Lab-Scale Methodology for New-Make Bourbon Whiskey Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Maize Samples
2.2. Whole Kernel Assessment
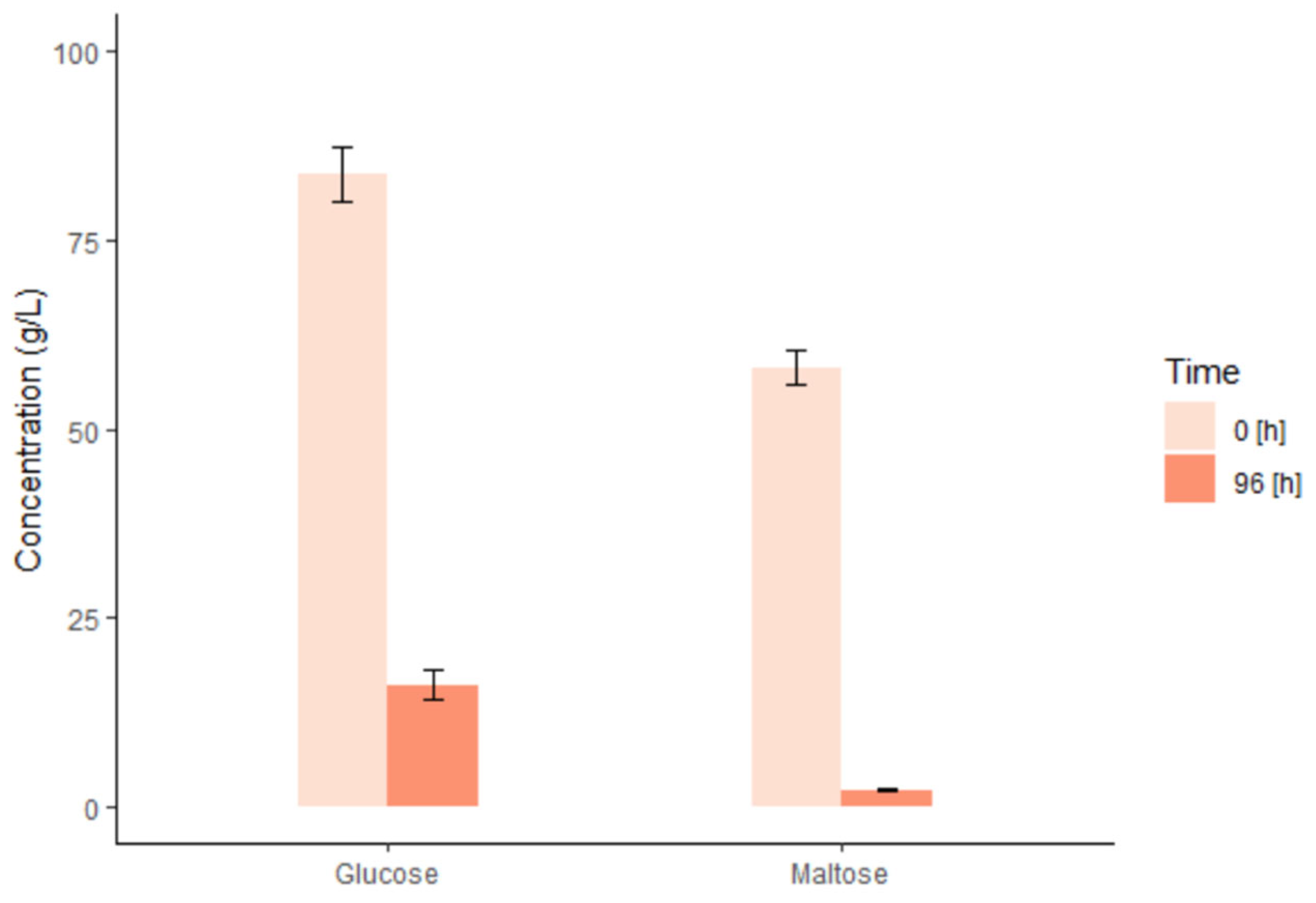
2.3. Mashing and Fermentation
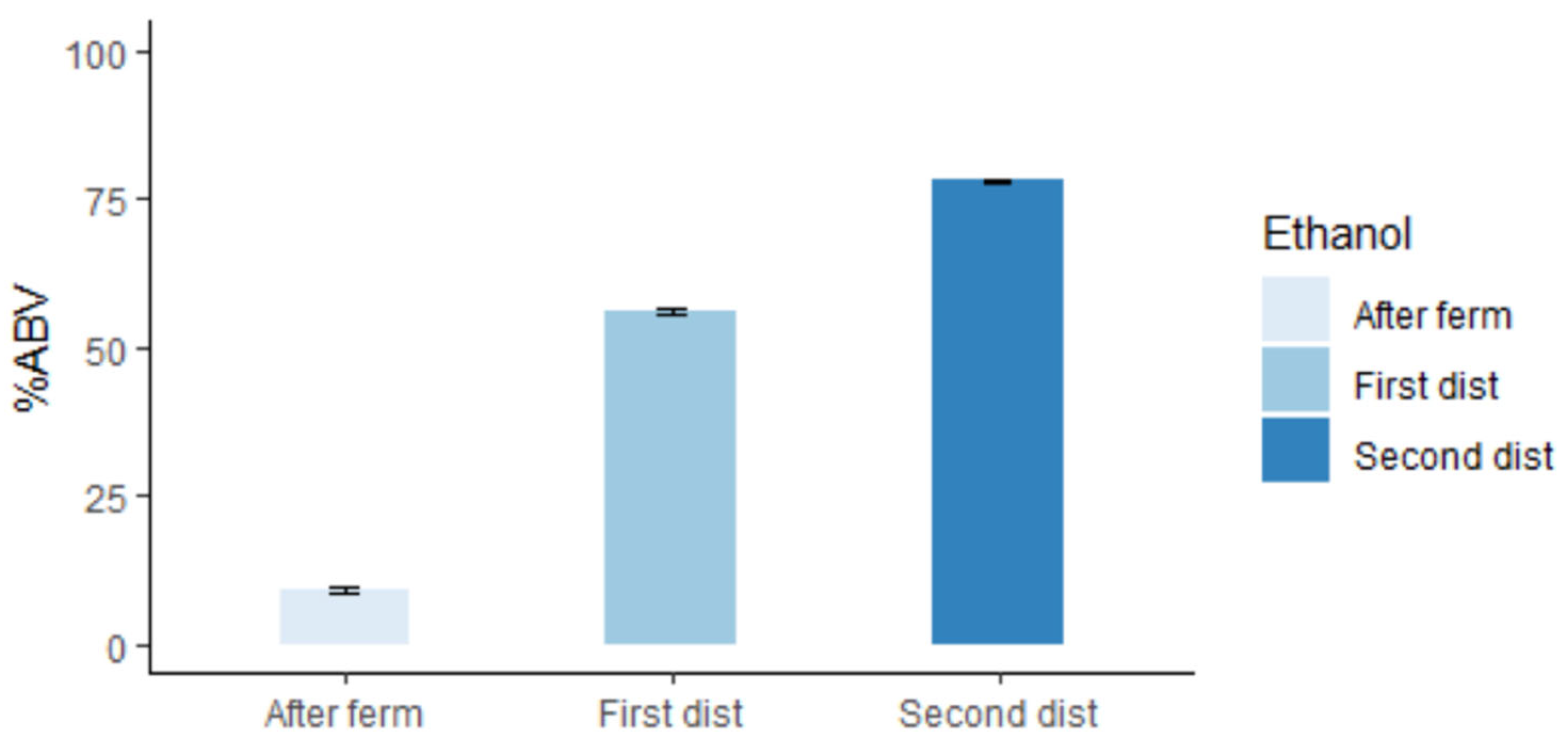
2.4. First Pass and Second Pass Distillation
2.5. HPLC Analysis
2.6. Data Analysis and Visualization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McMichael, A. Bourbon. In The SAGE Encyclopedia of Alcohol: Social, Cultural, and Historical Perspectives; SAGE Publications Inc.: Thousand Oaks, CA, USA, 2015; pp. 286–289. [Google Scholar] [CrossRef]
- Kentucky Corn Growers Association (KCCA). Kentucky Distillers Set Records for Bourbon Barrel Inventories, New Fills-And Punishing Production Taxes. 2022. Available online: https://www.kycorn.org/news/2022/9/29/kentucky-distillers-set-records-for-bourbon-barrel-inventories-new-fills-and-punishing-production-taxes (accessed on 10 November 2022).
- Distilled Spirits Council of the United States (DISCUS). On America’s Whiskey Trail. 2020. Available online: https://www.distilledspirits.org/wp-content/uploads/2020/04/American-Whiskey-2019.pdf (accessed on 18 November 2022).
- Kiss, A.A. Distillation technology-still young and full of breakthrough opportunities. J. Chem. Technol. Biotechnol. 2014, 89, 479–498. [Google Scholar] [CrossRef]
- Bamforth, C.W. Distilled Alcoholic Beverages. In Food, Fermentation and Micro-Organisms; John Wiley & Sons Inc: Hoboken, NJ, USA, 2005; pp. 122–132. [Google Scholar]
- Wright, S.; Pilkington, H. Whiskies of Canada and the United States. In Whisky and Other Spirits: Technology, Production and Marketing; Academic Press: Cambridge, MA, USA, 2021; pp. 87–104. [Google Scholar]
- Strickland, M.; Wright, S.; Pilkington, H. Craft distilling in North America. In Whisky and Other Spirits: Technology, Production and Marketing; Academic Press: Cambridge, MA, USA, 2021; pp. 105–123. [Google Scholar]
- Dawson, J.C.; Healy, G.K. Flavour Evaluation for Plant Breeders. Plant. Breed. Rev. 2018, 41, 215–261. [Google Scholar] [CrossRef]
- Arnold, R.J.; Ochoa, A.; Kerth, C.R.; Miller, R.K.; Murray, S.C. Assessing the impact of corn variety and Texas terroir on flavor and alcohol yield in new-make bourbon whiskey. PLoS ONE 2019, 14, e0220787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agu, R.C.; Bringhurst, T.A.; Brosnan, J.M. Production of grain whisky and ethanol from wheat, maize and other cereals. J. Inst. Brew. 2006, 112, 314–323. [Google Scholar] [CrossRef]
- Devantier, R.; Pedersen, S.; Olsson, L. Characterization of very high gravity ethanol fermentation of corn mash. Effect of glucoamylase dosage, pre-saccharification and yeast strain. Appl. Microbiol. Biotechnol. 2005, 68, 622–629. [Google Scholar] [CrossRef]
- Swanston, J.S.; Smith, P.L.; Gillespie, T.L.; Brosnan, J.M.; Bringhurst, T.A.; Agu, R.C. Associations between grain—Characteristics and alcohol yield among soft wheat varieties. J. Sci. Food Agric. 2007, 87, 676–683. [Google Scholar] [CrossRef]
- Green, D.I.G.; Agu, R.C.; Bringhurst, T.A.; Brosnan, J.M.; Jack, F.R.; Walker, G.M. Maximizing alcohol yields from wheat and maize and their co-products for distilling or bioethanol production. J. Inst. Brew. 2015, 121, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Agu, R.C.; Swanston, J.S.; Walker, J.W.; Pearson, S.Y.; Bringhurst, T.A.; Brosnan, J.M.; Jack, F.R. Predicting alcohol yield from UK soft winter wheat for grain distilling: Combined influence of hardness and nitrogen measurements. J. Inst. Brew. 2009, 115, 183–190. [Google Scholar] [CrossRef]
- Zoeller, K. Comparative Evaluation of Ethanol Zield from HTF Corn Varieties in the Whisky Production Process. Master’s Thesis, University of Louisville, Louisville, KY, USA, 2008. [Google Scholar] [CrossRef]
- Balcerek, M.; Pielech-Przybylska, K.; Dziekońska-Kubczak, U.; Patelski, P.; Strak, E. Fermentation results and chemical composition of agricultural distillates obtained from rye and barley grains and the corresponding malts as a source of amylolytic enzymes and starch. Molecules 2016, 21, 1320. [Google Scholar] [CrossRef] [Green Version]
- Kyraleou, M.; Herb, D.; O’Reilly, R.; Conway, N.; Bryan, T.; Kilcawley, K.N. The impact of terroir on the flavour of single malt whisk(e)y new make spirit. Foods 2021, 10, 443. [Google Scholar] [CrossRef]
- Bettenhausen, H.M.; Benson, A.; Fisk, S.; Herb, D.; Hernandez, J.; Lim, J.; Queisser, S.H.; Shellhammer, T.H.; Vega, V.; Yao, L.; et al. Variation in Sensory Attributes and Volatile Compounds in Beers Brewed from Genetically Distinct Malts: An Integrated Sensory and Non-Targeted Metabolomics Approach. J. Am. Soc. Brew. Chem. 2020, 78, 136–152. [Google Scholar] [CrossRef] [Green Version]
- Lallemand Distilling. Distilazyme AA. 2019. Available online: https://lallemanddistilling.com/wp-content/uploads/2018/10/TDS-DistilaZyme-AA-2.pdf (accessed on 18 November 2022).
- Dunaway, K.W.; Dasari, R.K.; Bennett, N.G.; Berson, R.E. Characterization of changes in viscosity and insoluble solids content during enzymatic saccharification of pretreated corn stover slurries. Bioresour. Technol. 2010, 101, 3575–3582. [Google Scholar] [CrossRef]
- Lallemand Distilling. DistilaMax RM. 2021. Available online: https://lallemanddistilling.com/wp-content/uploads/2012/12/TDS-DistilaMax-RM-20122021.pdf (accessed on 18 November 2022).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Dodge, L.A.; Kalinoski, R.M.; Lalitendu, D.; Bursavich, J.; Muley, P.; Boldor, D.; Shi, J. Sequential Extraction and Characterization of Lignin-Derived Compounds from Thermochemically Processed Biorefinery Lignins. Energy Fuels 2019, 33, 4322–4330. [Google Scholar] [CrossRef]
- Haefele, D.M.; Combs, E.E. Corn: Genetics, composition and quality. In The Alcohol Textbook; Lallemand Biofuels & Distilled Spirits: Duluth, GA, USA, 2017; pp. 42–54. [Google Scholar]
- Piggot, R. Maize: Receiving, storage and milling. In The Alcohol Textbook; Lallemand Biofuels & Distilled Spirits: Duluth, GA, USA, 2017; pp. 115–120. [Google Scholar]
- Buttery, R.G.; Ling, L.C.; Stern, D.J. Studies on Popcorn Aroma and Flavor Volatiles. J. Agric. Food Chem. 1997, 45, 837–843. [Google Scholar] [CrossRef]
- Piggott, J.R.; Conner, J.M. Whiskies. In Fermented Beverage Production; Springer: Boston, MA, USA, 2003; pp. 239–262. [Google Scholar] [CrossRef]
- Poisson, L.; Schieberle, P. Characterization of the most odor-active compounds in an American Bourbon whisky by application of the aroma extract dilution analysis. J. Agric. Food Chem. 2008, 56, 5813–5819. [Google Scholar] [CrossRef]
- Wanikawa, A. Flavors in Malt Whisky: A review. J. Am. Soc. Brew. Chem. 2020, 78, 260–278. [Google Scholar] [CrossRef]
- Willkie, H.F.; Prochaska, J.A. Fundamentals of Distillery Practice: A Handbook on the Manufacture of Ethyl Alcohol and Distillers’ Feed Products from Cereals; J.E. Seagram & Sons Inc.: Louisville, KY, USA, 1943. [Google Scholar]
- Agu, R.A.; Bringhurst, T.A.; Brosnan, J.M.; Jack, F.R. Effect of process conditions on alcohol yield of wheat, maize and other cereals. J. Inst. Brew. 2008, 114, 39–44. [Google Scholar] [CrossRef]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef]
- Ingledew, W.M. Yeast stress and fermentation. In The Alcohol Textbook; Lallemand Biofuels & Distilled Spirits: Duluth, GA, USA, 2017; pp. 273–285. [Google Scholar]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, M. Acetic acid and lactic acid inhibition of growth of Saccharomyces cerevisiae by different mechanisms. J. Am. Soc. Brew. Chem. 2001, 59, 187–194. [Google Scholar] [CrossRef]
- Madson, P.W. Alcohol distillation—Theory. In The Alcohol Textbook; Lallemand Biofuels & Distilled Spirits: Duluth, GA, USA, 2017; pp. 413–426. [Google Scholar]
- Nicol, D.A. Developments in distillery practices in response to external pressures. In Proceedings of the third Aviemore Conference on Malting, Brewing and Distilling, Aviemore, Scotland, 20–24 May 1990; Institute of Brewing: London, UK, 1990; pp. 117–137. [Google Scholar]
- Swanston, J.S.; Smith, P.L.; Thomas, W.T.B.; Sylvester-Bradley, R.; Kindred, D.; Brosnan, J.M.; Bringhurst, T.A.; Agu, R.C. Stability, across environments, of grain and alcohol yield, in soft wheat varieties grown for grain distilling or bioethanol production. J. Sci. Food Agric. 2014, 94, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Bringhurst, T.A.; Harrison, B.M.; Brosnan, J. Scotch whisky: Raw material selection and processing. In Whisky and Other Spirits: Technology, Production and Marketing; Academic Press: Cambridge, MA, USA, 2021; pp. 137–203. [Google Scholar]


| Sample | Moisture % | Protein % | Oil % | C. fiber % | Ash % | Starch % |
|---|---|---|---|---|---|---|
| 1 | 11.98 ± 0.06 | 7.27 ± 0.08 | 3.39 ± 0.05 | 1.70 ± 0.01 | 1.07 ± 0.01 | 65.21 ± 0.13 |
| 2 | 10.51 ± 0.17 | 7.57 ± 0.11 | 3.99 ± 0.51 | 1.66 ± 0.08 | 1.20 ± 0.02 | 66.61 ± 0.09 |
| 3 | 10.78 ± 0.18 | 7.23 ± 0.06 | 4.37 ± 0.41 | 2.68 ± 0.03 | 0.86 ± 0.06 | 65.85 ± 0.35 |
| 4 | 8.89 ± 0.17 | 8.10 ± 0.11 | 3.60 ± 0.03 | 1.91 ± 0.05 | 1.26 ± 0.01 | 66.26 ± 0.25 |
| 5 | 9.62 ± 0.01 | 7.39 ± 0.17 | 3.52 ± 0.17 | 1.93 ± 0.06 | 1.24 ± 0.01 | 66.93 ± 0.50 |
| 6 | 9.75 ± 0.03 | 7.61 ± 0.15 | 3.56 ± 0.15 | 2.02 ± 0.09 | 1.24 ± 0.01 | 66.02 ± 0.24 |
| 7 | 13.12 ± 0.16 | 7.30 ± 0.16 | 3.64 ± 0.16 | 1.58 ± 0.06 | 1.26 ± 0.03 | 65.40 ± 0.26 |
| 8 | 12.20 ± 0.27 | 7.68 ± 0.01 | 4.06 ± 0.01 | 1.57 ± 0.03 | 1.18 ± 0.06 | 64.54 ± 0.30 |
| Average | 10.87 ± 1.48 | 7.52 ± 0.29 | 3.76 ± 0.34 | 1.88 ± 0.36 | 1.16 ± 0.14 | 65.98 ± 0.89 |
| Sample | Glycerol (g/L) | Acetic Acid (g/L) | Lactic Acid (g/L) |
|---|---|---|---|
| 1 | 2.80 | 0.42 | 3.66 |
| 2 | 2.12 | 0.34 | 1.18 |
| 3 | 2.62 | 0.42 | 4.34 |
| 4 | 1.96 | 0.94 | 4.82 |
| 5 | 2.84 | 0.94 | 2.48 |
| 6 | 2.00 | 0.70 | 2.88 |
| 7 | 2.26 | 0.92 | 2.46 |
| 8 | 1.94 | 0.40 | 3.52 |
| Average | 2.32 ± 0.38 | 0.64 ± 0.27 | 3.17 ± 1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verges, V.L.; Gollihue, J.W.; Joyce, G.E.; DeBolt, S. Lab-Scale Methodology for New-Make Bourbon Whiskey Production. Foods 2023, 12, 457. https://doi.org/10.3390/foods12030457
Verges VL, Gollihue JW, Joyce GE, DeBolt S. Lab-Scale Methodology for New-Make Bourbon Whiskey Production. Foods. 2023; 12(3):457. https://doi.org/10.3390/foods12030457
Chicago/Turabian StyleVerges, Virginia L., Jarrad W. Gollihue, Glenna E. Joyce, and Seth DeBolt. 2023. "Lab-Scale Methodology for New-Make Bourbon Whiskey Production" Foods 12, no. 3: 457. https://doi.org/10.3390/foods12030457






