Analysis of Akkermansia muciniphila in Mulberry Galacto-Oligosaccharide Medium via Comparative Transcriptomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Strain
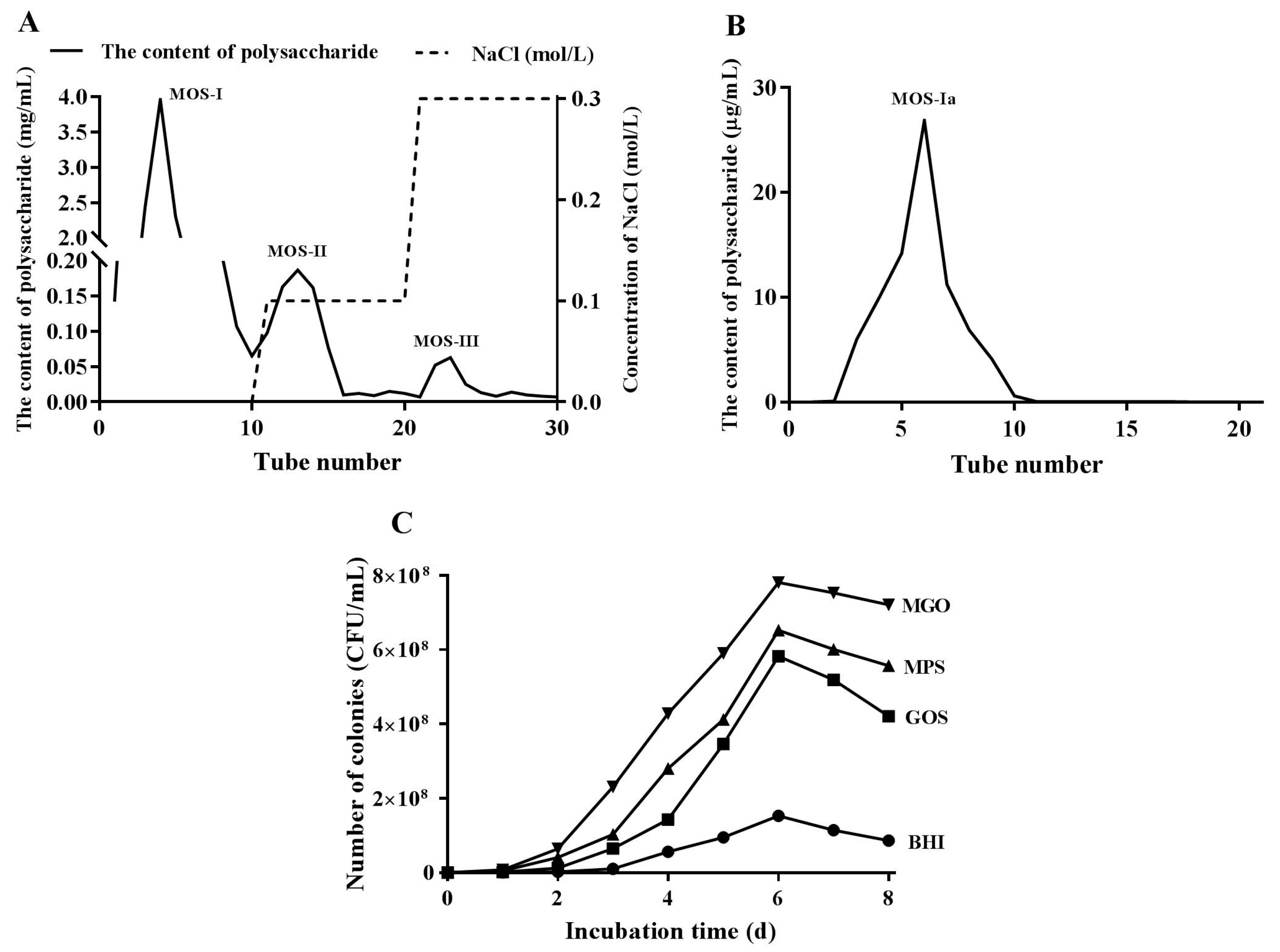
2.2. Preparation, Isolation, and Purification of Mulberry Oligosaccharides
2.3. Effects of Different Prebiotics on the Growth of A. muciniphila
2.4. RNA-Seq and Real-Time Quantitative PCR (RT-qPCR) Validation
2.4.1. Bioinformatic and Differential Expression Analyses
2.4.2. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
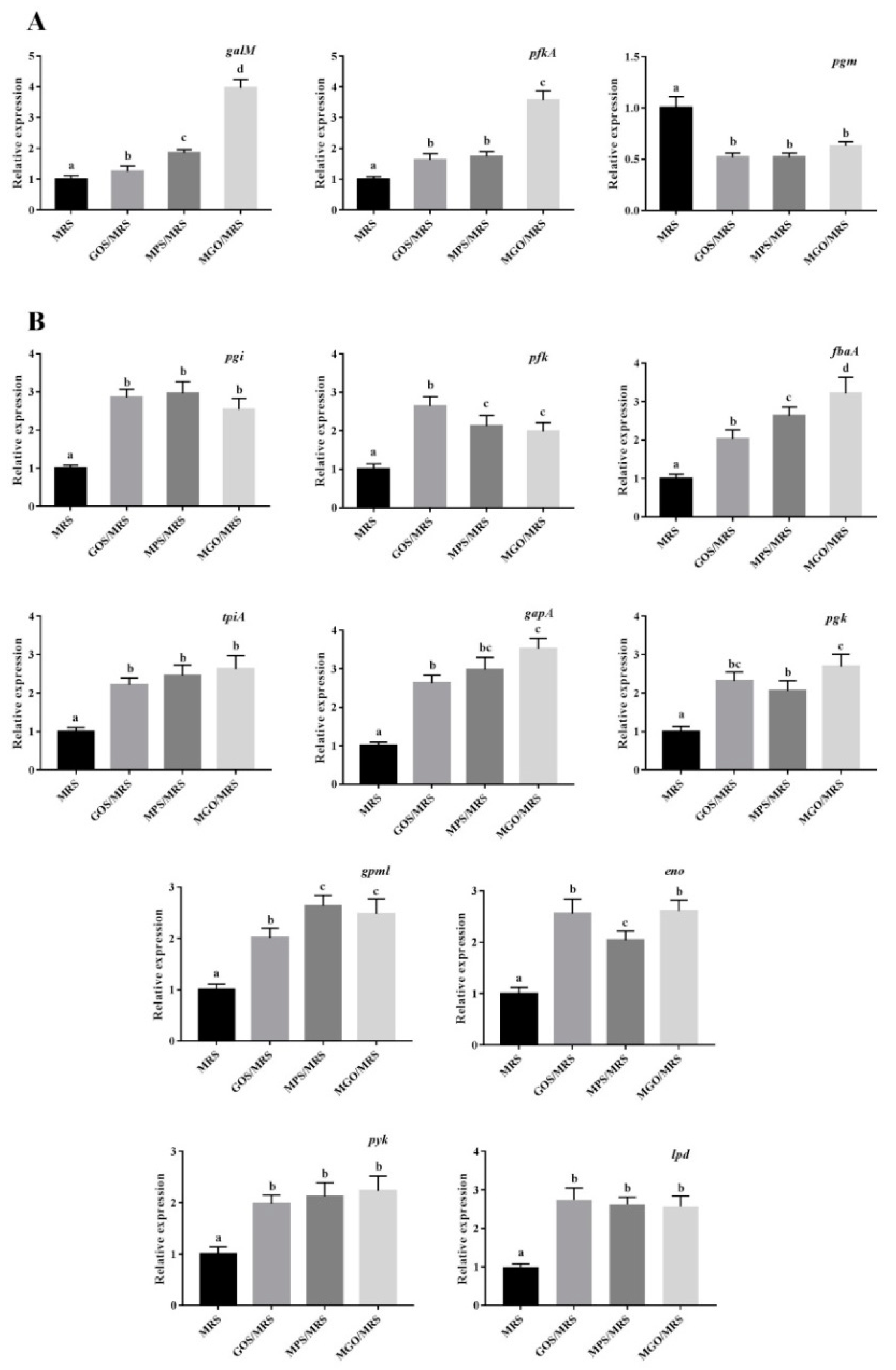
2.4.3. Validation of RNA-Seq Results by RT-qPCR
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proliferation of A. muciniphila Cultured with Different Carbohydrates
3.2. Differential Gene Expression for Analysis
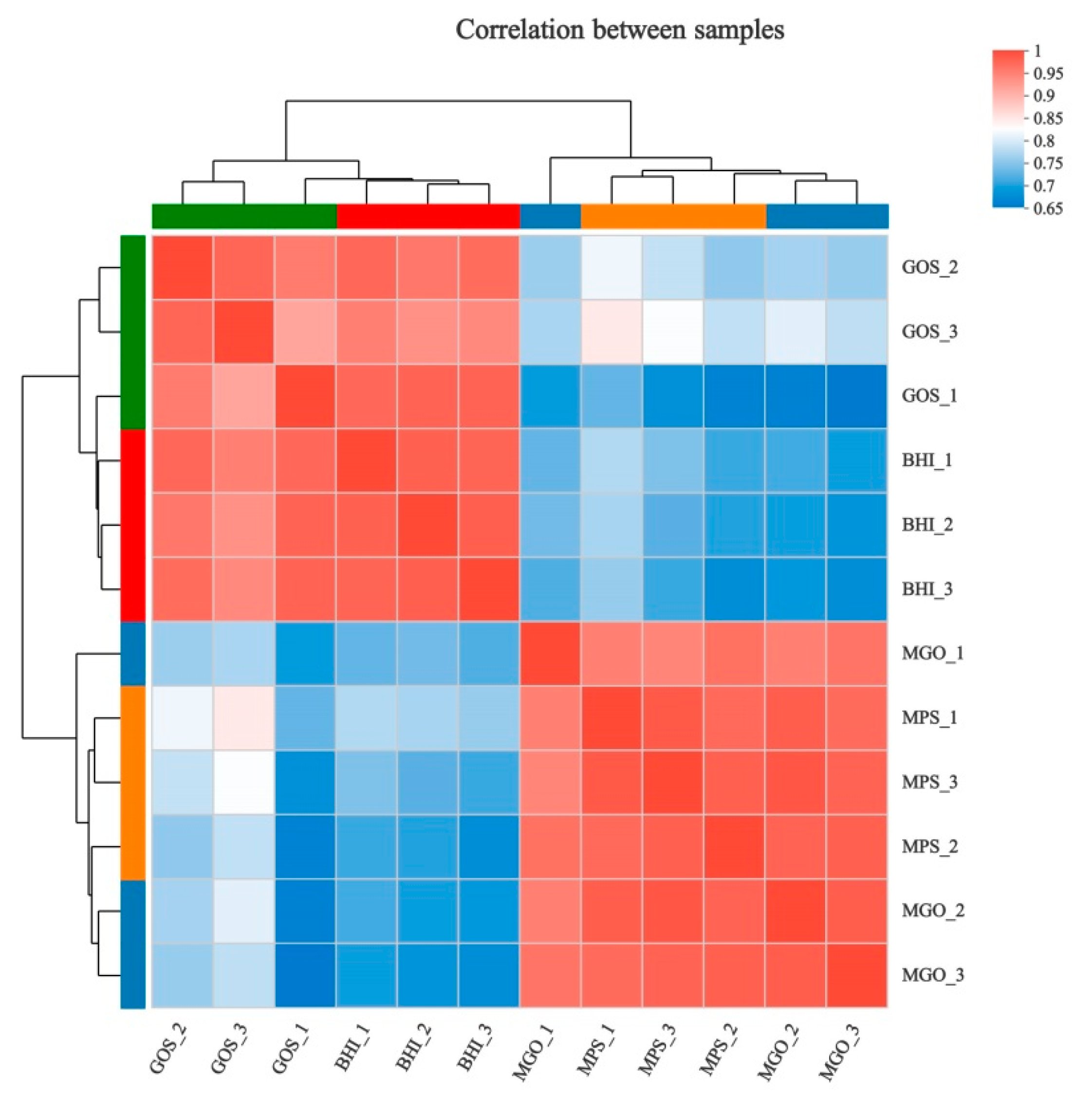
3.2.1. Cluster Analysis of Differential Gene Expression
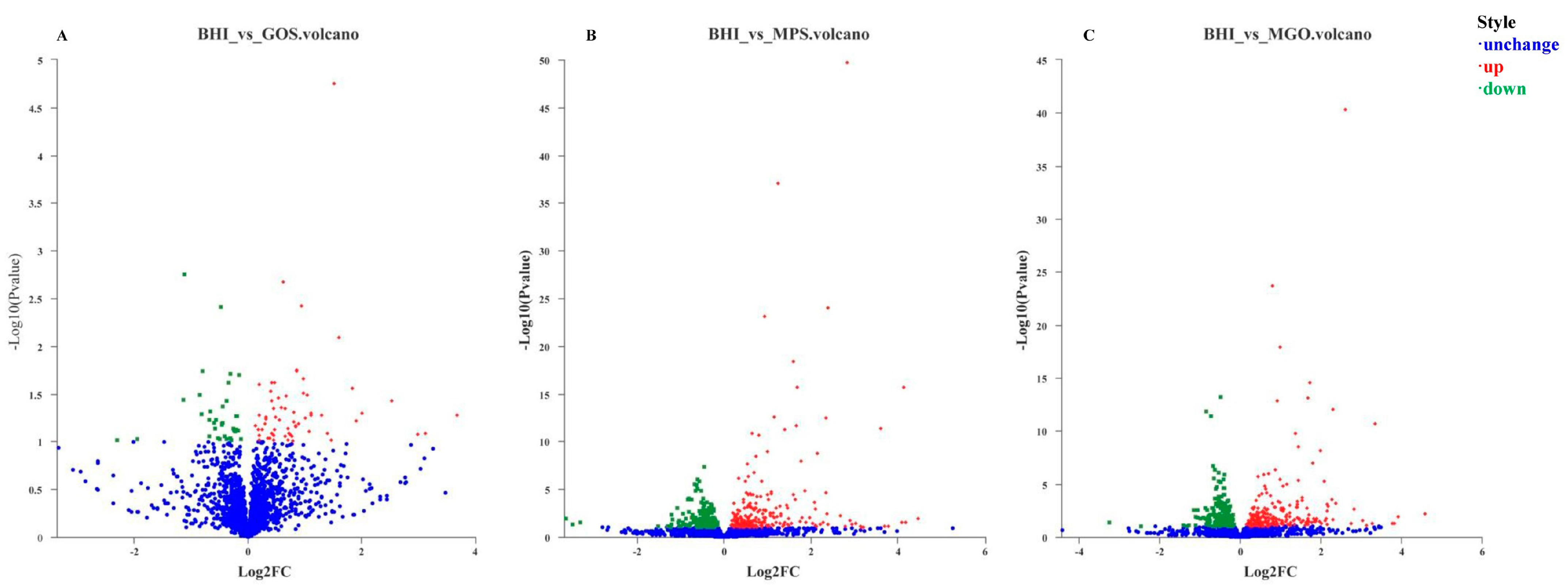
3.2.2. Identification of DEGs
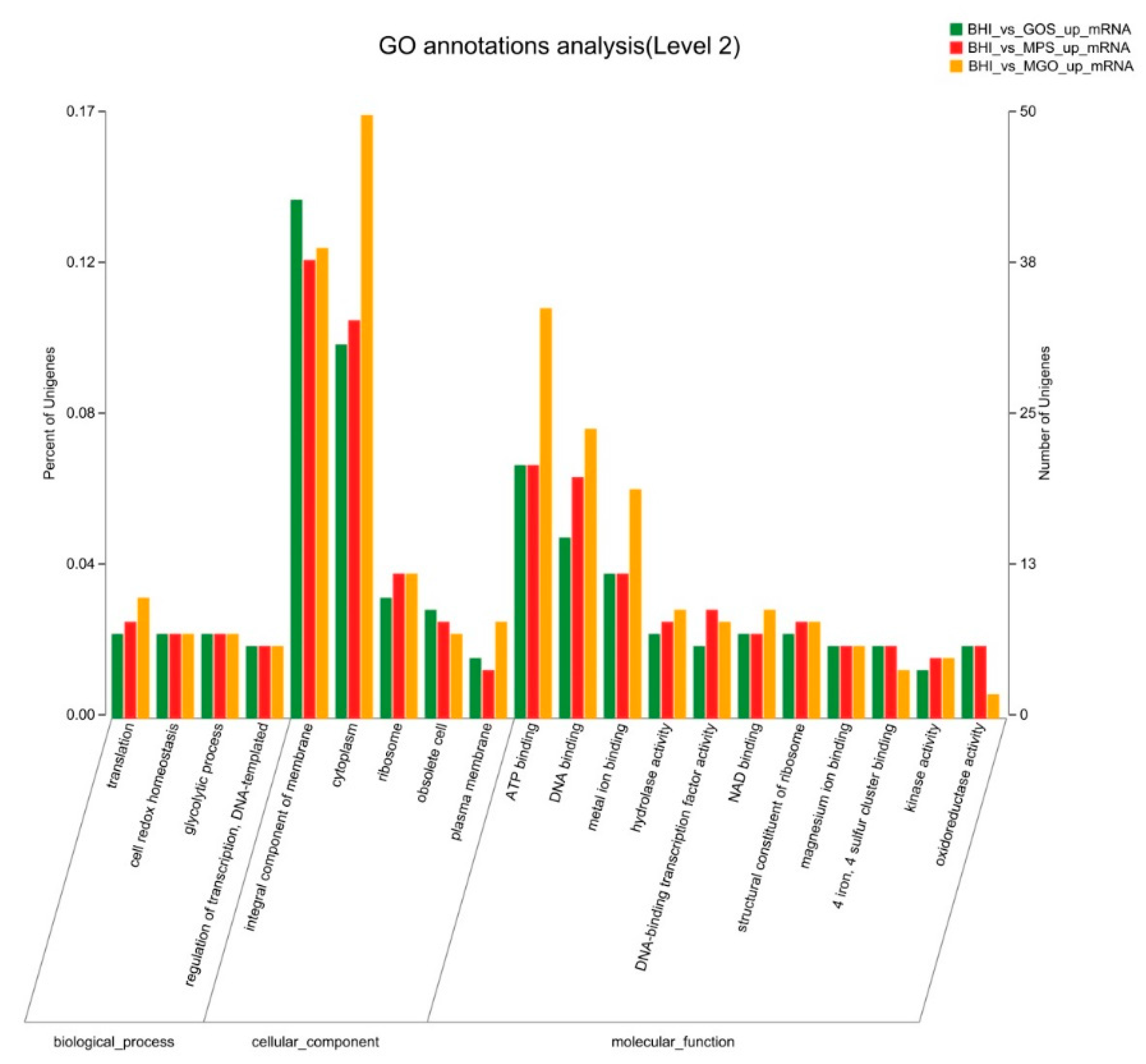
3.3. GO Annotation Analysis of DEGs
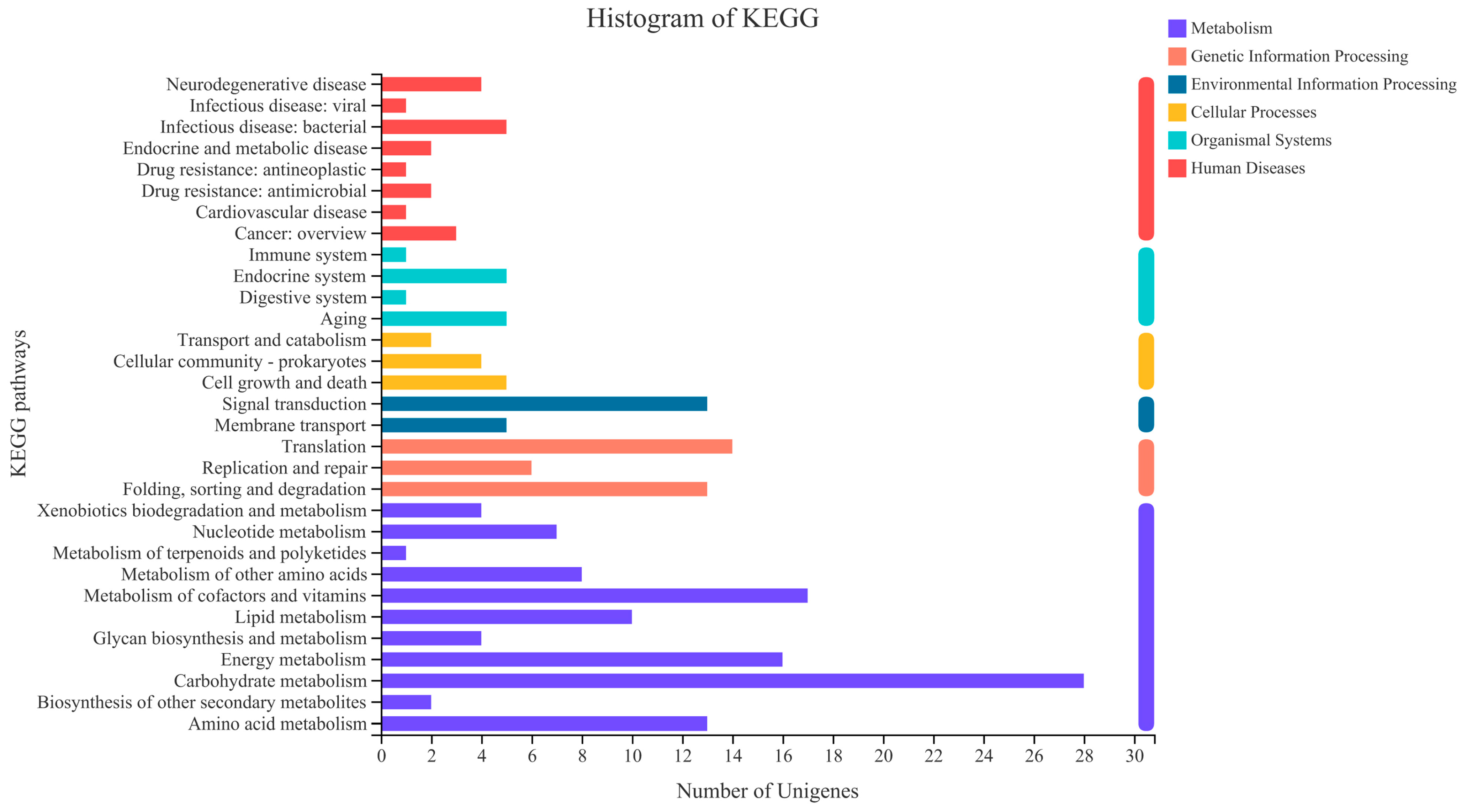
3.4. KEGG Analysis of DEGs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Villares, A.; Mateo-Vivaracho, L.; Guillamón, E. Structural features and healthy properties of polysaccharides occurring in mushrooms. Agriculture 2012, 2, 452–471. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-J.; Lin, J.-Y. Anti-inflammatory and anti-apoptotic effects of strawberry and mulberry fruit polysaccharides on lipopolysaccharide-stimulated macrophages through modulating pro-/anti-inflammatory cytokines secretion and Bcl-2/Bak protein ratio. Food Chem. Toxicol. 2012, 50, 3032–3039. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, P.-p.; Huang, Q.; You, L.-J.; Liu, R.H.; Zhao, M.-m.; Fu, X.; Luo, Z.-G. A comparison study on polysaccharides extracted from Fructus Mori using different methods: Structural characterization and glucose entrapment. Food Funct. 2019, 10, 3684–3695. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H.; Li, C. Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro. Food Funct. 2016, 7, 530–539. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet-and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef]
- Huebner, J.; Wehling, R.; Hutkins, R. Functional activity of commercial prebiotics. Int. Dairy J. 2007, 17, 770–775. [Google Scholar] [CrossRef]
- Li, E.; Zhu, Q.; Pang, D.; Liu, F.; Liao, S.; Zou, Y. Analysis of Lactobacillus rhamnosus GG in Mulberry Galacto-Oligosaccharide Medium by Comparative Transcriptomics and Metabolomics. Front. Nutr. 2022, 9, 853271. [Google Scholar] [CrossRef]
- Subhan, F.B.; Hashemi, Z.; Herrera, M.C.A.; Turner, K.; Windeler, S.; Gänzle, M.G.; Chan, C.B. Ingestion of isomalto-oligosaccharides stimulates insulin and incretin hormone secretion in healthy adults. J. Funct. Foods 2020, 65, 103730. [Google Scholar] [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012, 46, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Long, X.; Liao, S.; Pang, D.; Li, Q.; Zou, Y. Effect of mulberry galacto-oligosaccharide isolated from mulberry on glucose metabolism and gut microbiota in a type 2 diabetic mice. J. Funct. Foods 2021, 87, 104836. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben Amor, K.; Salminen, S.; De Vos, W. The Mucin Degrader Akkermansia muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [Green Version]
- Ellekilde, M.; Krych, L.; Hansen, C.; Hufeldt, M.; Dahl, K.; Hansen, L.; Sørensen, S.; Vogensen, F.; Nielsen, D.; Hansen, A. Characterization of the gut microbiota in leptin deficient obese mice–Correlation to inflammatory and diabetic parameters. Res. Vet. Sci. 2014, 96, 241–250. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; Fu, X.; Liu, R.H. In vitro fermentation of mulberry fruit polysaccharides by human fecal inocula and impact on microbiota. Food Funct. 2016, 7, 4637–4643. [Google Scholar] [CrossRef]
- Li, E.; Yang, S.; Zou, Y.; Cheng, W.; Li, B.; Hu, T.; Li, Q.; Wang, W.; Liao, S.; Pang, D. Purification, Characterization, Prebiotic Preparations and Antioxidant Activity of Oligosaccharides from Mulberries. Molecules 2019, 24, 2329. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Nie, P.; Sun, B. Characterization of two membrane-associated protease genes obtained from screening out-membrane protein genes of Flavobacterium columnare G4. J. Fish Dis. 2004, 27, 719–729. [Google Scholar] [CrossRef]
- Agarwal, S.; Choudhury, B. Presence of multiple acyltranferases with diverse substrate specificity in Bacillus smithii strain IITR6b2 and characterization of unique acyltransferase with nicotinamide. J. Mol. Catal. B Enzym. 2014, 107, 64–72. [Google Scholar] [CrossRef]
- Cameron, B.; Blanche, F.; Rouyez, M.; Bisch, D.; Famechon, A.; Couder, M.; Cauchois, L.; Thibaut, D.; Debussche, L.; Crouzet, J. Genetic analysis, nucleotide sequence, and products of two Pseudomonas denitrificans cob genes encoding nicotinate-nucleotide: Dimethylbenzimidazole phosphoribosyltransferase and cobalamin (5’-phosphate) synthase. J. Bacteriol. 1991, 173, 6066–6073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Zhao, B.; Zhou, X.; Lu, D.; Wang, Y.; Chen, Y.; Xiao, D. Analysis of the molecular basis of Saccharomyces cerevisiae mutant with high nucleic acid content by comparative transcriptomics. Food Res. Int. 2021, 142, 110188. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Qiu, X.; Zhang, Y.; Ma, J.; Gao, Y.; Zhang, X.; Niu, L.; Teng, M. Crystallization and preliminary X-ray diffraction analysis of the putative aldose 1-epimerase YeaD from Escherichia coli. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2010, 66, 951–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoden, J.B.; Holden, H.M. High resolution X-ray structure of galactose mutarotase from Lactococcus lactis. J. Biol. Chem. 2002, 277, 20854–20861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keston, A.S.; Meeuwisse, G.W.; Fredrikzon, B. Evidence for participation of mutarotase in sugar transport: Absence of the enzyme in a case of glucose galactose malabsorption. Biochem. Biophys. Res. Commun. 1982, 108, 1574–1580. [Google Scholar] [CrossRef]
- Banford, S.; Timson, D.J. The structural and molecular biology of type IV galactosemia. Biochimie 2021, 183, 13–17. [Google Scholar] [CrossRef]
- Ventura, J.-R.S.; Hu, H.; Jahng, D. Enhanced butanol production in Clostridium acetobutylicum ATCC 824 by double overexpression of 6-phosphofructokinase and pyruvate kinase genes. Appl. Microbiol. Biotechnol. 2013, 97, 7505–7516. [Google Scholar] [CrossRef]
- Mutuku, J.M.; Nose, A. High activities and mRNA expression of pyrophosphate-fructose-6-phosphate-phosphotransferase and 6-phosphofructokinase are induced as a response to Rhizoctonia solani infection in rice leaf sheaths. Physiol. Mol. Plant Pathol. 2012, 77, 41–51. [Google Scholar] [CrossRef]
- Suo, Y.; Fu, H.; Ren, M.; Liao, Z.; Ma, Y.; Wang, J. Enhanced butyric acid production in Clostridium tyrobutyricum by overexpression of rate-limiting enzymes in the Embden-Meyerhof-Parnas pathway. J. Biotechnol. 2018, 272, 14–21. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, J.; Yu, K.; Zhang, W.; Cai, Z.; Sun, M.; Hu, Y.; Zhao, X.; Xiong, C.; Niu, Q. Comparative Transcriptome Investigation of Nosema ceranae Infecting Eastern Honey Bee Workers. Insects 2022, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Han, J.; Yang, Z.; Zhu, J.; Ren, A.; Shi, L.; Yu, H.; Zhao, M. Cloning and characterization of phosphoglucose isomerase in Lentinula edodes. J. Basic Microbiol. 2022, 62, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Li, C.; Zheng, L.; Jiang, B.; Zhang, T.; Chen, J. Enhanced biosynthesis of d-tagatose from maltodextrin through modular pathway engineering of recombinant Escherichia coli. Biochem. Eng. J. 2022, 178, 108303. [Google Scholar] [CrossRef]
- Zhou, Y.; Du, C.; Odiba, A.S.; He, R.; Ahamefule, C.S.; Wang, B.; Jin, C.; Fang, W. Phosphoglucose Isomerase Plays a Key Role in Sugar Homeostasis, Stress Response, and Pathogenicity in Aspergillus flavus. Front. Cell. Infect. Microbiol. 2021, 11, 777266. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Deng, X.; Sun, R.; Luo, M.; Liang, M.; Gu, B.; Zhang, T.; Peng, Z.; Lu, Y.; Tian, C. GPI Is a Prognostic Biomarker and Correlates with Immune Infiltrates in Lung Adenocarcinoma. Front. Oncol. 2021, 11, 752642. [Google Scholar] [CrossRef]
- Meek, R.; Cadby, I.; Lovering, A. Bdellovibrio bacteriovorus phosphoglucose isomerase structures reveal novel rigidity in the active site of a selected subset of enzymes upon substrate binding. Open Biol. 2021, 11, 210098. [Google Scholar] [CrossRef]
- Olivares-Illana, V.; Riveros-Rosas, H.; Cabrera, N.; Tuena de Gómez-Puyou, M.; Pérez-Montfort, R.; Costas, M.; Gómez-Puyou, A. A guide to the effects of a large portion of the residues of triosephosphate isomerase on catalysis, stability, druggability, and human disease. Proteins Struct. Funct. Bioinform. 2017, 85, 1190–1211. [Google Scholar] [CrossRef]
- Jin, E.S.; Sherry, A.D.; Malloy, C.R. Interaction between the pentose phosphate pathway and gluconeogenesis from glycerol in the liver. J. Biol. Chem. 2014, 289, 32593–32603. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Sun, S.-J.; Ai, Y.-J.; Feng, X.; Zheng, Y.-M.; Gao, Y.; Zhang, J.-Y.; Zhang, L.; Sun, Y.-P.; Xiong, Y. Elevated nuclear localization of glycolytic enzyme TPI1 promotes lung adenocarcinoma and enhances chemoresistance. Cell Death Dis. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Pancholi, V. Multifunctional α-enolase: Its role in diseases. Cell. Mol. Life Sci. CMLS 2001, 58, 902–920. [Google Scholar] [CrossRef]
- Piast, M.; Kustrzeba-Wójcicka, I.; Matusiewicz, M.; Banaś, T. Molecular evolution of enolase. Acta Biochim. Pol. 2005, 52, 507–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansdon, E.B.; Fisher, A.J.; Segel, I.H. Human 3 ‘-phosphoadenosine 5 ‘-phosphosulfate synthetase (isoform 1, brain): Kinetic properties of the adenosine triphosphate sulfurylase and adenosine 5 ‘-phosphosulfate kinase domains. Biochemistry 2004, 43, 4356–4365. [Google Scholar] [CrossRef]
- Ramos, A.; Neves, A.R.; Ventura, R.; Maycock, C.; Lopez, P.; Santos, H. Effect of pyruvate kinase overproduction on glucose metabolism of Lactococcus lactis. Microbiology 2004, 150, 1103–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, C.; Froehlich, J.E.; Moll, M.R.; Benning, C. A heteromeric plastidic pyruvate kinase complex involved in seed oil biosynthesis in Arabidopsis. Plant Cell 2007, 19, 2006–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xiao, W.; Luo, L.; Pang, J.; Rong, W.; He, C. Downregulation of OsPK1, a cytosolic pyruvate kinase, by T-DNA insertion causes dwarfism and panicle enclosure in rice. Planta 2012, 235, 25–38. [Google Scholar] [CrossRef]
- Zhong, W.H.; Cui, L.; Goh, B.C.; Cai, Q.X.; Ho, P.Y.; Chionh, Y.H.; Yuan, M.; El Sahili, A.; Fothergill-Gilmore, L.A.; Walkinshaw, M.D.; et al. Allosteric pyruvate kinase-based “logic gate” synergistically senses energy and sugar levels in Mycobacterium tuberculosis. Nat. Commun. 2017, 8, 1986. [Google Scholar] [CrossRef] [Green Version]
- Zoraghi, R.; Worrall, L.; See, R.H.; Strangman, W.; Popplewell, W.L.; Gong, H.; Samaai, T.; Swayze, R.D.; Kaur, S.; Vuckovic, M. Methicillin-resistant Staphylococcus aureus (MRSA) pyruvate kinase as a target for bis-indole alkaloids with antibacterial activities. J. Biol. Chem. 2011, 286, 44716–44725. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| AMUC_RS07000 (galM) | CCTCACCAACCACGCTTACT | TATAGGCGGAGGCCCGTATC |
| AMUC_RS01180 (pfkA) | TATTCCGGCCACGATTGACA | GCCGTATCACGAACGGAATC |
| AMUC_RS00900 (pgm) | TGAAGTGGTCAACGTGCTCA | GCGTTTGTTCCTCGCTCATC |
| AMUC_RS10555 (pgi) | AGTAAGCGTGGTTGGTGAGG | AAACGCCCTGTCTATCCGTC |
| AMUC_RS07935 (pfk) | TTATGCCGTGGAACTGGTGG | TTCCTCAATGGGTACGGCAG |
| AMUC_RS03915 (fbaA) | TAGGCAATTCCGCCCTTGG | TGTGAAGGGCTACGAGAACG |
| AMUC_RS03080 (tpiA) | TGGAACCCGTGCTGGAAATC | TAGGCGATGACCAGGTTGGA |
| AMUC_RS07575 (gapA) | TTGCTCCGATGGTGAAGGTG | GCTGGTCGTTCGTGTAGGAG |
| AMUC_RS07580 (pgk) | TGAAATGGACTGCTTCGCCA | CGCCGCCTACAATGGAAATG |
| AMUC_RS01755 (gpml) | TGTGGAGCAGTGTTATGCGA | TTATCCCTCACGCGCTGTTC |
| AMUC_RS04575 (eno) | CTGGAAGCCACGGAACAAAC | AAACACCCAGAATGGCGTTG |
| AMUC_RS02385 (pyk) | AAACGCCTGTCTGATCGTCT | GGTCATTGCTGAAGGCGAAG |
| AMUC_RS09020 (lpd) | CTACTGTCATCGGCTCCAGG | AATTCCGTGCCGATAGCTCC |
| AMUC _16SrRNA | AAGGGTTTCGGCTCGTAAAA | TGCACTCAAGTTTCCCAGTT |
| Carbohydrate | Concentration (%) | A. muciniphila Proliferation |
|---|---|---|
| GOS | 1 | 178% ± 8.3a |
| 3 | 269% ± 7.5b | |
| 5 | 582% ± 10.2c | |
| IMO | 1 | 156% ± 5.3a |
| 3 | 204% ± 9.4b | |
| 5 | 351% ± 11.2c | |
| MPS | 1 | 159% ± 5.4a |
| 3 | 208% ± 6.8b | |
| 5 | 256% ± 7.2c | |
| MPS hydrolyzed via pectinase | 1 | 151% ± 4.3a |
| 3 | 201% ± 7.3b | |
| 5 | 330% ± 9.5c | |
| MPS hydrolyzed via glucoamylase | 1 | 185% ± 3.5a |
| 3 | 241% ± 6.3b | |
| 5 | 484% ± 8.9c | |
| MPS hydrolyzed via β-mannanase | 1 | 202%± 5.4a |
| 3 | 405% ± 7.2b | |
| 5 | 652% ± 9.9c | |
| MPS hydrolyzed via xylanase | 1 | 103% ± 2.3a |
| 3 | 135% ± 4.4b | |
| 5 | 198% ± 5.2c | |
| MPS hydrolyzed via β-glucanase | 1 | 158% ± 3.9a |
| 3 | 221% ± 4.5b | |
| 5 | 412% ± 5.1c | |
| MPS hydrolyzed via α-amylase | 1 | 109% ± 2.2a |
| 3 | 125% ± 3.1b | |
| 5 | 132% ± 2.8c |
| Carbohydrate | Concentration (%) | A. muciniphila Proliferation |
|---|---|---|
| MOS-I | 1 | 254% ± 3.2a |
| 3 | 497% ± 5.8b | |
| 5 | 781% ± 10.5c | |
| MOS-II | 1 | 125% ± 4.2a |
| 3 | 198% ± 3.5b | |
| 5 | 245% ± 6.8c | |
| MOS-III | 1 | 132% ± 2.3a |
| 3 | 201% ± 4.8b | |
| 5 | 253% ± 6.1c |
| Gene ID (KEGG Name) | Gene Description | FC | Style | ||
|---|---|---|---|---|---|
| GOS/BHI | MPS/BHI | MGO/BHI | |||
| galactose metabolism | |||||
| AMUC_RS07000 (galM) | galactose mutarotase | 1.18 | 1.36 | 3.47 | up |
| AMUC_RS01180 (pfkA) | 6-phosphofructokinase | 1.30 | 1.32 | 3.16 | up |
| AMUC_RS00900 (pgm) | phospho-sugar mutase | 0.42 | 0.62 | 0.52 | down |
| glycolysis/gluconeogenesis | |||||
| AMUC_RS07000 (galM) | galactose mutarotase | 1.18 | 1.36 | 3.47 | up |
| AMUC_RS10555 (pgi) | glucose-6-phosphate isomerase | 2.24 | 2.52 | 2.31 | up |
| AMUC_RS07935 (pfk) | 6-phosphofructokinase | 2.34 | 1.85 | 1.68 | up |
| AMUC_RS03915 (fbaA) | class II fructose-bisphosphate aldolase | 1.87 | 2.21 | 2.85 | up |
| AMUC_RS03080 (tpiA) | triose-phosphate isomerase | 1.98 | 2.12 | 1.87 | up |
| AMUC_RS07575 (gapA) | type I glyceraldehyde-3-phosphate dehydrogenase | 2.12 | 2.54 | 2.74 | up |
| AMUC_RS07580 (pgk) | phosphoglycerate kinase | 2.01 | 1.84 | 2.32 | up |
| AMUC_RS01755 (gpml) | 2%2C3-bisphosphoglycerate-independent phosphoglycerate mutase | 1.87 | 2.31 | 2.14 | up |
| AMUC_RS04575 (eno) | phosphopyruvate hydratase | 2.24 | 1.89 | 2.18 | up |
| AMUC_RS02385 (pyk) | pyruvate kinase | 1.63 | 1.85 | 1.77 | up |
| AMUC_RS09020 (lpd) | dihydrolipoyl dehydrogenase | 2.32 | 2.11 | 2.25 | up |
| AMUC_RS00900 (pgm) | phospho-sugar mutase | 0.56 | 0.49 | 0.47 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, E.; Li, S.; Liu, F.; Li, Q.; Pang, D.; Wang, H.; Liao, S.; Zou, Y. Analysis of Akkermansia muciniphila in Mulberry Galacto-Oligosaccharide Medium via Comparative Transcriptomics. Foods 2023, 12, 440. https://doi.org/10.3390/foods12030440
Li E, Li S, Liu F, Li Q, Pang D, Wang H, Liao S, Zou Y. Analysis of Akkermansia muciniphila in Mulberry Galacto-Oligosaccharide Medium via Comparative Transcriptomics. Foods. 2023; 12(3):440. https://doi.org/10.3390/foods12030440
Chicago/Turabian StyleLi, Erna, Shipei Li, Fan Liu, Qian Li, Daorui Pang, Hong Wang, Sentai Liao, and Yuxiao Zou. 2023. "Analysis of Akkermansia muciniphila in Mulberry Galacto-Oligosaccharide Medium via Comparative Transcriptomics" Foods 12, no. 3: 440. https://doi.org/10.3390/foods12030440
APA StyleLi, E., Li, S., Liu, F., Li, Q., Pang, D., Wang, H., Liao, S., & Zou, Y. (2023). Analysis of Akkermansia muciniphila in Mulberry Galacto-Oligosaccharide Medium via Comparative Transcriptomics. Foods, 12(3), 440. https://doi.org/10.3390/foods12030440





