Influence of the Maillard Reaction on Properties of Air-Assisted Electrospun Gelatin/Zein/Glucose Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Solution Preparation
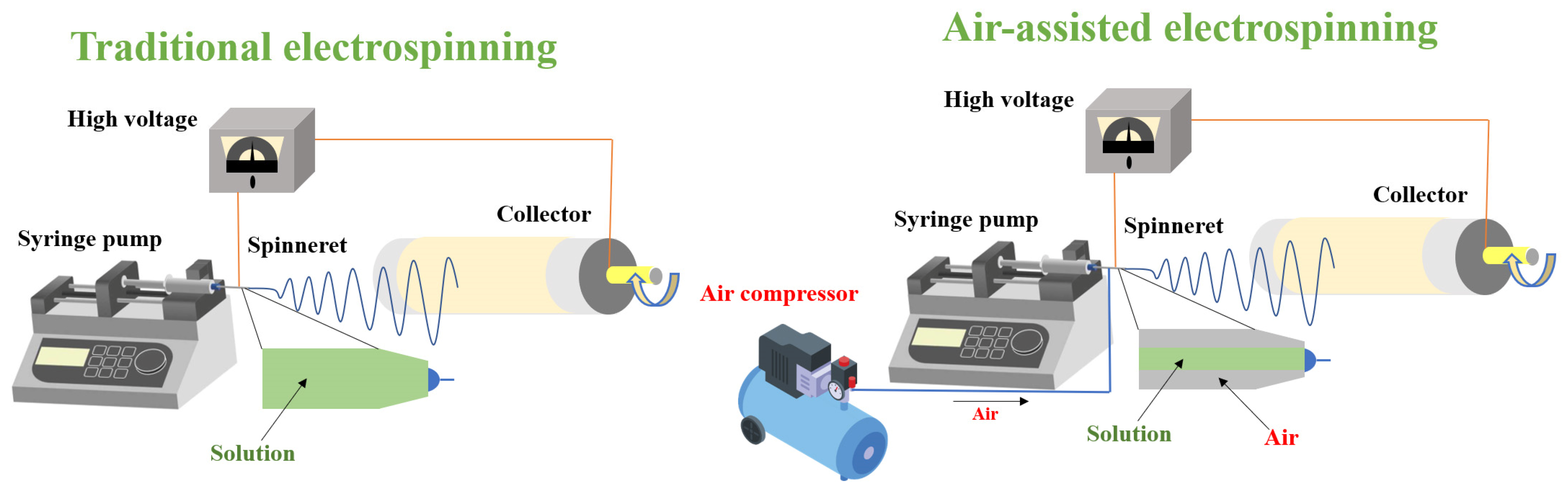
2.3. Air-Assisted Electrospinning
2.4. Maillard Reaction
2.5. Fiber Morphologies
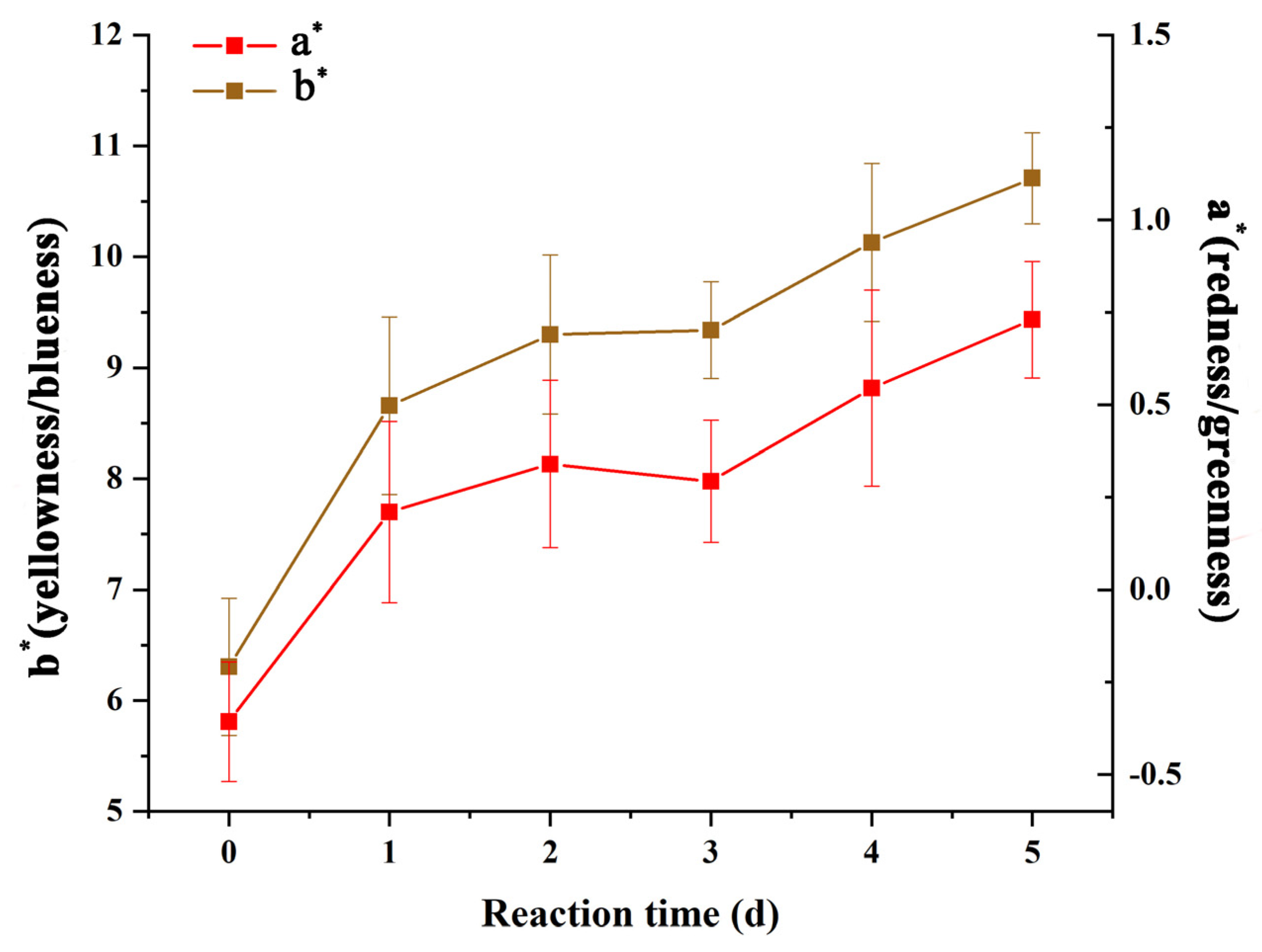
2.6. Color Analysis
2.7. Infrared Spectroscopy
2.8. Thermal Properties Analysis
2.9. Surface Elemental Analysis
2.10. Water Contact Angle Tests
2.11. Mechanical Properties
2.12. Water Vapor Permeability
2.13. Stability to Water
2.14. Statistical Analysis
3. Results and Discussion
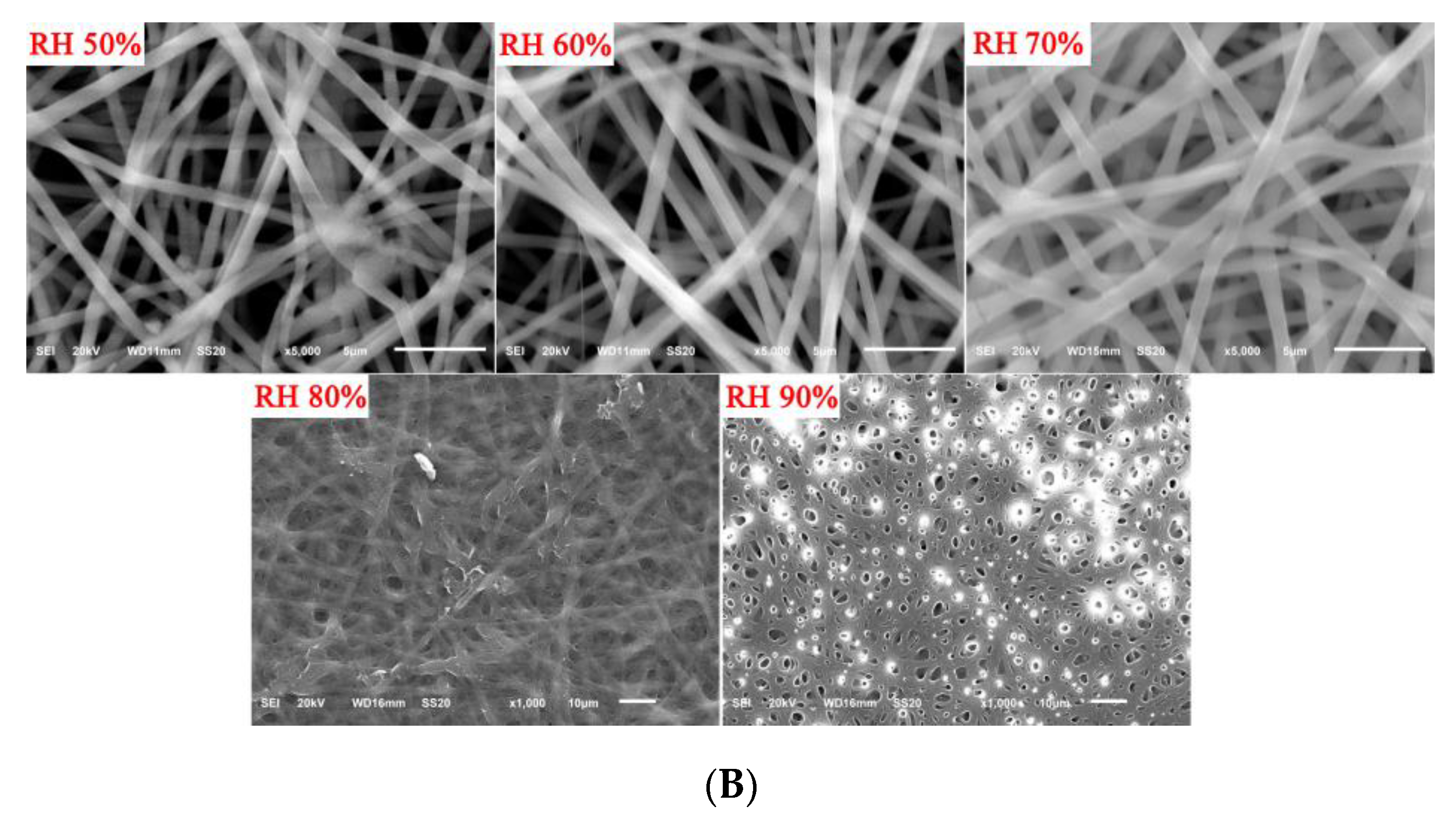
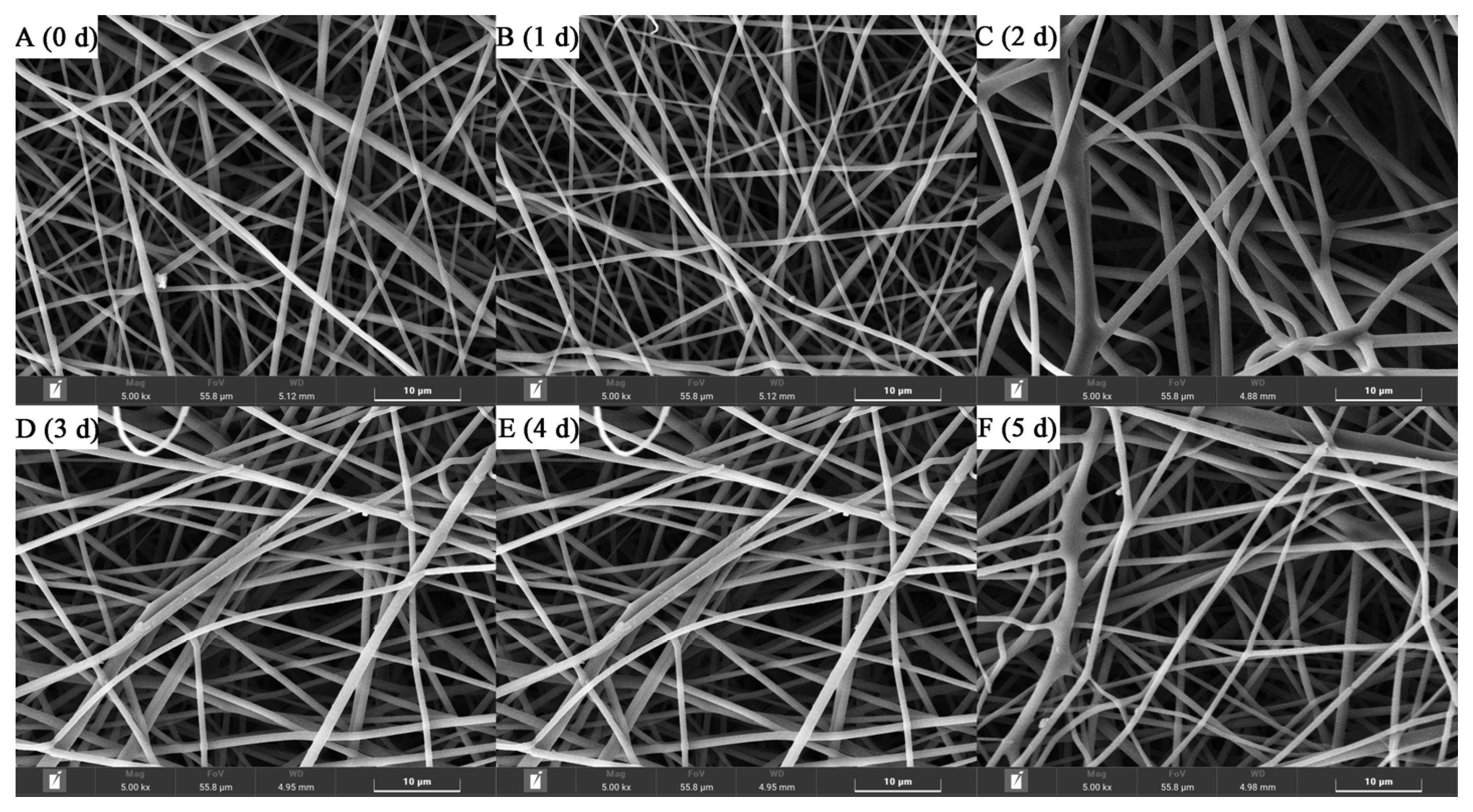
3.1. Fiber Morphologies
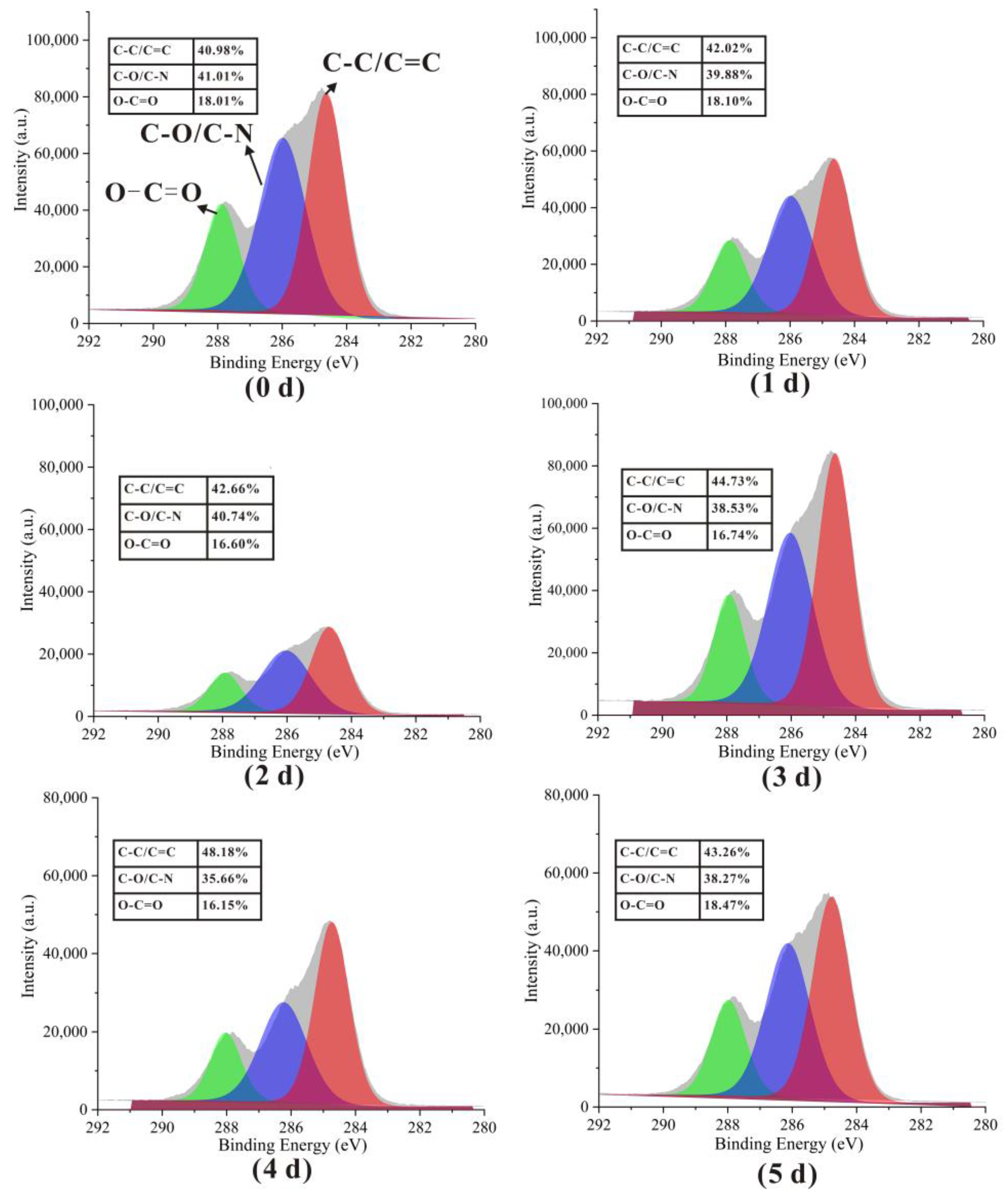
3.2. Infrared Spectroscopy
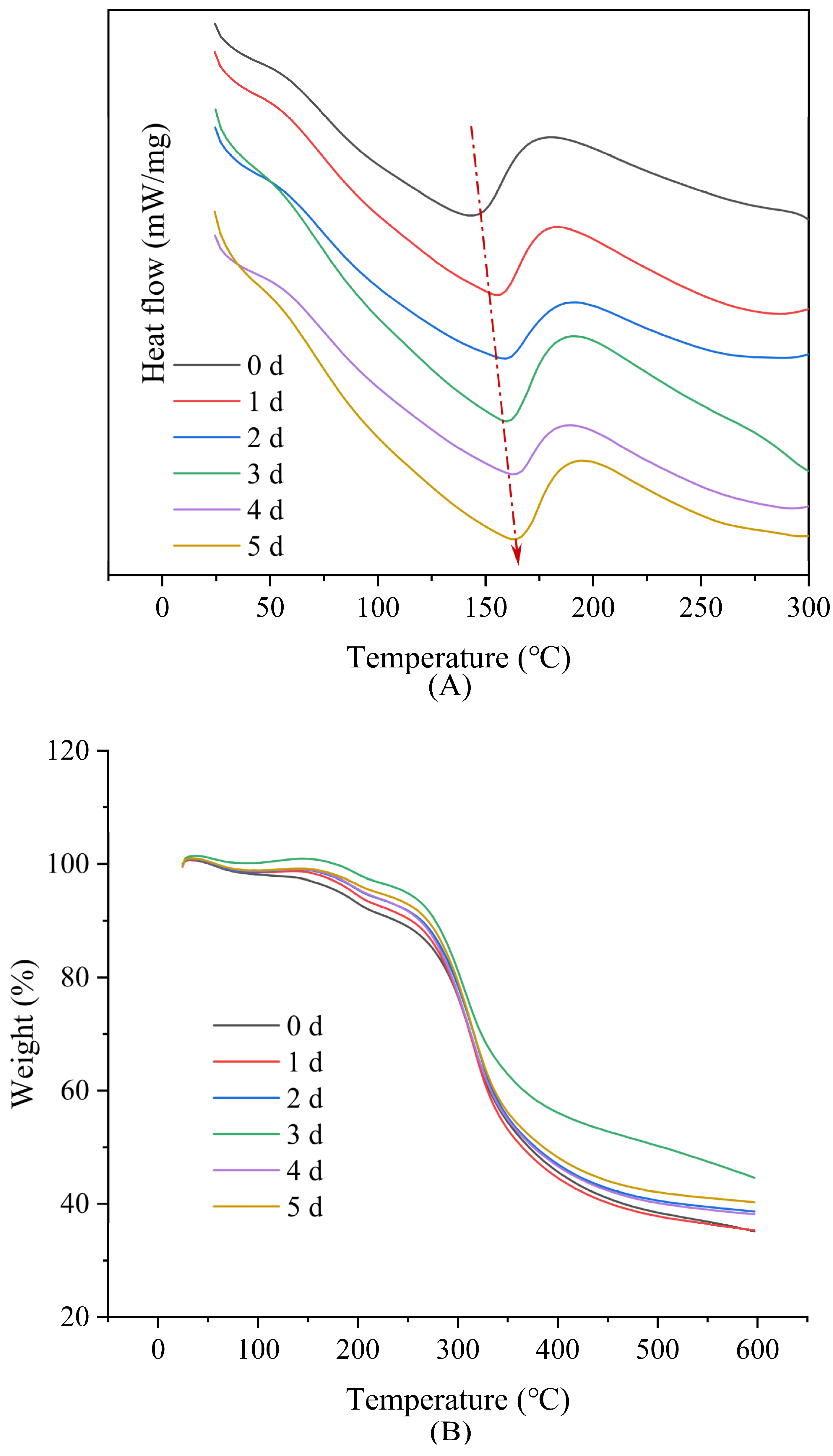
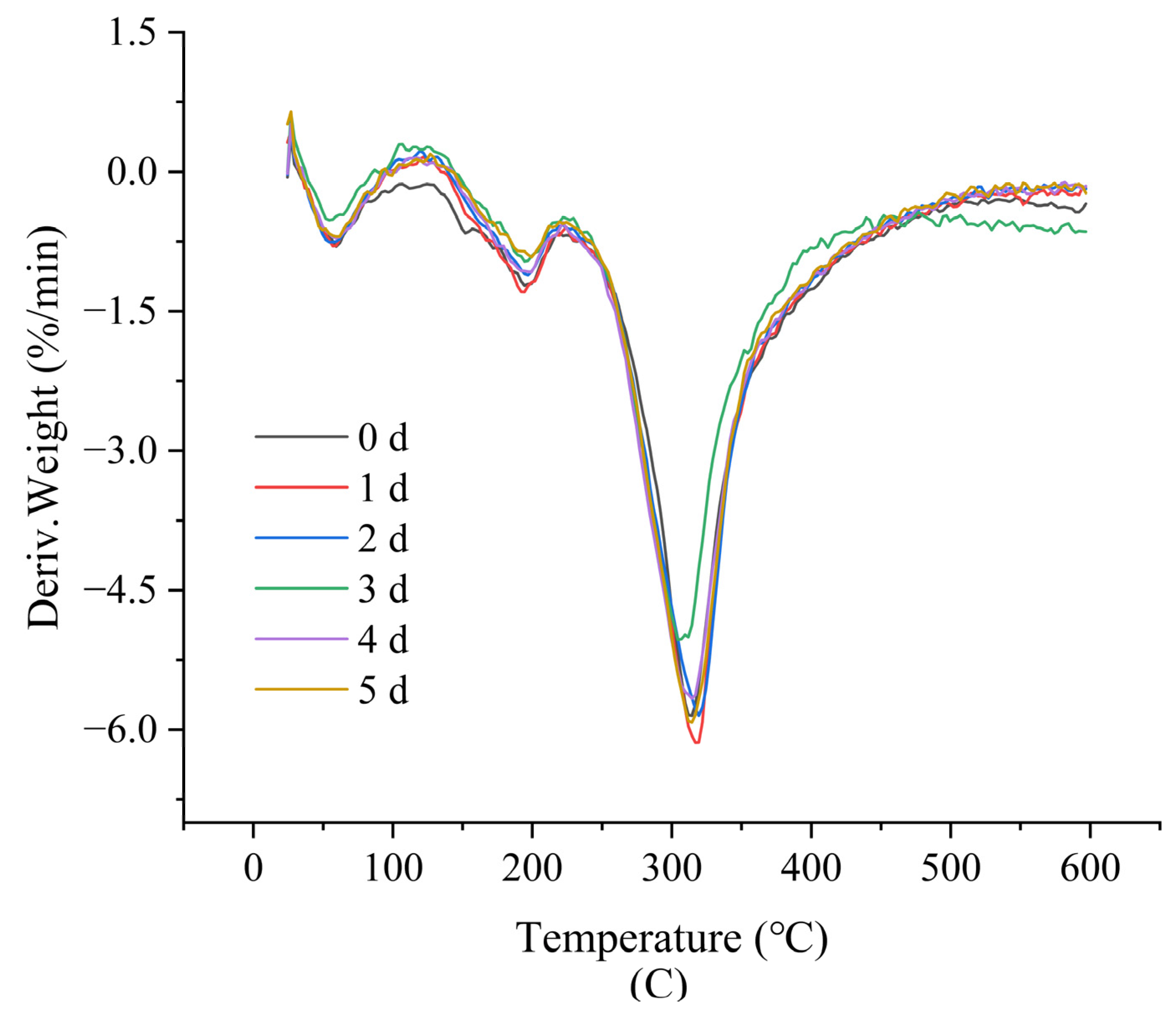
3.3. Thermal Analysis
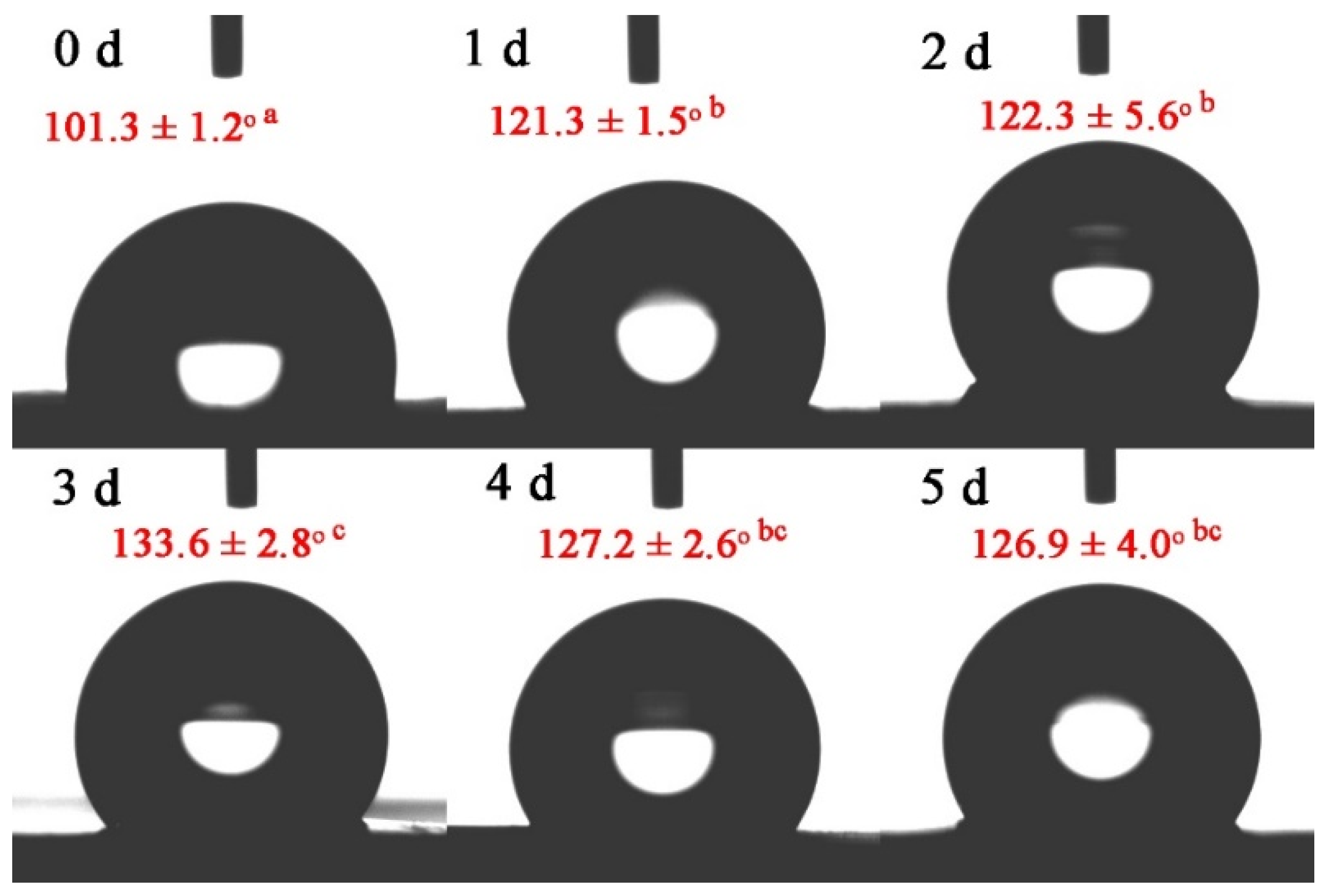
3.4. Water Contact Angle Analysis
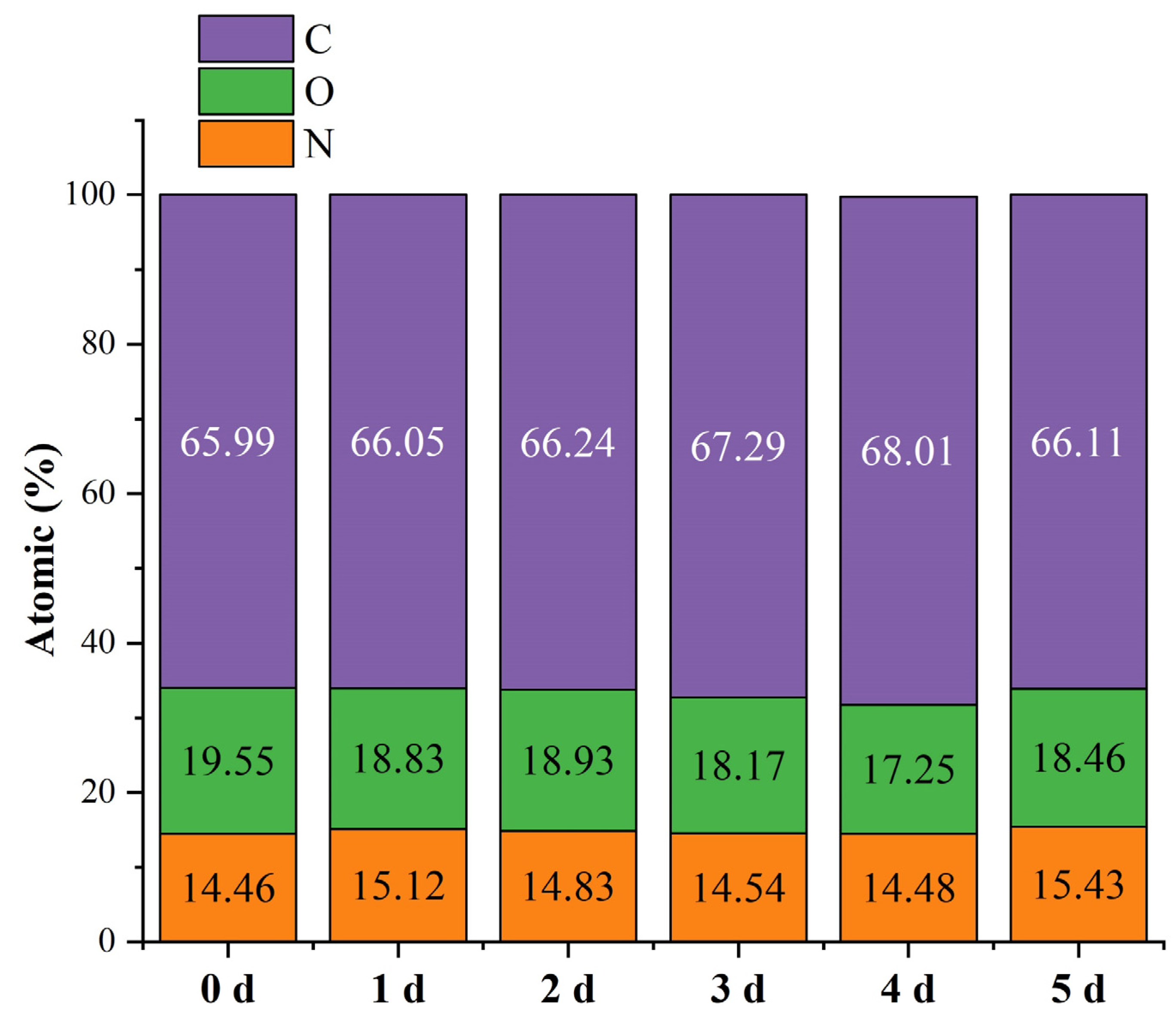
3.5. Surface Elemental Analysis
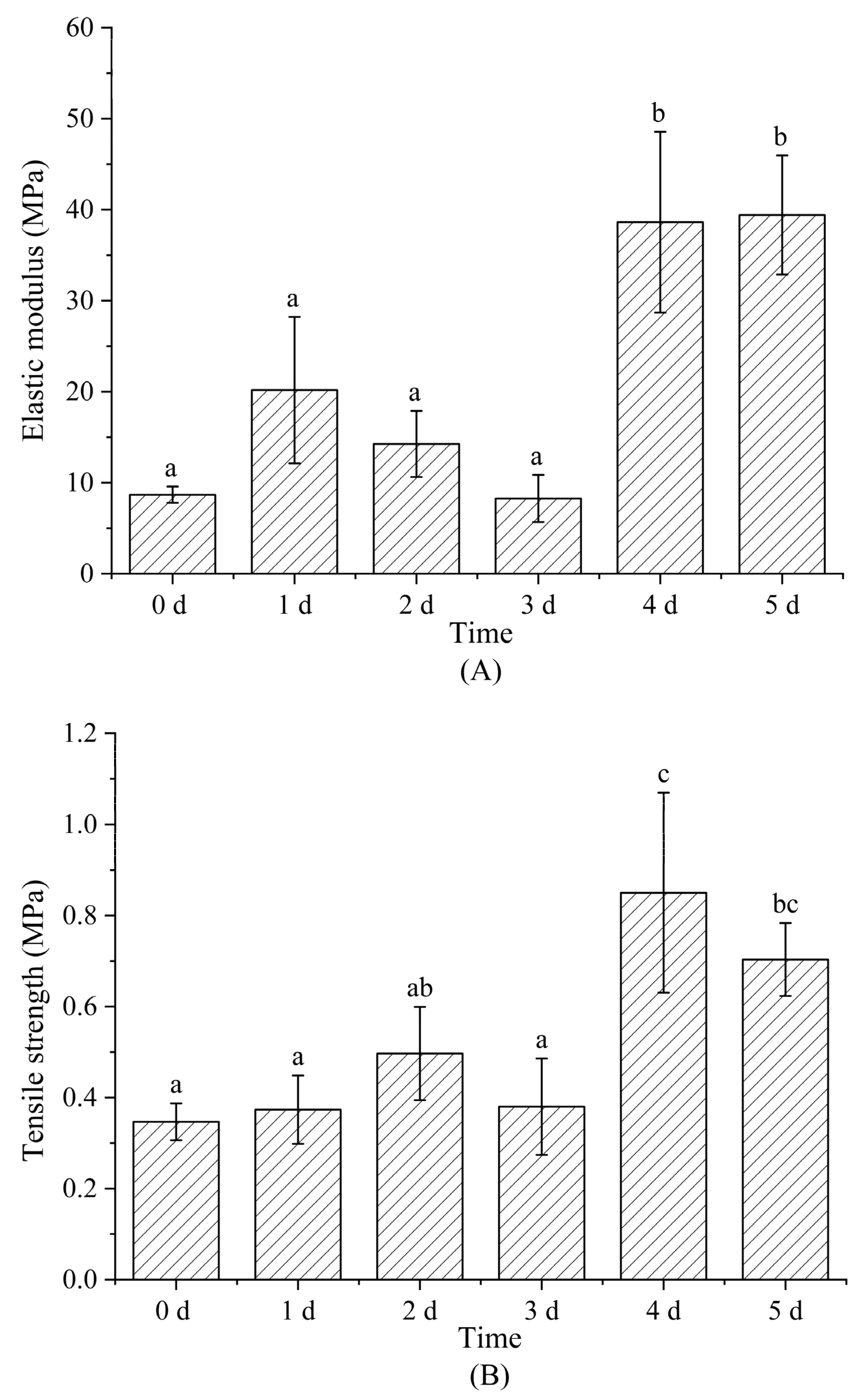
3.6. Mechanical Property Analysis
3.7. Water Vapor Permeability and Water Stability Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mohammadi, M.A.; Ramezani, S.; Hosseini, H.; Mortazavian, A.M.; Hosseini, S.M.; Ghorbani, M. Electrospun Antibacterial and Antioxidant Zein/Polylactic Acid/Hydroxypropyl Methylcellulose Nanofibers as an Active Food Packaging System. Food Bioprocess Technol. 2021, 14, 1529–1541. [Google Scholar] [CrossRef]
- Li, T.; Shen, Y.; Chen, H.; Xu, Y.; Wang, D.; Cui, F.; Han, Y.; Li, J. Antibacterial Properties of Coaxial Spinning Membrane of Methyl ferulate/zein and Its Preservation Effect on Sea Bass. Foods 2021, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Tayebi-Moghaddam, S.; Khatibi, R.; Taklavi, S.; Hosseini-Isfahani, M.; Rezaeinia, H. Sustained-release modeling of clove essential oil in brine to improve the shelf life Iranian white cheese by bioactive electrospun zein. Int. J. of Food Microbiol. 2021, 355, 109337. [Google Scholar] [CrossRef]
- Haghju, S.; Bari, M.R.; Khaled-Abad, M.A. Affecting parameters on fabrication of beta-D-galactosidase immobilized chitosan/poly (vinyl alcohol) electrospun nanofibers. Carbohydr. Polym. 2018, 200, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Tian, W.; Bai, H.; Zhang, J.; Wang, S.; Zhang, J. Amine-responsive cellulose-based ratiometric fluorescent materials for real-time and visual detection of shrimp and crab freshness. Nat. Commun. 2019, 10, 795. [Google Scholar] [CrossRef]
- Quek, S.Y.; Hadi, J.; Tanambell, H. Application of Electrospinning as Bioactive Delivery System. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 145–149. [Google Scholar]
- Roy, S.; Rhim, J.-W. Gelatin/agar-based functional film integrated with Pickering emulsion of clove essential oil stabilized with nanocellulose for active packaging applications. Colloids Surf. A 2021, 627, 127220. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of cinnamic aldehyde using zein nanofibers by novel needle-less electrospinning: Production, characterization and their application to reduce nitrite in sausages. J. Food Eng. 2021, 288, 110140. [Google Scholar] [CrossRef]
- Ansarifar, E.; Moradinezhad, F. Encapsulation of thyme essential oil using electrospun zein fiber for strawberry preservation. Chem. Biol. Technol. Agric. 2022, 9, 2. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Fabrication of eugenol loaded gelatin nanofibers by electrospinning technique as active packaging material. LWT 2021, 139, 110800. [Google Scholar] [CrossRef]
- Morsy, R.; Hosny, M.; Reicha, F.; Elnimr, T. Developing and physicochemical evaluation of cross-linked electrospun gelatin–glycerol nanofibrous membranes for medical applications. J. Mol. Struct. 2017, 1135, 222–227. [Google Scholar] [CrossRef]
- Panzavolta, S.; Gioffre, M.; Focarete, M.L.; Gualandi, C.; Foroni, L.; Bigi, A. Electrospun gelatin nanofibers: Optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomater. 2011, 7, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, S.R.; Rostamabadi, H.; Samborska, K.; Mirarab, S.; Rashidinejhad, A.; Jafari, S.M. Protein-polysaccharide interactions for the fabrication of bioactive-loaded nanocarriers: Chemical conjugates and physical complexes. Pharmacol. Res. 2022, 178, 106164. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Nasri, M.; Debeaufort, F. Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocoll. 2019, 97, 105196. [Google Scholar] [CrossRef]
- Kwak, H.W.; Park, J.; Yun, H.; Jeon, K.; Kang, D.-W. Effect of crosslinkable sugar molecules on the physico-chemical and antioxidant properties of fish gelatin nanofibers. Food Hydrocoll. 2021, 111, 106259. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Feng, F.; Zhang, H. Study on wettability, mechanical property and biocompatibility of electrospun gelatin/zein nanofibers cross-linked by glucose. Food Hydrocoll. 2019, 87, 1–10. [Google Scholar] [CrossRef]
- Naik, R.R.; Wang, Y.; Selomulya, C. Improvements of plant protein functionalities by Maillard conjugation and Maillard reaction products. Crit. Rev. Food Sci. Nutr. 2021, 62, 7036–7061. [Google Scholar] [CrossRef] [PubMed]
- Aminyan, R.; Bazgir, S. Fabrication and characterization of nanofibrous polyacrylic acid superabsorbent using gas-assisted electrospinning technique. React. Funct. Polym. 2019, 141, 133–144. [Google Scholar] [CrossRef]
- Duan, G.; Greiner, A. Air-Blowing-Assisted Coaxial Electrospinning toward High Productivity of Core/Sheath and Hollow Fibers. Macromol. Mater. Eng. 2019, 304, 1800669. [Google Scholar] [CrossRef]
- Li, L.; Kang, W.; Zhuang, X.; Shi, J.; Zhao, Y.; Cheng, B. A comparative study of alumina fibers prepared by electro-blown spinning (EBS) and solution blowing spinning (SBS). Mater. Lett. 2015, 160, 533–536. [Google Scholar] [CrossRef]
- Siimon, K.; Reemann, P.; Poder, A.; Pook, M.; Kangur, T.; Kingo, K.; Jaks, V.; Maeorg, U.; Jarvekulg, M. Effect of glucose content on thermally cross-linked fibrous gelatin scaffolds for tissue engineering. Mater. Sci. Eng. C 2014, 42, 538–545. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Zhang, C.; Chen, K.; Feng, F.; Zhang, H. Comparison of ethyl cellulose-gelatin composite films fabricated by electrospinning versus solvent casting. J. Appl. Polym. Sci. 2018, 135, 46824. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Improvement of barrier properties of fish gelatin films promoted by gelatin glycation with lactose at high temperatures. LWT 2015, 63, 315–321. [Google Scholar] [CrossRef]
- Shi, Z.; Ju, J.; Liang, Y.; Huang, W.; Kang, W.; Cheng, B. A Comparative Study of Poly(tetrafluoroethylene) Ultrafine Fibrous Porous Membranes Prepared by Electrospinning, Solution Blowing Spinning, and Electroblown Spinning. Chem. Lett. 2017, 46, 131–134. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, X.; Che, X.; Yang, M.; Zhai, G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C 2018, 90, 750–763. [Google Scholar] [CrossRef]
- Hsiao, H.-Y.; Huang, C.-M.; Liu, Y.-Y.; Kuo, Y.-C.; Chen, H. Effect of air blowing on the morphology and nanofiber properties of blowing-assisted electrospun polycarbonates. J. Appl. Polym. Sci. 2011, 124, 4904–4914. [Google Scholar] [CrossRef]
- Gibis, M.; Pribek, F.; Kutzli, I.; Weiss, J. Influence of the Protein Content on Fiber Morphology and Heat Treatment of Electrospun Potato Protein–Maltodextrin Fibers. Appl. Sci. 2021, 11, 7896. [Google Scholar] [CrossRef]
- Wang, D.; Sun, J.; Li, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/zein nanofiber films loaded with perillaldehyde, thymol, or varepsilon-polylysine and evaluation of their effects on the preservation of chilled chicken breast. Food Chem. 2022, 373, 131439. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Y.; Sun, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Fabrication and Characterization of Gelatin/Zein Nanofiber Films Loading Perillaldehyde for the Preservation of Chilled Chicken. Foods 2021, 10, 1277. [Google Scholar] [CrossRef]
- Siimon, K.; Siimon, H.; Jarvekulg, M. Mechanical characterization of electrospun gelatin scaffolds cross-linked by glucose. J. Mater. Sci. Mater. Med. 2015, 26, 5375. [Google Scholar] [CrossRef]
- Deng, L.; Kang, X.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein films fabricated by electrospinning vs solvent casting. Food Hydrocoll. 2018, 74, 324–332. [Google Scholar] [CrossRef]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of Plant Proteins via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application. Foods 2021, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.-H.; Léonard, M.-L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Salerno, A.; Zeppetelli, S.; Maio, E.D.; Iannace, S.; Netti, P.A. Novel 3D porous multi-phase composite scaffolds based on PCL, thermoplastic zein and ha prepared via supercritical CO2 foaming for bone regeneration. Compos. Sci. Technol. 2010, 70, 1838–1846. [Google Scholar] [CrossRef]
- Wang, L.; Wu, M.; Liu, H.M. Emulsifying and physicochemical properties of soy hull hemicelluloses-soy protein isolate conjugates. Carbohydr. Polym. 2017, 163, 181–190. [Google Scholar] [CrossRef]
- Radunz, M.; Mota Camargo, T.; Dos Santos Hackbart, H.C.; Paes Nunes, C.F.; Araujo Ribeiro, J.; da Rosa Zavareze, E. Characterization of ultrafine zein fibers incorporated with broccoli, kale, and cauliflower extracts by electrospinning. J. Sci. Food Agric. 2022, 102, 4210–4217. [Google Scholar] [CrossRef]
- Ruggeri, M.; Bianchi, E.; Rossi, S.; Boselli, C.; Cornaglia, A.I.; Malavasi, L.; Carzino, R.; Suarato, G.; Sanchez-Espejo, R.; Athanassiou, A.; et al. Maltodextrin-amino acids electrospun scaffolds cross-linked with Maillard-type reaction for skin tissue engineering. Biomater. Adv. 2022, 133, 112593. [Google Scholar] [CrossRef]
- Wang, L.; Mu, R.-J.; Li, Y.; Lin, L.; Lin, Z.; Pang, J. Characterization and antibacterial activity evaluation of curcumin loaded konjac glucomannan and zein nanofibril films. LWT 2019, 113, 108293. [Google Scholar] [CrossRef]
- Qin, Z.; Jia, X.; Liu, Q.; Kong, B.; Wang, H. Enhancing physical properties of chitosan/pullulan electrospinning nanofibers via green crosslinking strategies. Carbohydr. Polym. 2020, 247, 116734. [Google Scholar] [CrossRef]
- Oh, S.; Park, J.; Nam, J.; Hyun, Y.; Jin, H.-J.; Kwak, H.W. Antioxidant and UV-blocking glucose-crosslinked sericin films with enhanced structural integrity. React. Funct. Polym. 2021, 165, 104942. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, P.; Zhang, H. Electrospinning of nanofibers: Potentials and perspectives for active food packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 479–502. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Deng, Z.; Rao, J.; Yang, Z.; Li, Y.; Wu, D.; Chen, K. Characterization of glycosylated gelatin/pullulan nanofibers fabricated by multi-fluid mixing solution blow spinning. Int. J. Biol. Macromol. 2022, 214, 512–521. [Google Scholar] [CrossRef] [PubMed]

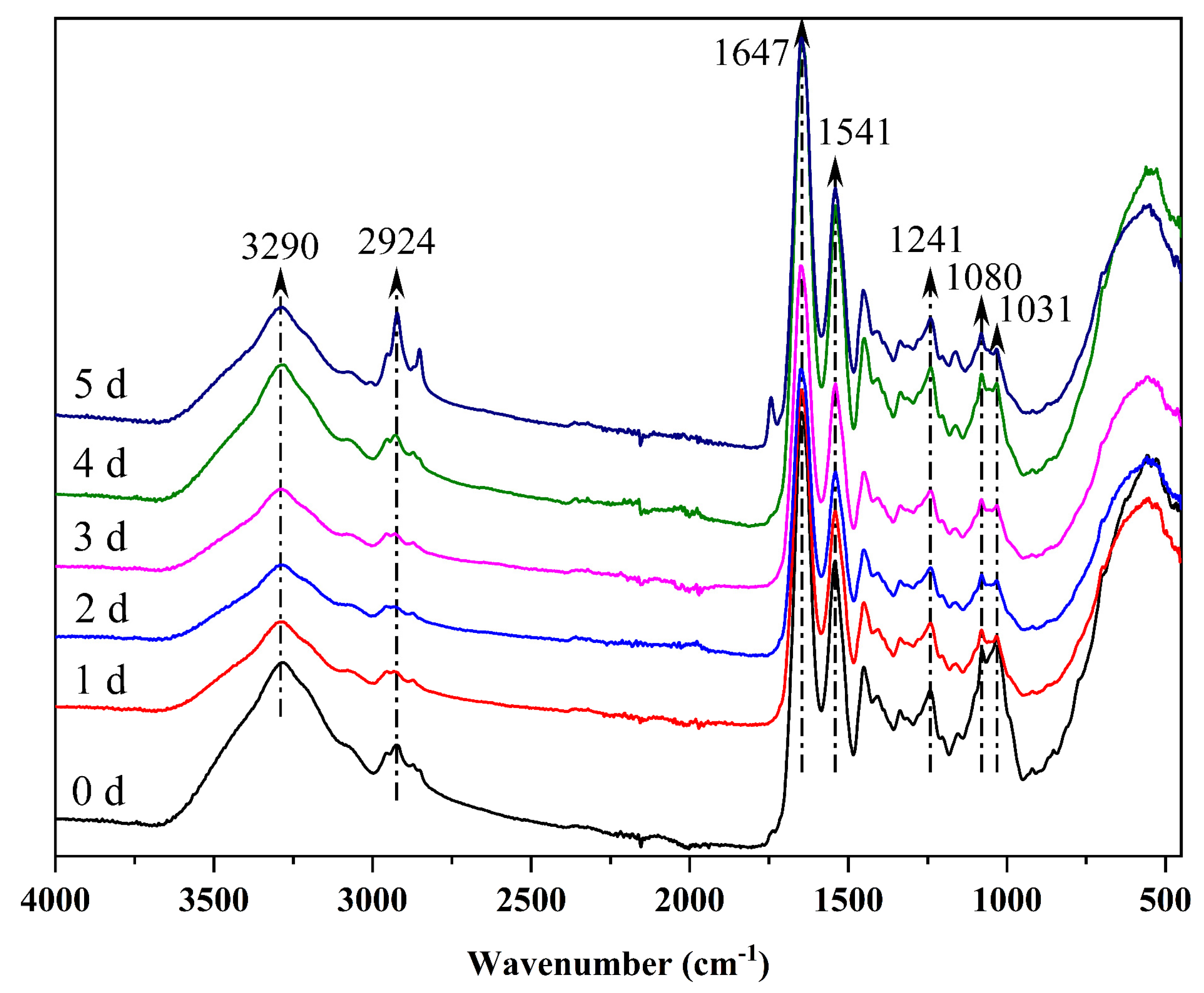


| Wavenumber/cm−1 | 3290 | 2924 | 1647 | 1541 | 1450 | 1407 | 1337 | 1241 | 1080 | 1031 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 d | 9.17% | 5.41% | 19.86% | 13.71% | 8.95% | 7.76% | 7.15% | 7.94% | 10.01% | 10.04% |
| 1 d | 8.41% | 4.86% | 22.78% | 15.05% | 9.30% | 7.69% | 7.05% | 7.88% | 8.50% | 8.47% |
| 2 d | 8.61% | 6.88% | 21.25% | 14.40% | 9.35% | 8.35% | 7.03% | 7.94% | 8.23% | 7.95% |
| 3 d | 8.35% | 6.22% | 22.44% | 15.01% | 9.45% | 7.67% | 7.11% | 7.99% | 8.11% | 7.65% |
| 4 d | 8.27% | 5.05% | 22.40% | 15.17% | 9.40% | 7.78% | 7.15% | 7.99% | 8.51% | 8.28% |
| 5 d | 8.22% | 5.05% | 22.96% | 15.28% | 9.41% | 7.72% | 7.10% | 7.99% | 8.25% | 8.03% |
| DSC | TGA | ||||||
|---|---|---|---|---|---|---|---|
| T (°C) | ∆H (J/g) | Peak1 (°C) | Weight Loss (%) | Peak2 (°C) | Weight Loss (%) | Residue at 600 °C (%) | |
| 0 d | 143.5 | −3.346 | 194.4 | 9.23 | 313.3 | 56.29 | 35.13 |
| 1 d | 155.4 | −4.241 | 193.3 | 6.14 | 318.2 | 57.13 | 35.35 |
| 2 d | 158.6 | −4.042 | 196.6 | 4.96 | 319.5 | 55.25 | 38.63 |
| 3 d | 160.8 | −5.455 | 195.6 | 4.73 | 305.6 | 52.12 | 44.56 |
| 4 d | 162.7 | −4.180 | 198.5 | 4.99 | 315.0 | 55.75 | 38.17 |
| 5 d | 163.9 | −5.752 | 198.9 | 3.67 | 313.3 | 55.14 | 40.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Luo, S.; Li, Y.; Zhang, H.; Yuan, Z.; Shang, L.; Deng, L. Influence of the Maillard Reaction on Properties of Air-Assisted Electrospun Gelatin/Zein/Glucose Nanofibers. Foods 2023, 12, 451. https://doi.org/10.3390/foods12030451
Liu S, Luo S, Li Y, Zhang H, Yuan Z, Shang L, Deng L. Influence of the Maillard Reaction on Properties of Air-Assisted Electrospun Gelatin/Zein/Glucose Nanofibers. Foods. 2023; 12(3):451. https://doi.org/10.3390/foods12030451
Chicago/Turabian StyleLiu, Songqi, Shiyuan Luo, Yuanli Li, Huange Zhang, Zhihe Yuan, Longchen Shang, and Lingli Deng. 2023. "Influence of the Maillard Reaction on Properties of Air-Assisted Electrospun Gelatin/Zein/Glucose Nanofibers" Foods 12, no. 3: 451. https://doi.org/10.3390/foods12030451
APA StyleLiu, S., Luo, S., Li, Y., Zhang, H., Yuan, Z., Shang, L., & Deng, L. (2023). Influence of the Maillard Reaction on Properties of Air-Assisted Electrospun Gelatin/Zein/Glucose Nanofibers. Foods, 12(3), 451. https://doi.org/10.3390/foods12030451








