olive Oil with Various Edible Oils. Grasas Aceites 2017, 68, e173. [Google Scholar] [CrossRef] [Green Version]
- Mendez, J.; Mendoza, L.; Cruz-Tirado, J.P.; Quevedo, R.; Siche, R. Trends in Application of NIR and Hyperspectral Imaging for Food Authentication. Sci. Agropecu. 2019, 10, 143–161. [Google Scholar] [CrossRef]
- Baiano, A. Applications of Hyperspectral Imaging for Quality Assessment of Liquid Based and Semi-Liquid Food Products: A Review. J. Food Eng. 2017, 214, 10–15. [Google Scholar] [CrossRef]
- Choi, J.Y.; Moon, K.D. Non-Destructive Discrimination of Sesame Oils via Hyperspectral Image Analysis. J. Food Compos. Anal. 2020, 90, 103505. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Q.; He, Y. Identification of Different Varieties of Sesame Oil Using Near-Infrared Hyperspectral Imaging and Chemometrics Algorithms. PLoS ONE 2014, 9, e98522. [Google Scholar] [CrossRef]
- Martínez Gila, D.M.; Cano Marchal, P.; Gámez García, J.; Gómez Ortega, J. On-Line System Based on Hyperspectral Information to Estimate Acidity, Moisture and Peroxides in Olive Oil Samples. Comput. Electron. Agric. 2015, 116, 1–7. [Google Scholar] [CrossRef]
- Romaniello, R.; Baiano, A. Discrimination of Flavoured Olive Oil Based on Hyperspectral Imaging. J. Food Sci. Technol. 2018, 55, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, C. Near Infrared Spectroscopy: A Mature Analytical Technique with New Perspectives—A Review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Sánchez-Rodríguez, M.I.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Caridad, J.M.; Moalem, M. Chemometric Study of Andalusian Extra Virgin Olive Oils Raman Spectra: Qualitative and Quantitative Information. Talanta 2016, 156–157, 180–190. [Google Scholar] [CrossRef]
- Nikkhah, A.; Van Haute, S.; Jovanovic, V.; Jung, H.; Dewulf, J.; Cirkovic Velickovic, T.; Ghnimi, S. Life Cycle Assessment of Edible Insects (Protaetia Brevitarsis Seulensis Larvae) as a Future Protein and Fat Source. Sci. Rep. 2021, 11, 14030. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.H.; Friedman, J.H. Springer Series in Statistics The Elements of Statistical Learning. Math. Intell. 2009, 27, 83–85. [Google Scholar]
- Uncu, O.; Ozen, B.; Tokatli, F. Use of FTIR and UV-Visible Spectroscopy in Determination of Chemical Characteristics of Olive Oils. Talanta 2019, 201, 65–73. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Meyer, H.; Bendix, J. Hsdar: Manage, Analyse and Simulate Hyperspectral Data in R; R Package Version 0.4; 2022. Available online: https://cran.r-project.org/package=hsdar (accessed on 20 September 2022).
- Boulesteix, A.-L.; Durif, G.; Lambert-Lacroix, S.; Peyre, J.; Strimmer, K. Plsgenomics: PLS Analyses for Genomics. 2018. Available online: https://cran.r-project.org/package=plsgenomics (accessed on 20 September 2022).
- Liland, K.H.; Mevik, B.H.; Wehrens, R.; Hiemstra, P. PLS: Partial Least Squares and Principal Component Regression; R Package Version 2; 2022. Available online: https://CRAN.R-project.org/package=pls (accessed on 20 September 2022).
- Codex Stan 33-1981; Codex Alimentarius (International Food Standards). Standard for Olive Oils and Olive Pomace oils. Codex Alimentarius. FAO: Rome, Italy; WHO: Geneva, Switzerland, Adopted in 1981. Revised in 1989, 2003, 2015, 2017. Amended in 2009, 2013, 2021. Available online: https://www.fao.org/fao-who-codexalimentarius (accessed on 5 August 2022).
- Codex Alimentarius Commission. Codex Standard for Named Vegetable Oils. Codex Stan 2001, 8, 11–25. [Google Scholar]
- Xiaobo, Z.; Jiewen, Z.; Povey, M.J.W.; Holmes, M.; Hanpin, M. Variables Selection Methods in Near-Infrared Spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive Measurement of Fruit and Vegetable Quality by Means of NIR Spectroscopy: A Review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Afseth, N.K.; Wold, J.P.; Segtnan, V.H. The Potential of Raman Spectroscopy for Characterisation of the Fatty Acid Unsaturation of Salmon. Anal. Chim. Acta 2006, 572, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hashem, H.; Almoselhy, R.I.M.; Magdy, A. Rapid Authentication of Extra Virgin Olive Oil Using UV and FTIR Spectroscopy. Middle East J. Appl. Sci. 2020, 10, 263–271. [Google Scholar] [CrossRef]
- de la Mata, P.; Dominguez-Vidal, A.; Bosque-Sendra, J.M.; Ruiz-Medina, A.; Cuadros-Rodríguez, L.; Ayora-Cañada, M.J. Olive Oil Assessment in Edible Oil Blends by Means of ATR-FTIR and Chemometrics. Food Control 2012, 23, 449–455. [Google Scholar] [CrossRef]
- Yang, Y.; Ferro, M.D.; Cavaco, I.; Liang, Y. Detection and Identification of Extra Virgin Olive Oil Adulteration by GC-MS Combined with Chemometrics. J. Agric. Food Chem. 2013, 61, 3693–3702. [Google Scholar] [CrossRef]
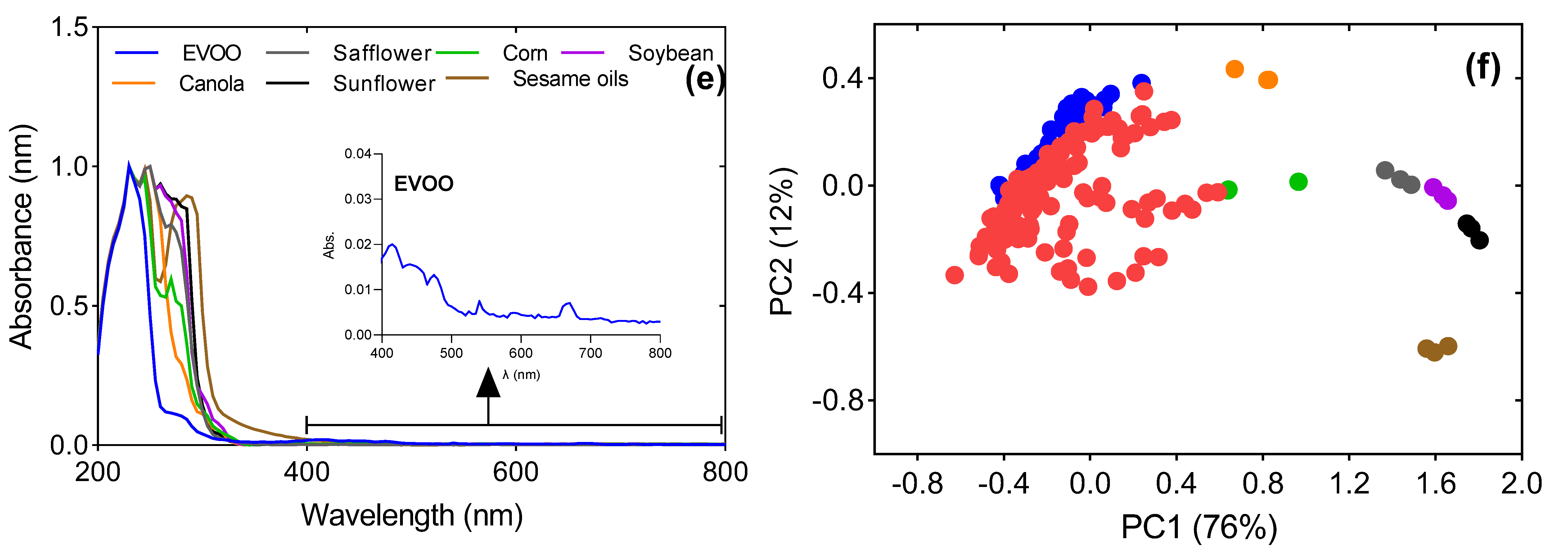
| FAME | EVOO | Safflower Oil | Corn Oil | Soybean Oil | Canola Oil | Sunflower Oil | Sesame Oil |
|---|---|---|---|---|---|---|---|
| Palmitic (C16:0) | 16.49 ± 0.49 | 8.99 ± 0.50 | 13.58 ± 0.81 | 12.53 ± 1.10 | 11.52 ± 0.40 | 8.88 ± 0.58 | 8.27 ± 1.22 |
| Palmitoleic (C16:1) | 0.11 ± 0.0 | nd | nd | nd | 0.06 ± 0.01 | nd | nd |
| Stearic (C18:0) | 4.17 ± 0.31 | 3.86 ± 0.42 | 2.36 ± 0.16 | 6.71 ± 0.41 | 2.40 ± 0.19 | 3.67 ± 0.47 | 5.51 ± 0.67 |
| Oleic (C18:1 cis-9) | 75.69 ± 0.73 | 10.38 ± 1.03 | 25.50 ± 1.47 | 17.76 ± 1.87 | 57.99 ± 0.58 | 20.18 ± 0.52 | 44.82 ± 0.65 |
| Elaidic acid (C18:1 trans-9) | nd | 0.13 ± 0.02 | 0.05 ± 0.02 | 0.46 ± 0.10 | 2.96 ± 0.21 | 1.01 ± 0.04 | 0.92 ± 0.26 |
| Linoleic (C18:2) | 3.48 ± 0.46 | 76.50 ± 1.88 | 58.30 ± 1.49 | 58.62 ± 1.56 | 17.57 ± 0.45 | 66.26 ± 0.44 | 40.49 ± 0.26 |
| Linolenic (C18:3) | 0.06 ± 0.02 | 0.14 ± 0.03 | 0.21 ± 0.03 | 3.93 ± 0.48 | 7.49 ± 0.53 | nd | nd |
| SFA | 20.66 ± 0.48 | 12.85 ± 0.90 | 15.94 ± 0.90 | 19.24 ± 1.16 | 13.92 ± 0.49 | 12.55 ± 0.41 | 13.78 ± 0.63 |
| MUFA | 75.80 ± 0.72 | 10.38 ± 1.03 | 25.50 ± 1.47 | 17.76 ± 1.87 | 58.05 ± 0.59 | 20.18 ± 0.44 | 44.82 ± 0.26 |
| PUFA | 3.54 ± 0.46 | 76.64 ± 1.89 | 58.52 ± 1.48 | 62.54 ± 1.80 | 25.06 ± 0.87 | 66.26 ± 0.44 | 40.49 ± 0.26 |
| Wavenumber (cm−1) | Molecule/Group | Vibrational Mode |
|---|---|---|
| 868 | –(CH2)n– | C–C stretching |
| 968 | trans RHC=CHR | C=C bending |
| 1008 | HC–CH3 | CH3 bending |
| 1150 | –(CH2)n– | C–C stretching |
| 1265 | cis RHC=CHR | =C–H bending (scissoring) |
| 1300 | –(CH)2 | C–H bending (twisting) |
| 1440 | –(CH)2 | C–H bending (scissoring) |
| 1525 | RHC=CHR | C=C stretching |
| 1650 | cis RHC=CHR | C=C stretching |
| 1750 | RC=OOR | C=O stretching |
| Technique | LVs | Classification as Pure EVOO, Adulterant (Type of Edible Oil) or Category of Adulterated EVOO * (Details in Table 4) | Classification as Pure EVOO or Adulterated Olive Oil |
|---|---|---|---|
| HSI | 25 | 93.83 ± 0.34 | 100 |
| Raman | 14 | 87.76 ± 0.48 | 96.56 ± 0.16 |
| UV-vis | 26 | 96.72 ± 0.24 | 99.56 ± 0.18 |
| FTIR | 33 | 91.97 ± 0.47 | 99.78 ± 0.09 |
| GC-MS | 7 | 77.60 ± 0.32 | 93.72 ± 0.22 |
| Predicted | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Method | Class | EVOO | EVOO + Safflower | EVOO + Corn | EVOO + Soybean | EVOO + Canola | EVOO + Sunflower | EVOO + Sesame | |
| Measured | HSI | EVOO | 390 (100%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| EVOO + safflower | 0.0 | 200 (95.2%) | 10 (4.8%) | 0.0 | 0.0 | 0.0 | 0.0 | ||
| EVOO + corn | 0.0 | 18 (8.6%) | 175 (83.3%) | 17 (8.1%) | 0.0 | 0.0 | 0.0 | ||
| EVOO + soybean | 0.0 | 6 (2.9%) | 7 (3.3%) | 197 (93.8%) | 0.0 | 0.0 | 0.0 | ||
| EVOO + canola | 0.0 | 0.0 | 0.0 | 0.0 | 210 (100%) | 0.0 | 0.0 | ||
| EVOO + sunflower | 0.0 | 0.0 | 0.0 | 0.0 | 45 (21.4%) | 158 (75.2%) | 7 (3.3%) | ||
| EVOO + sesame | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 210 (100%) | ||
| Raman | EVOO | 335 (85.9%) | 30 (7.7%) | 17 (4.4%) | 8 (2.1%) | 0.0 | 0.0 | 0.0 | |
| EVOO + safflower | 6 (2.9%) | 180 (85.7%) | 4 (1.9%) | 16 (7.6%) | 0.0 | 0.0 | 4 (3.8%) | ||
| EVOO + corn | 0.0 | 0.0 | 162 (77.1%) | 48 (22.9%) | 0.0 | 0.0 | 0.0 | ||
| EVOO + soybean | 1 (0.5%) | 0.0 | 31 (14.8%) | 138 (65.7%) | 40 (19.0%) | 0.0 | 0.0 | ||
| EVOO + canola | 0.0 | 0.0 | 0.0 | 0.0 | 207 (98.6%) | 3 (1.4%) | 0.0 | ||
| EVOO + sunflower | 0.0 | 6 (2.9%) | 1 (0.5%) | 0 | 6 (2.9%) | 197 (93.8%) | 0.0 | ||
| EVOO + sesame | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 210 (100%) | ||
| UV-vis | EVOO | 382 (97.9%) | 0.0 | 0.0 | 0.0 | 6 (1.5%) | 2 (0.5%) | 0.0 | |
| EVOO + safflower | 0.0 | 210 (100.0%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| EVOO + corn | 0.0 | 0.0 | 187 (89.0%) | 23 (11.0%) | 0.0 | 0.0 | 0.0 | ||
| EVOO + soybean | 0.0 | 3 (1.4%) | 16 (7.6%) | 190 (90.5%) | 1 (0.5%) | 0.0 | 0.0 | ||
| EVOO + canola | 0.0 | 0.0 | 0.0 | 0.0 | 210 (100%) | 0.0 | 0.0 | ||
| EVOO + sunflower | 0.0 | 0.0 | 0.0 | 0.0 | 2 (1.0%) | 204 (97.1%) | 4 (1.9%) | ||
| EVOO + sesame | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 210 (100%) | ||
| FTIR | EVOO | 386 (99.0%) | 4 (1.0%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| EVOO + safflower | 0.0 | 190 (90.5%) | 20 (9.5%) | 0.0 | 0.0 | 0.0 | 0.0 | ||
| EVOO + corn | 0.0 | 0.0 | 194 (88.3%) | 16 (9.2%) | 0.0 | 0.0 | 0.0 | ||
| EVOO + soybean | 0.0 | 0.0 | 6 (2.9%) | 194 (92.4%) | 10 (4.8%) | 0.0 | 0.0 | ||
| EVOO + canola | 0.0 | 0.0 | 0.0 | 0.0 | 193 (91.9%) | 17 (8.1%) | 0.0 | ||
| EVOO + sunflower | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 180 (85.7%) | 30 (14.3%) | ||
| EVOO + sesame | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 5 (2.4%) | 205 (97.6%) | ||
| GC-MS | EVOO | 364 (93.3%) | 0.0 | 11 (2.8%) | 0.0 | 0.0 | 15 (3.8%) | 0.0 | |
| EVOO + safflower | 39 (18.6%) | 100 (47.6%) | 55 (26.2%) | 0.0 | 0.0 | 16 (7.6%) | 0.0 | ||
| EVOO + corn | 0.0 | 59 (28.1%) | 110 (52.4%) | 1 (0.5%) | 0.0 | 40 (19.0%) | 0.0 | ||
| EVOO + soybean | 0.0 | 10 (4.8%) | 32 (15.2%) | 158 (75.2%) | 0.0 | 0.0 | 10 (4.8%) | ||
| EVOO + canola | 10 (4.8%) | 20 (9.5%) | 16 (7.6%) | 0.0 | 160 (76.2%) | 4 (1.9%) | 0.0 | ||
| EVOO + sunflower | 25 (11.9%) | 0.0 | 13 (6.2%) | 0.0 | 0.0 | 162 (77.1%) | 10 (4.8%) | ||
| EVOO + sesame | 15 (7.1%) | 5 (2.4%) | 1 (0.5%) | 0.0 | 0.0 | 0.0 | 90 (84.2%) | ||
| Training Set (Cross-Validation) | Test Set (Considering PLS-DA Classification Errors) a | Test Set (Known Adulterant Identity) b | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | PLS-Regression Model | LVs | R2CV | Predicted vs. Measured Fitted Line | RMSECV (%) | RPD | LVs | R2pred | Predicted vs. Measured Fitted Line | RMSEP (%) | RPD | LVs | R2pred | Predicted vs. Measured Fitted Line | RMSEP (%) | RPD |
| HSI | Overall | 19 | 0.97 | y = 0.98x + 0.17 | 1.17 | 5.80 | 17 | 0.97 | y = 0.98x + 0.21 | 1.14 | 5.91 | 18 | 0.97 | y = 0.98 + 0.16 | 1.14 | 5.98 |
| EVOO + safflower | 10 | 0.99 | y = 0.98x + 0.18 | 0.53 | 13.14 | 9 | 0.95 | y = 0.90x + 0.62 | 1.62 | 4.42 | 10 | 1.00 | y = 0.98x + 0.22 | 0.48 | 14.23 | |
| EVOO + corn | 9 | 0.98 | y = 0.98x + 0.11 | 0.87 | 8.06 | 9 | 0.98 | y = 0.95x + 0.59 | 0.89 | 7.26 | 9 | 0.99 | y = 0.99x + 0.38 | 0.76 | 8.90 | |
| EVOO + soybean | 8 | 0.98 | y = 0.97x + 0.44 | 1.09 | 6.38 | 7 | 0.97 | y = 1.00x − 0.03 | 1.14 | 6.00 | 7 | 0.98 | y = 0.97x + 0.33 | 1.06 | 6.45 | |
| EVOO + canola | 7 | 0.99 | y = 0.99x + 0.09 | 0.62 | 11.28 | 7 | 0.98 | y = 0.99x + 0.23 | 0.86 | 7.80 | 6 | 1.00 | y = 0.99x + 0.10 | 0.45 | 14.96 | |
| EVOO + sunflower | 4 | 0.99 | y = 0.98x + 0.21 | 0.84 | 8.35 | 4 | 0.98 | y = 0.97x + 0.24 | 0.91 | 7.10 | 4 | 0.98 | y = 0.98x + 0.23 | 0.88 | 7.67 | |
| EVOO + sesame | 6 | 0.99 | y = 0.98x + 0.17 | 0.69 | 10.13 | 5 | 0.98 | y = 1.00x + 0.11 | 0.90 | 8.48 | 6 | 0.99 | y = 0.98x + 0.20 | 0.57 | 12.00 | |
| Raman | Overall | 8 | 0.92 | y = 0.94x + 0.61 | 1.95 | 3.49 | 8 | 0.92 | y = 0.94x + 0.62 | 1.96 | 3.48 | 8 | 0.92 | y = 0.94x + 0.52 | 1.95 | 3.46 |
| EVOO + safflower | 2 | 0.90 | y = 0.90x + 0.93 | 2.17 | 3.21 | 2 | 0.86 | y = 0.88x + 1.01 | 2.39 | 2.69 | 2 | 0.90 | y = 0.94x + 0.40 | 2.17 | 3.12 | |
| EVOO + corn | 6 | 0.97 | y = 0.95x + 0.51 | 1.16 | 6.03 | 2 | 0.95 | y = 0.96x + 0.41 | 1.43 | 4.58 | 6 | 0.97 | y = 0.94x + 0.52 | 1.20 | 5.65 | |
| EVOO + soybean | 5 | 0.98 | y = 0.97x + 0.20 | 1.00 | 7.00 | 4 | 0.97 | y = 0.93x + 0.46 | 1.29 | 5.23 | 5 | 0.98 | y = 0.97x + 0.15 | 1.00 | 6.79 | |
| EVOO + canola | 3 | 0.96 | y = 0.92x + 0.63 | 1.35 | 5.18 | 3 | 0.92 | y = 1.09x − 0.03 | 2.55 | 2.86 | 3 | 0.97 | y = 0.91x + 0.81 | 1.27 | 5.33 | |
| EVOO + sunflower | 3 | 0.93 | y = 0.92x + 0.66 | 1.84 | 3.80 | 3 | 0.93 | y = 0.98x + 0.09 | 1.78 | 3.76 | 3 | 0.93 | y = 0.97x + 0.06 | 1.80 | 3.77 | |
| EVOO + sesame | 5 | 0.96 | y = 0.92x + 0.61 | 1.39 | 5.03 | 5 | 0.96 | y = 0.95x + 0.35 | 1.30 | 5.21 | 5 | 0.96 | y = 0.94x + 0.40 | 1.29 | 5.27 | |
| UV-vis | Overall | 8 | 0.63 | y = 0.67x + 2.77 | 4.15 | 1.64 | 8 | 0.64 | y = 0.65x + 3.13 | 4.06 | 1.66 | 8 | 0.64 | y = 0.68x + 2.75 | 4.10 | 1.66 |
| EVOO + safflower | 6 | 0.94 | y = 0.96x + 0.11 | 1.76 | 3.97 | 6 | 0.94 | y = 0.97x + 0.12 | 1.70 | 4.02 | 9 | 0.96 | y = 0.97x + 0.06 | 1.45 | 4.69 | |
| EVOO + corn | 4 | 0.95 | y = 0.99x + 0.19 | 1.67 | 4.18 | 2 | 0.90 | y = 0.83x + 2.21 | 2.46 | 2.99 | 4 | 0.94 | y = 0.97x + 0.35 | 1.69 | 4.00 | |
| EVOO + soybean | 9 | 0.98 | y = 0.98x + 0.09 | 0.96 | 7.30 | 9 | 0.99 | y = 0.99x | 0.77 | 8.76 | 9 | 0.98 | y = 0.98x + 0.10 | 0.89 | 7.83 | |
| EVOO + canola | 9 | 0.97 | y = 1.02x − 0.05 | 1.19 | 5.91 | 8 | 0.93 | y = 1.01x − 0.14 | 1.60 | 5.77 | 9 | 0.99 | y = 1.01x + 0.02 | 0.84 | 8.11 | |
| EVOO + sunflower | 4 | 0.93 | y = 0.92x + 0.44 | 1.91 | 3.66 | 4 | 0.94 | y = 0.91x + 1.01 | 1.64 | 4.11 | 4 | 0.94 | y = 0.90x + 1.07 | 1.65 | 4.14 | |
| EVOO + sesame | 4 | 0.98 | y = 1.00x − 0.07 | 1.01 | 6.98 | 3 | 0.86 | y = 0.91x + 0.63 | 2.40 | 3.39 | 5 | 0.98 | y = 0.99x + 0.01 | 0.83 | 8.15 | |
| FTIR | Overall | 16 | 0.69 | y = 0.78x + 1.94 | 3.87 | 1.78 | 19 | 0.77 | y = 0.85x + 1.41 | 3.26 | 2.07 | 19 | 0.77 | y = 0.85x + 1.34 | 3.31 | 2.06 |
| EVOO + safflower | 4 | 0.68 | y = 0.74x + 2.00 | 3.98 | 1.76 | 5 | 0.62 | y = 0.62x + 2.00 | 3.68 | 1.64 | 5 | 0.71 | y = 0.80x + 1.99 | 3.79 | 1.81 | |
| EVOO + corn | 4 | 0.63 | y = 0.74x + 2.27 | 4.34 | 1.62 | 2 | 0.17 | y = 0.25x + 6.65 | 6.50 | 1.07 | 10 | 0.79 | y = 0.93x + 0.53 | 3.32 | 2.04 | |
| EVOO + soybean | 2 | 0.53 | y = 0.57x + 3.4 | 4.81 | 1.45 | 2 | 0.21 | y = 0.29x + 5.56 | 6.19 | 1.11 | 2 | 0.45 | y = 0.49x + 4.56 | 5.02 | 1.35 | |
| EVOO + canola | 8 | 0.84 | y = 0.90x + 0.75 | 2.76 | 2.58 | 8 | 0.60 | y = 0.75x + 1.41 | 4.59 | 1.73 | 8 | 0.87 | y = 0.90x + 0.72 | 2.45 | 2.77 | |
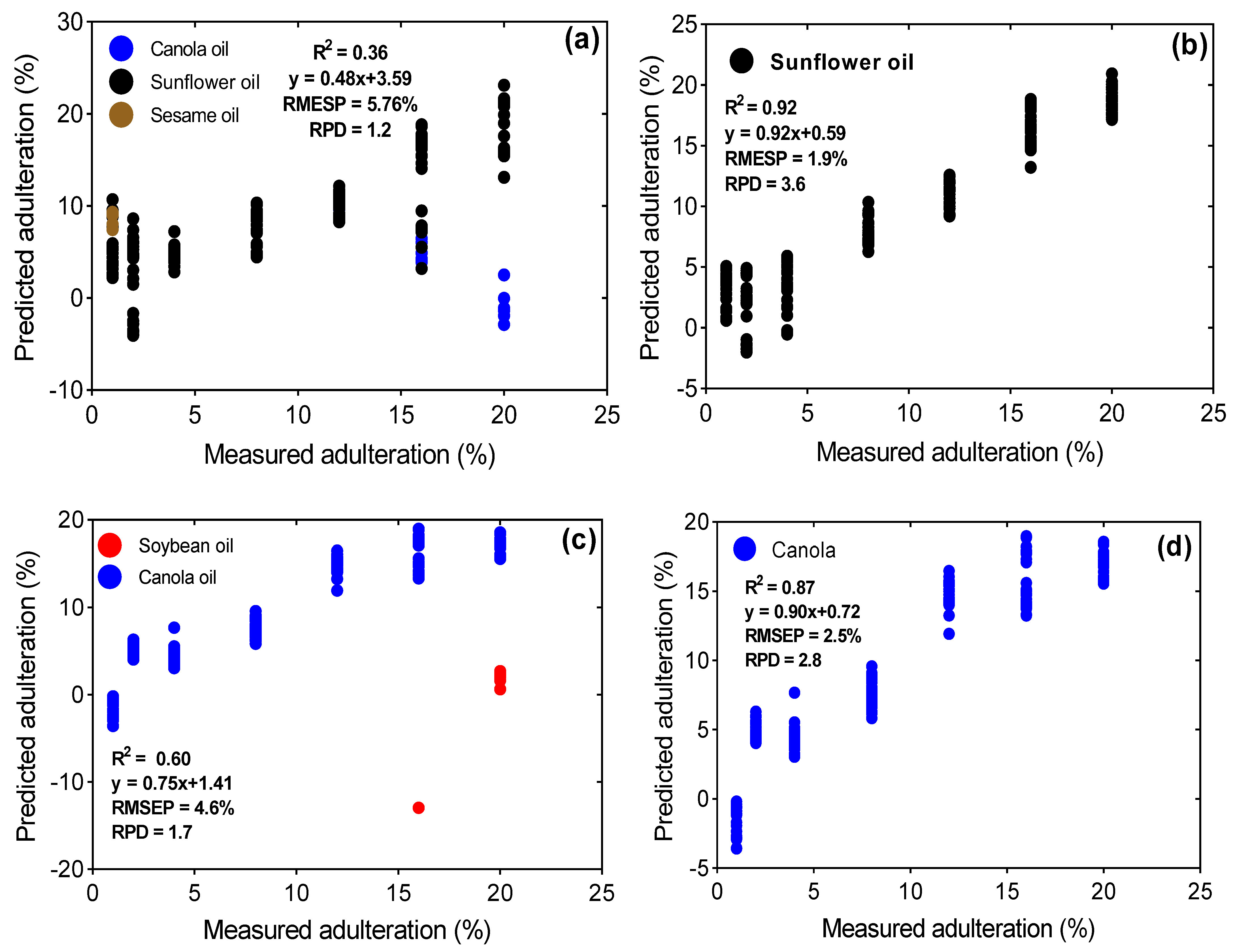
| EVOO + sunflower | 10 | 0.91 | y = 0.92x + 0.57 | 2.04 | 3.41 | 3 | 0.36 | y = 0.48x + 3.59 | 5.76 | 1.21 | 10 | 0.92 | y = 0.92x + 0.59 | 1.89 | 3.60 | |
| EVOO + sesame | 7 | 0.74 | y = 0.87x + 1.18 | 3.72 | 1.89 | 4 | 0.43 | y = 0.53x + 3.36 | 5.36 | 1.28 | 7 | 0.77 | y = 0.84x + 1.61 | 3.26 | 2.08 | |
| GC-MS | Overall | 6 | 0.90 | y = 0.93x + 1.13 | 7.70 | 3.04 | 6 | 0.53 | y = 0.68x + 3.45 | 4.85 | 1.38 | 6 | 0.90 | y = 0.92x + 1.21 | 7.49 | 3.14 |
| EVOO + safflower | 5 | 0.92 | y = 0.96x + 1.31 | 11.52 | 3.70 | 2 | 0.22 | y = 0.37x + 9.79 | 6.50 | 1.04 | 2 | 0.54 | y = 0.63x + 6.09 | 5.41 | 1.25 | |
| EVOO + corn | 5 | 0.92 | y = 0.96x + 1.08 | 11.44 | 3.69 | 4 | 0.27 | y = 0.84x + 4.20 | 7.60 | 0.63 | 2 | 0.24 | y = 0.43x + 7.30 | 6.77 | 0.99 | |
| EVOO + soybean | 5 | 0.89 | y = 0.94x + 1.94 | 13.63 | 3.08 | 3 | 0.55 | y = 1.02 + 2.44 | 6.33 | 0.99 | 3 | 0.60 | y = 0.60x + 3.88 | 6.05 | 1.13 | |
| EVOO + canola | 3 | 0.88 | y = 0.92x + 2.99 | 14.44 | 2.87 | 3 | 0.53 | y = 0.95x + 2.52 | 6.24 | 1.05 | 2 | 0.29 | y = 0.55x + 9.08 | 8.38 | 0.81 | |
| EVOO + sunflower | 4 | 0.91 | y = 0.95x + 1.71 | 12.43 | 3.35 | 5 | 0.19 | y = 0.53x + 3.81 | 6.86 | 0.82 | 2 | 0.24 | y = 0.55x + 5.89 | 7.48 | 0.91 | |
| EVOO + sesame | 4 | 0.89 | y = 0.94x + 2.01 | 13.65 | 3.09 | 3 | 0.51 | y = 0.66x + 4.92 | 5.28 | 1.33 | 2 | 0.19 | y = 0.30x + 7.79 | 6.45 | 1.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malavi, D.; Nikkhah, A.; Raes, K.; Van Haute, S. Hyperspectral Imaging and Chemometrics for Authentication of Extra Virgin Olive Oil: A Comparative Approach with FTIR, UV-VIS, Raman, and GC-MS. Foods 2023, 12, 429. https://doi.org/10.3390/foods12030429
Malavi D, Nikkhah A, Raes K, Van Haute S. Hyperspectral Imaging and Chemometrics for Authentication of Extra Virgin Olive Oil: A Comparative Approach with FTIR, UV-VIS, Raman, and GC-MS. Foods. 2023; 12(3):429. https://doi.org/10.3390/foods12030429
Chicago/Turabian StyleMalavi, Derick, Amin Nikkhah, Katleen Raes, and Sam Van Haute. 2023. "Hyperspectral Imaging and Chemometrics for Authentication of Extra Virgin Olive Oil: A Comparative Approach with FTIR, UV-VIS, Raman, and GC-MS" Foods 12, no. 3: 429. https://doi.org/10.3390/foods12030429






