Impact of Solubilized Substances on the Techno-Functional, Pasting and Rheological Properties of Ultrasound-Modified Rice, Tef, Corn and Quinoa Flours
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrasound Treatment
2.3. Characterization of Soluble Compounds and Ash Content
2.4. Particle Size Distribution
2.5. Color
2.6. Amylose and Starch Content
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Techno-Functional Properties
2.9. Thermal Properties
2.10. Pasting Properties
2.11. Gels’ Rheological Properties
2.12. Statistical Analysis
3. Results
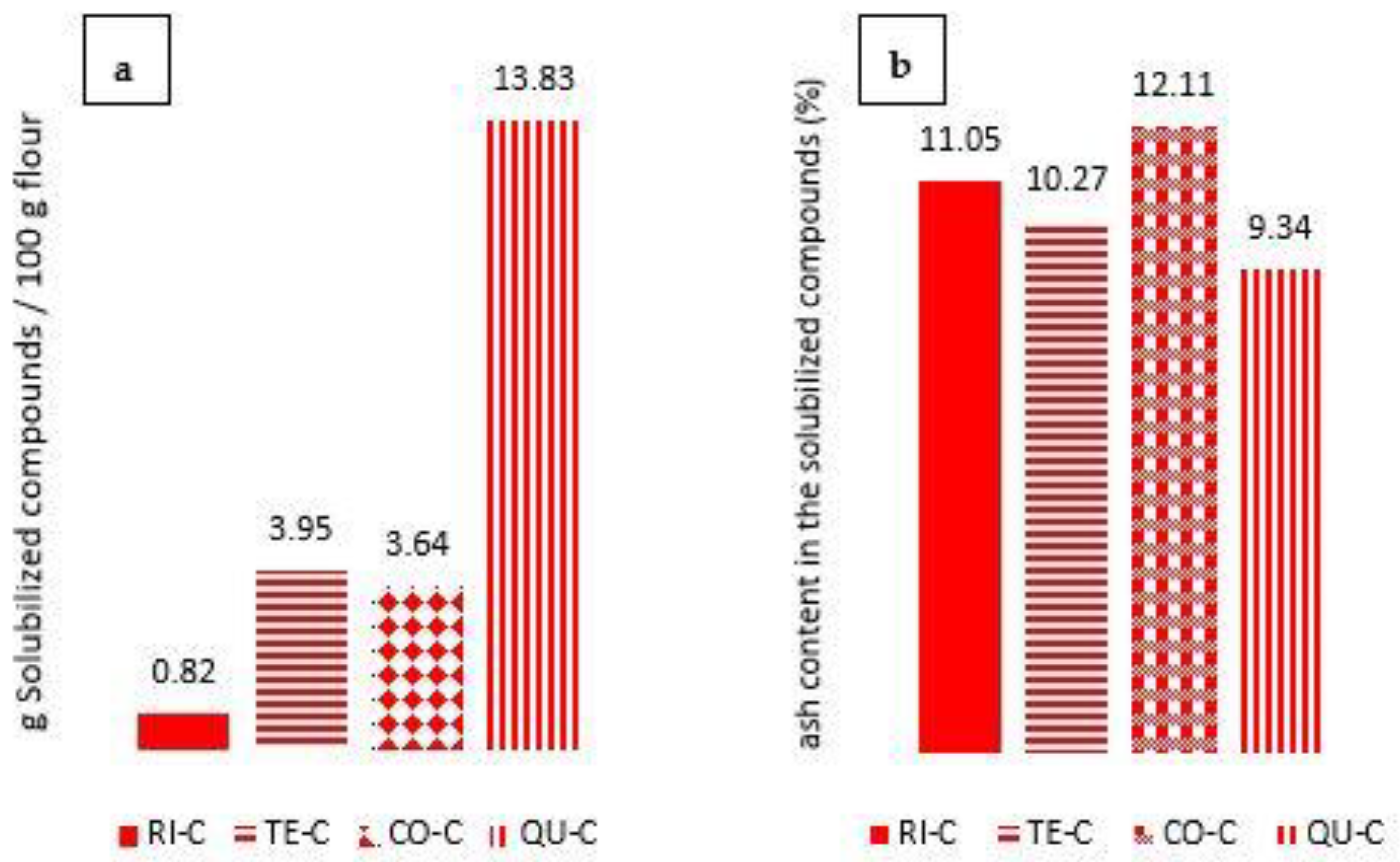
3.1. Characterization of the Supernatant Obtained from Centrifuged Samples
3.2. Particle Size
3.3. Color
3.4. Amylose and Starch Content
3.5. FTIR Spectroscopy
3.6. Techno-Functional Properties
3.7. Thermal Properties
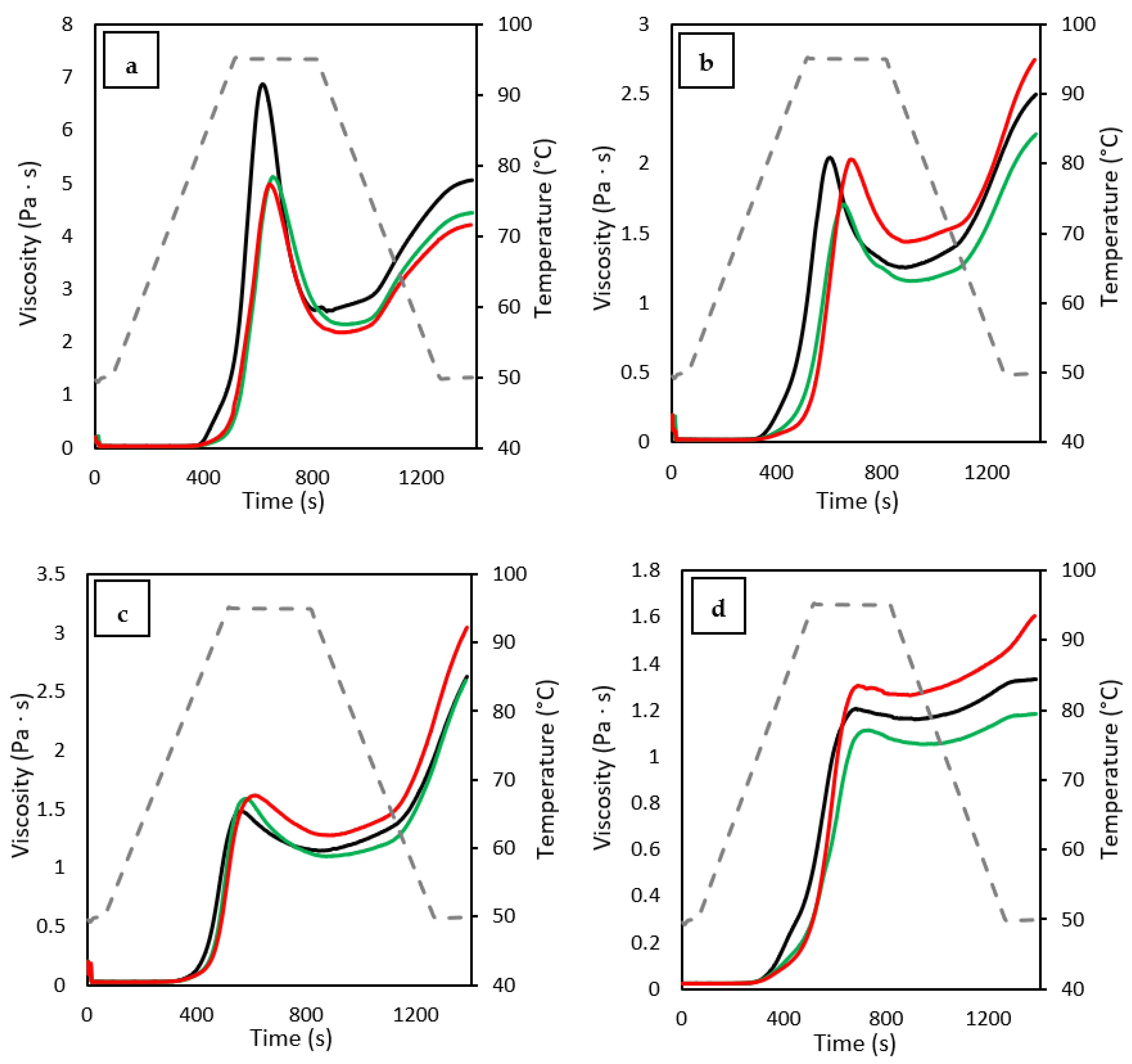
3.8. Pasting Properties
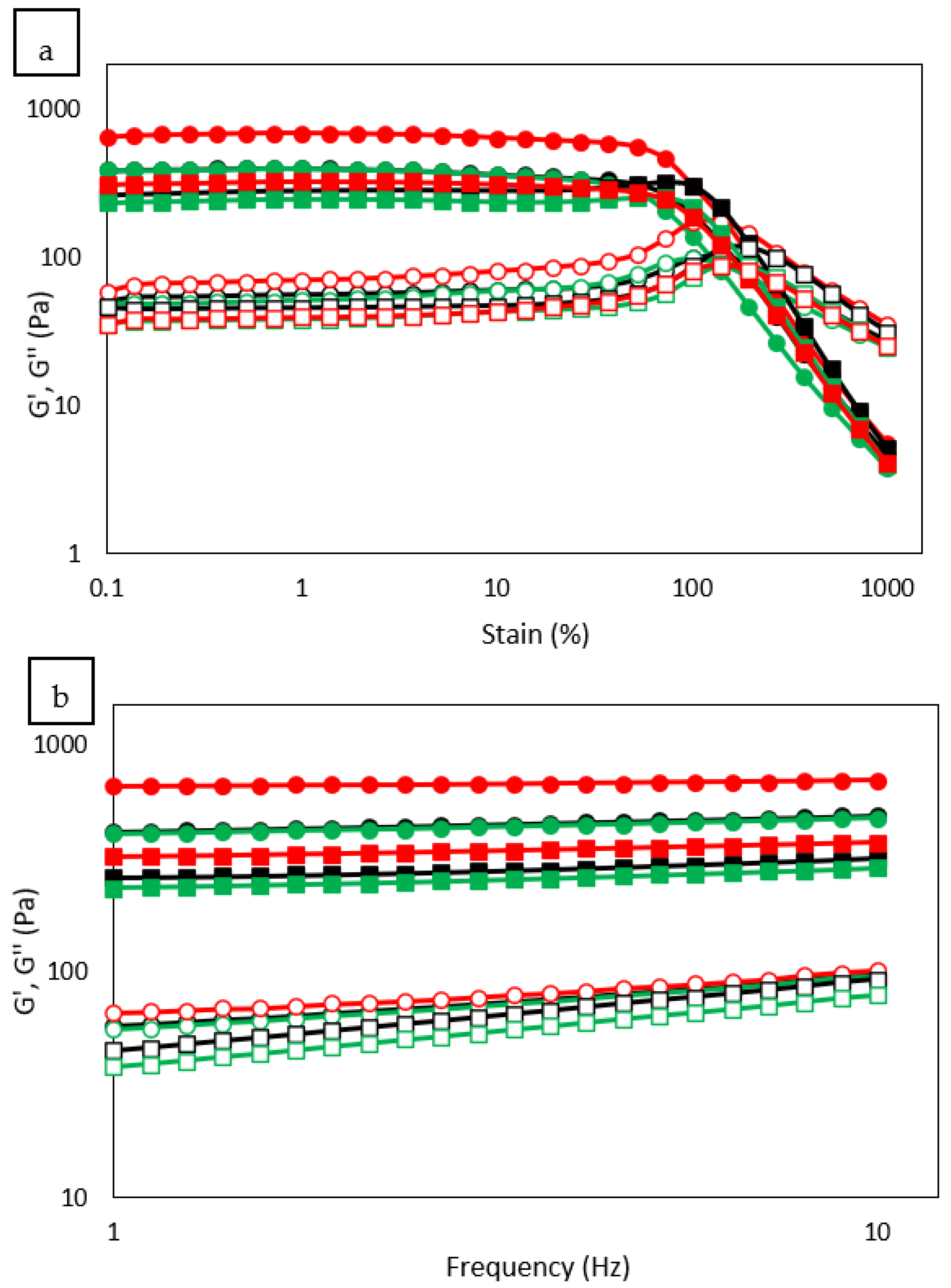
3.9. Rheological Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, A.; Li, Y.; Zheng, J. Dual-Frequency Ultrasonic Effect on the Structure and Properties of Starch with Different Size. LWT 2019, 106, 254–262. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, Y.; Wu, X.; Luo, F.; Lin, Q.; Ding, Y. Changes in Structural, Digestive, and Rheological Properties of Corn, Potato, and Pea Starches as Influenced by Different Ultrasonic Treatments. Int. J. Biol. Macromol. 2021, 185, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Monroy, Y.; Rivero, S.; García, M.A. Microstructural and Techno-Functional Properties of Cassava Starch Modified by Ultrasound. Ultrason. Sonochem. 2018, 42, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zhu, F. Effect of Ultrasound on Structural and Physicochemical Properties of Sweetpotato and Wheat Flours. Ultrason. Sonochem. 2020, 66, 105118. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Herceg, Z.; Šubarić, D.; Babić, J.; Brnčić, M.; Brnčić, S.R.; Bosiljkov, T.; Čvek, D.; Tripalo, B.; Gelo, J. Ultrasound Effect on Physical Properties of Corn Starch. Carbohydr. Polym. 2010, 79, 91–100. [Google Scholar] [CrossRef]
- Flores-Silva, P.C.; Roldan-Cruz, C.A.; Chavez-Esquivel, G.; Vernon-Carter, E.J.; Bello-Perez, L.A.; Alvarez-Ramirez, J. In Vitro Digestibility of Ultrasound-Treated Corn Starch. Starch Stärke 2017, 69, 1700040. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Zhu, C. Effect of Ultrasound Pretreatment on Enzymolysis and Physicochemical Properties of Corn Starch. Int. J. Biol. Macromol. 2018, 111, 848–856. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Villanueva, M.; Vela, A.J.; Ronda, F. Protein and Lipid Enrichment of Quinoa (Cv.Titicaca) by Dry Fractionation. Techno-Functional, Thermal and Rheological Properties of Milling Fractions. Food Hydrocoll 2020, 105, 105770. [Google Scholar] [CrossRef]
- Kaur, H.; Gill, B.S. Effect of High-Intensity Ultrasound Treatment on Nutritional, Rheological and Structural Properties of Starches Obtained from Different Cereals. Int. J. Biol. Macromol. 2019, 126, 367–375. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Rostamabadi, H.; Rostamabadi, M.M.; Hamedi, H.; Hosseini, S.M.H. Preparation of Physically Modified Oat Starch with Different Sonication Treatments. Food Hydrocoll. 2019, 89, 311–320. [Google Scholar] [CrossRef]
- Karwasra, B.L.; Kaur, M.; Gill, B.S. Impact of Ultrasonication on Functional and Structural Properties of Indian Wheat (Triticum Aestivum L.) Cultivar Starches. Int. J. Biol. Macromol. 2020, 164, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Ronda, F.; Villanueva, M.; Collar, C. Influence of Acidification on Dough Viscoelasticity of Gluten-Free Rice Starch-Based Dough Matrices Enriched with Exogenous Protein. LWT Food Sci. Technol. 2014, 59, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, P.; Kumar, V.; Sharma, H.K. Comparative Study of Physicochemical, Functional, Antinutritional and Pasting Properties of Taro (Colocasia Esculenta), Rice (Oryza Sativa) Flour, Pigeonpea (Cajanus Cajan) Flour and Their Blends. LWT 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Abebe, W.; Collar, C.; Ronda, F. Impact of Variety Type and Particle Size Distribution on Starch Enzymatic Hydrolysis and Functional Properties of Tef Flours. Carbohydr. Polym. 2015, 115, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Amini, A.M.; Razavi, S.M.A.; Mortazavi, S.A. Morphological, Physicochemical, and Viscoelastic Properties of Sonicated Corn Starch. Carbohydr. Polym. 2015, 122, 282–292. [Google Scholar] [CrossRef]
- Chan, H.T.; Bhat, R.; Karim, A.A. Effects of Sodium Dodecyl Sulphate and Sonication Treatment on Physicochemical Properties of Starch. Food Chem. 2010, 120, 703–709. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Rokita, B.; Lotfy, S.; Ulanski, P.; Rosiak, J.M. Degradation of Chitosan and Starch by 360-KHz Ultrasound. Carbohydr. Polym. 2005, 60, 175–184. [Google Scholar] [CrossRef]
- Minakawa, A.F.K.; Faria-Tischer, P.C.S.; Mali, S. Simple Ultrasound Method to Obtain Starch Micro- and Nanoparticles from Cassava, Corn and Yam Starches. Food Chem. 2019, 283, 11–18. [Google Scholar] [CrossRef]
- Zhu, F.; Li, H. Modification of Quinoa Flour Functionality Using Ultrasound. Ultrason. Sonochem. 2019, 52, 305–310. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the Secondary Structure of Proteins by Deconvolved FTIR Spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Fevzioglu, M.; Ozturk, O.K.; Hamaker, B.R.; Campanella, O.H. Quantitative Approach to Study Secondary Structure of Proteins by FT-IR Spectroscopy, Using a Model Wheat Gluten System. Int. J. Biol. Macromol. 2020, 164, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- AACC. Method 76-21.02. General Pasting Method for Wheat or Rye Flour of Starch Using the Rapid Visco Analyser. In AACC International Approved Methods; Cereals & Grains Association: St. Paul, MN, USA, 2017. [Google Scholar] [CrossRef]
- Harasym, J.; Satta, E.; Kaim, U. Ultrasound Treatment of Buckwheat Grains Impacts Important Functional Properties of Resulting Flour. Molecules 2020, 25, 3012. [Google Scholar] [CrossRef] [PubMed]
- Vela, A.J.; Villanueva, M.; Solaesa, Á.G.; Ronda, F. Impact of High-Intensity Ultrasound Waves on Structural, Functional, Thermal and Rheological Properties of Rice Flour and Its Biopolymers Structural Features. Food Hydrocoll. 2021, 113, 106480. [Google Scholar] [CrossRef]
- Yang, W.; Kong, X.; Zheng, Y.; Sun, W.; Chen, S.; Liu, D.; Zhang, H.; Fang, H.; Tian, J.; Ye, X. Controlled Ultrasound Treatments Modify the Morphology and Physical Properties of Rice Starch Rather than the Fine Structure. Ultrason. Sonochem. 2019, 59, 104709. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Luo, F.; Lin, Q. Insights into the Relations between the Molecular Structures and Digestion Properties of Retrograded Starch after Ultrasonic Treatment. Food Chem. 2019, 294, 248–259. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Liu, Y.; Ouyang, J. Effect of Ultrasonic and Microwave Dual-Treatment on the Physicochemical Properties of Chestnut Starch. Polymers 2020, 12, 1718. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Ohanenye, I.C.; Ahmed, T.; Udenigwe, C.C. Microwave Treatment Increased Protein Digestibility of Pigeon Pea (Cajanus Cajan) Flour: Elucidation of Underlying Mechanisms. Food Chem. 2020, 329, 127196. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, S.; Oladejo, A.O.; Ruan, S.; Wang, Y.; Huang, S.; Ma, H. Effects of Ultrasound Pretreatment with Different Frequencies and Working Modes on the Enzymolysis and the Structure Characterization of Rice Protein. Ultrason. Sonochem. 2017, 38, 19–28. [Google Scholar] [CrossRef]
- Vela, A.J.; Villanueva, M.; Ronda, F. Low-Frequency Ultrasonication Modulates the Impact of Annealing on Physicochemical and Functional Properties of Rice Flour. Food Hydrocoll. 2021, 120, 106933. [Google Scholar] [CrossRef]
- Wang, H.; Xu, K.; Ma, Y.; Liang, Y.; Zhang, H.; Chen, L. Impact of Ultrasonication on the Aggregation Structure and Physicochemical Characteristics of Sweet Potato Starch. Ultrason. Sonochem. 2020, 63, 104868. [Google Scholar] [CrossRef] [PubMed]
- Herceg, I.L.; Jambrak, A.R.; Šubarić, D.; Brnčić, M.; Brnčić, S.R.; Badanjak, M.; Tripalo, B.; Ježek, D.; Novotni, D.; Herceg, Z. Texture and Pasting Properties of Ultrasonically Treated Corn Starch. Czech J. Food Sci. 2010, 28, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Li, L.; Fu, X. Ultrasound Effects on the Structure and Chemical Reactivity of Cornstarch Granules. Starch Stärke 2007, 59, 371–378. [Google Scholar] [CrossRef]
- Luo, Z.; Fu, X.; He, X.; Luo, F.; Gao, Q.; Yu, S. Effect of Ultrasonic Treatment on the Physicochemical Properties of Maize Starches Differing in Amylose Content. Starch Stärke 2008, 60, 646–653. [Google Scholar] [CrossRef]
- Zuo, J.Y.; Knoerzer, K.; Mawson, R.; Kentish, S.; Ashokkumar, M. The Pasting Properties of Sonicated Waxy Rice Starch Suspensions. Ultrason. Sonochem. 2009, 16, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, Y.; Ge, Y.; Zhang, Y.; Sun, T.; Jiao, Y.; Zheng, X.Q. Effects of Ultrasound Processing on the Thermal and Retrogradation Properties of Nonwaxy Rice Starch. J. Food Process. Eng. 2013, 36, 793–802. [Google Scholar] [CrossRef]
- Babu, A.S.; Mohan, R.J.; Parimalavalli, R. Effect of Single and Dual-Modifications on Stability and Structural Characteristics of Foxtail Millet Starch. Food Chem. 2019, 271, 457–465. [Google Scholar] [CrossRef]
- Eliasson, A.-C. Interactions between Starch and Lipids Studied by DSC. Thermochim. Acta 1994, 246, 343–356. [Google Scholar] [CrossRef]
- Cao, M.; Gao, Q. Effect of Dual Modification with Ultrasonic and Electric Field on Potato Starch. Int. J. Biol. Macromol. 2020, 150, 637–643. [Google Scholar] [CrossRef]
- Park, D.J.; Han, J.A. Quality Controlling of Brown Rice by Ultrasound Treatment and Its Effect on Isolated Starch. Carbohydr. Polym. 2016, 137, 30–38. [Google Scholar] [CrossRef]
- Pinto, V.Z.; Vanier, N.L.; Deon, V.G.; Moomand, K.; El Halal, S.L.M.; Zavareze, E.D.R.; Lim, L.T.; Dias, A.R.G. Effects of Single and Dual Physical Modifications on Pinhão Starch. Food Chem. 2015, 187, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meadows, F. Pasting Process in Rice Flour Using Rapid Visco Analyser Curves and First Derivatives. Cereal Chem. 2002, 79, 559–562. [Google Scholar] [CrossRef]
| Sample | Native | Freeze-Dried | Centrifuged |
|---|---|---|---|
| Rice | RI-N | RI-F | RI-C |
| Tef | TE-N | TE-F | TE-C |
| Corn | CO-N | CO-F | CO-C |
| Quinoa | QU-N | QU-F | QU-C |
| Sample | D50 (μm) | (D90-D10)/D50 | L* | a* | b* | C* | h | ΔE | Amylose (%) | Starch (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| RI-N | 210 c | 1.51 a | 86.2 a | 0.49 b | 5.11 c | 5.13 c | 84.5 a | --- | 36.8 c | 72.4 a |
| RI-F | 130 a | 2.40 c | 88.2 b | −0.08 a | 3.41 b | 3.41 b | 91.3 b | 2.69 | 35.2 b | 73.1 ab |
| RI-C | 165 b | 2.11 b | 88.7 b | −0.06 a | 3.23 a | 3.23 a | 91.1 b | 3.18 | 29.5 a | 74.8 b |
| SE | 1 | 0.01 | 0.2 | 0.02 | 0.03 | 0.03 | 0.2 | --- | 0.4 | 0.6 |
| p-value | *** | *** | *** | *** | *** | *** | *** | --- | ** | * |
| TE-N | 121.4 c | 2.44 a | 59.1 a | 8.1 c | 11.8 c | 14.3 c | 55.6 a | --- | 39.0 b | 64.8 a |
| TE-F | 84.1 a | 3.06 b | 65.6 b | 5.7 b | 10.1 b | 11.6 b | 60.7 b | 7.13 | 41.9 c | 64.5 a |
| TE-C | 95.4 b | 3.16 c | 72.5 c | 3.8 a | 6.6 a | 7.6 a | 60.1 b | 15.00 | 36.8 a | 70.7 b |
| SE | 0.6 | 0.01 | 0.4 | 0.1 | 0.1 | 0.2 | 0.3 | --- | 0.2 | 0.6 |
| p-value | *** | *** | *** | *** | *** | *** | *** | --- | *** | ** |
| CO-N | 184 b | 2.09 b | 77.8 b | 5.82 c | 31.1 c | 31.8 c | 79.5 b | --- | 37.1 a | 62.5 a |
| CO-F | 144 a | 2.45 c | 78.4 b | 4.60 a | 29.3 b | 29.6 b | 81.1 c | 2.26 | 42.1 b | 62.9 a |
| CO-C | 223 c | 1.80 a | 75.9 a | 5.07 b | 25.6 a | 26.1 a | 78.8 a | 5.87 | 37.8 a | 70.2 b |
| SE | 3 | 0.01 | 0.3 | 0.08 | 0.3 | 0.3 | 0.1 | --- | 0.7 | 0.4 |
| p-value | *** | *** | *** | *** | *** | *** | *** | --- | * | *** |
| QU-N | 161.1 b | 2.23 b | 75.3 a | 3.57 c | 13.4 c | 13.82 c | 75.1 b | --- | 22.3 b | 46.2 a |
| QU-F | 142.7 a | 2.29 c | 75.0 a | 3.25 b | 12.7 b | 13.08 b | 75.6 c | 0.83 | 25.9 c | 44.5 a |
| QU-C | 161.8 b | 2.06 a | 76.7 b | 3.05 a | 10.4 a | 10.81 a | 73.6 a | 3.35 | 17.2 a | 54.5 b |
| SE | 0.6 | 0.01 | 0.3 | 0.03 | 0.1 | 0.06 | 0.1 | --- | 0.4 | 0.6 |
| p-value | *** | *** | ** | *** | *** | *** | *** | --- | ** | *** |
| Starch | Proteins Secondary Structure | |||||
|---|---|---|---|---|---|---|
| Sample | 1047/1022 | 1022/995 | LF-β-Sheet | Random Coil + α-Helix | β-Turns | HF-β-Sheet |
| RI-N | 0.714 b | 0.877 a | 39.3 c | 37.5 a | 21.2 a | 1.93 a |
| RI-F | 0.680 a | 0.894 b | 31.9 a | 45.0 b | 21.2 a | 1.91 a |
| RI-C | 0.676 a | 0.889 b | 34.1 b | 43.1 b | 20.9 a | 1.86 a |
| SE | 0.002 | 0.002 | 0.5 | 0.7 | 0.3 | 0.03 |
| p-value | *** | *** | *** | *** | ns | ns |
| TE-N | 0.793 c | 0.891 a | 32.7 b | 44.9 a | 20.3 b | 2.09 b |
| TE-F | 0.699 b | 0.886 a | 29.0 a | 46.1 b | 22.3 c | 2.73 c |
| TE-C | 0.679 a | 0.878 a | 33.0 a | 46.7 b | 19.1 a | 1.21 a |
| SE | 0.001 | 0.005 | 0.5 | 0.4 | 0.1 | 0.08 |
| p-value | *** | * | ** | * | *** | *** |
| CO-N | 0.739 c | 0.880 a | 39.1 c | 42.8 a | 15.5 a | 2.64 b |
| CO-F | 0.729 b | 0.904 b | 32.2 a | 48.2 b | 17.3 b | 2.21 ab |
| CO-C | 0.704 a | 0.882 a | 34.5 b | 46.8 b | 16.8 b | 1.90 a |
| SE | 0.002 | 0.002 | 0.6 | 0.7 | 0.3 | 0.17 |
| p-value | *** | *** | *** | ** | ** | ns |
| QU-N | 0.769 c | 0.938 c | 33.8 a | 44.1 a | 19.3 b | 2.79 a |
| QU-F | 0.692 b | 0.919 b | 32.9 a | 48.9 b | 16.2 a | 1.97 a |
| QU-C | 0.672 a | 0.905 a | 32.9 a | 50.3 c | 14.7 a | 2.14 a |
| SE | 0.002 | 0.003 | 0.6 | 0.3 | 0.6 | 0.13 |
| p-value | *** | *** | ns | *** | ** | ns |
| Sample | WAC | WAI | WSI | SP | EA | ES | FC | FS |
|---|---|---|---|---|---|---|---|---|
| RI-N | 1.31 b | 7.2 a | 1.6 a | 7.3 a | --- | --- | 3.0 a | 31 a |
| RI-F | 1.19 a | 9.2 b | 1.9 a | 9.4 b | --- | --- | 2.5 a | 35 a |
| RI-C | 1.22 a | 8.8 b | 2.1 a | 8.9 b | --- | --- | 3.5 a | 60 a |
| SE | 0.02 | 0.2 | 0.2 | 0.5 | --- | --- | 0.6 | 18 |
| p-value | ** | *** | ns | *** | --- | --- | ns | ns |
| TE-N | 1.13 b | 5.4 a | 5.4 c | 5.7 a | 48.2 b | 6 a | 6.5 b | 54 b |
| TE-F | 1.01 a | 7.2 b | 4.5 b | 7.5 b | 52.3 c | 29 b | 7.5 b | 46 b |
| TE-C | 1.23 c | 9.2 c | 2.3 a | 9.4 c | 40.6 a | 25 b | 2.5 a | 0 a |
| SE | 0.01 | 0.1 | 0.3 | 0.2 | 0.7 | 3 | 0.5 | 3 |
| p-value | *** | *** | *** | *** | *** | *** | * | ** |
| CO-N | 1.61 b | 6.0 a | 4.9 b | 6.4 a | 40.6 b | 5.9 b | 4.5 b | 90 b |
| CO-F | 1.56 a | 6.8 c | 6.0 c | 7.3 c | 45.1 c | 16.5 c | 4.5 b | 100 b |
| CO-C | 1.62 b | 6.4 b | 4.2 a | 6.7 b | 10.0 a | 0.0 a | 1.5 a | 0 a |
| SE | 0.02 | 0.1 | 0.1 | 0.3 | 0.2 | 0.3 | 0.5 | 6 |
| p-value | ns | *** | *** | *** | *** | *** | * | ** |
| QU-N | 0.92 b | 8.1 a | 6.2 b | 8.6 a | 57.7 a | 55.6 a | 6.0 a | 83 b |
| QU-F | 0.85 a | 8.3 a | 7.3 c | 9.0 a | 57.1 a | 56.2 a | 9.0 b | 23 a |
| QU-C | 1.01 c | 8.2 a | 5.1 a | 8.6 a | 57.4 a | 55.4 a | 4.0 a | 88 b |
| SE | 0.01 | 0.1 | 0.3 | 0.1 | 0.4 | 0.7 | 0.6 | 7 |
| p-value | *** | ns | * | ns | ns | ns | * | * |
| Sample | ΔHgel (J/g) | TO-gel (°C) | TP-gel (°C) | TC-gel (°C) | ΔHam-lip (J/g) | TP-am-lip (°C) | ΔHret (J/g) | TO-ret (°C) | TP-ret (°C) | TC-ret (°C) | ΔHam-lip-ret (J/g) | TP-am-lip-ret (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI-N | 14.3 a | 69.1 b | 74.4 b | 80.3 c | 1.30 a | 96.9 b | 8.5 a | 34.7 b | 50.7 a | 62.9 a | 3.0 a | 98.5 a |
| RI-F | 14.2 a | 68.0 a | 73.2 a | 78.9 b | 1.58 b | 96.4 ab | 9.5 b | 34.1 a | 51.1 a | 63.3 a | 3.2 a | 98.4 a |
| RI-C | 14.0 a | 68.1 a | 73.2 a | 78.5 a | 1.57 b | 96.1 a | 9.4 b | 35.0 b | 50.9 a | 62.7 a | 2.8 a | 97.9 a |
| SE | 0.1 | 0.1 | 0.1 | 0.1 | 0.05 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 |
| p-value | ns | ** | *** | *** | * | ns | * | * | ns | ns | ns | ns |
| TE-N | 13.2 b | 64.8 c | 71.1 c | 78.8 b | 0.82 a | 97.1 b | 6.8 ab | 38.3 b | 51.3 a | 63.2 a | 2.4 a | 95.8 a |
| TE-F | 12.3 a | 64.4 b | 70.7 b | 77.4 a | 0.95 a | 95.3 a | 7.4 b | 35.9 a | 50.6 a | 63.7 a | 2.2 a | 96.4 a |
| TE-C | 12.4 a | 64.0 a | 70.3 a | 77.1 a | 1.23 b | 96.2 ab | 5.9 a | 37.7 b | 50.8 a | 63.5 a | 1.9 a | 95.9 a |
| SE | 0.2 | 0.1 | 0.1 | 0.1 | 0.05 | 0.2 | 0.2 | 0.3 | 0.4 | 0.2 | 0.1 | 0.6 |
| p-value | * | ** | ** | ** | * | * | * | * | ns | ns | ns | ns |
| CO-N | 8.8 a | 62.9 c | 70.7 c | 77.6 b | 1.2 a | 96.8 a | 6.3 a | 38.1 a | 51.2 a | 62.7 a | 3.5 a | 89.1 a |
| CO-F | 8.5 a | 62.0 b | 70.2 b | 76.9 ab | 1.1 a | 97.7 a | 6.2 a | 38.8 a | 50.6 a | 62.5 a | 3.0 a | 89.5 a |
| CO-C | 9.7 a | 61.0 a | 69.3 a | 76.3 a | 0.9 a | 97.8 a | 5.5 a | 37.8 a | 49.9 a | 62.4 a | 2.9 a | 90.3 a |
| SE | 0.5 | 0.1 | 0.1 | 0.2 | 0.2 | 0.5 | 0.1 | 0.2 | 0.4 | 0.4 | 0.1 | 0.6 |
| p-value | ns | ** | ** | ns | ns | ns | * | ns | ns | ns | ns | ns |
| QU-N | 16.0 b | 62.3 a | 70.4 b | 79.6 b | 1.28 a | 94.6 a | 1.8 b | 25.2 a | 46.3 a | 61.0 a | 3.0 a | 89.5 b |
| QU-F | 15.3 b | 62.2 a | 70.6 b | 79.3 b | 1.14 a | 95.0 a | 0.9 a | 30.6 b | 47.8 a | 59.7 a | 2.9 a | 86.2 a |
| QU-C | 13.0 a | 61.8 a | 69.5 a | 78.3 a | 1.46 b | 94.2 a | 0.9 a | 31.2 b | 47.6 a | 60.3 a | 3.4 a | 86.5 a |
| SE | 0.3 | 0.3 | 0.1 | 0.2 | 0.08 | 0.2 | 0.1 | 0.4 | 0.8 | 0.5 | 0.1 | 0.5 |
| p-value | * | ns | * | * | * | ns | ** | * | ns | ns | ns | * |
| Sample | PT (°C) | PV (Pa · s) | TV (Pa · s) | BV (Pa · s) | FV (Pa · s) | SV (Pa · s) | τmax(Pa) | Cross over (Pa) | G₁′ (Pa) | a | G₁″ (Pa) | b | tan (δ)₁ | c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI-N | 82.1 a | 6.52 b | 2.43 a | 4.04 b | 5.03 c | 2.61 b | 219 a | 315 a | 214 b | 0.103 a | 37 b | 0.321 a | 0.172 a | 0.218 a |
| RI-F | 85.0 b | 5.10 a | 2.25 a | 2.83 a | 4.48 b | 2.22 a | 224 a | 294 a | 164 a | 0.107 a | 28 a | 0.341 b | 0.171 a | 0.233 b |
| RI-C | 84.8 b | 5.02 a | 2.22 a | 2.80 a | 4.25 a | 2.03 a | 226 a | 318 a | 141 a | 0.115 a | 26 a | 0.343 b | 0.183 a | 0.228 ab |
| SE | 0.2 | 0.07 | 0.08 | 0.09 | 0.03 | 0.08 | 7 | 9 | 10 | 0.004 | 1 | 0.002 | 0.004 | 0.004 |
| p-value | ** | ** | ns | ** | *** | * | ns | ns | * | ns | ** | ** | ns | ns |
| TE-N | 78.2 a | 2.04 b | 1.25 b | 0.78 c | 2.49 b | 1.24 b | 231 c | 322 c | 254 a | 0.08 a | 45 b | 0.314 a | 0.176 b | 0.23 a |
| TE-F | 80.3 b | 1.64 a | 1.11 a | 0.53 a | 2.10 a | 0.99 a | 187 b | 232 b | 240 a | 0.05 a | 34 a | 0.310 a | 0.126 a | 0.27 a |
| TE-C | 82.1 c | 2.11 b | 1.43 c | 0.71 b | 2.75 c | 1.32 b | 147 a | 210 a | 315 b | 0.07 a | 39 a | 0.317 a | 0.122 a | 0.25 a |
| SE | 0.3 | 0.05 | 0.03 | 0.02 | 0.07 | 0.04 | 4 | 4 | 10 | 0.03 | 1 | 0.007 | 0.005 | 0.01 |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** | ns | * | ns | *** | ns |
| CO-N | 79.1 a | 1.48 a | 1.08 a | 0.32 a | 2.55 a | 1.47 a | 166 b | 228 b | 399 a | 0.071 b | 56 a | 0.227 b | 0.139 c | 0.157 a |
| CO-F | 80.4 b | 1.56 ab | 1.09 a | 0.48 b | 2.60 a | 1.51 a | 90 a | 165 a | 394 a | 0.066 b | 52 a | 0.241 c | 0.132 b | 0.175 b |
| CO-C | 80.9 c | 1.60 b | 1.29 b | 0.31 a | 3.06 b | 1.77 b | 225 c | 348 c | 645 b | 0.030 a | 65 b | 0.189 a | 0.101 a | 0.161 a |
| SE | 0.2 | 0.03 | 0.02 | 0.02 | 0.04 | 0.03 | 7 | 9 | 17 | 0.003 | 1 | 0.003 | 0.002 | 0.003 |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | *** | *** | ** |
| QU-N | 75.7 a | 1.21 a | 1.16 b | 0.04 a | 1.34 b | 0.176 b | 24 b | 53 b | 276 b | 0.040 a | 28 b | 0.261 a | 0.100 c | 0.22 a |
| QU-F | 76.8 b | 1.13 a | 1.07 a | 0.06 a | 1.20 a | 0.132 a | 23 ab | 43 a | 217 a | 0.040 a | 17 a | 0.318 b | 0.079 a | 0.28 b |
| QU-C | 77.8 c | 1.36 b | 1.32 c | 0.05 a | 1.66 c | 0.350 c | 16 a | 43 a | 295 b | 0.046 b | 26 b | 0.253 a | 0.089 b | 0.21 a |
| SE | 0.2 | 0.03 | 0.03 | 0.01 | 0.03 | 0.004 | 2 | 3 | 13 | 0.001 | 1 | 0.009 | 0.002 | 0.01 |
| p-value | *** | *** | *** | ns | *** | *** | ns | ** | ** | ** | *** | ** | *** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vela, A.J.; Villanueva, M.; Náthia-Neves, G.; Ronda, F. Impact of Solubilized Substances on the Techno-Functional, Pasting and Rheological Properties of Ultrasound-Modified Rice, Tef, Corn and Quinoa Flours. Foods 2023, 12, 484. https://doi.org/10.3390/foods12030484
Vela AJ, Villanueva M, Náthia-Neves G, Ronda F. Impact of Solubilized Substances on the Techno-Functional, Pasting and Rheological Properties of Ultrasound-Modified Rice, Tef, Corn and Quinoa Flours. Foods. 2023; 12(3):484. https://doi.org/10.3390/foods12030484
Chicago/Turabian StyleVela, Antonio J., Marina Villanueva, Grazielle Náthia-Neves, and Felicidad Ronda. 2023. "Impact of Solubilized Substances on the Techno-Functional, Pasting and Rheological Properties of Ultrasound-Modified Rice, Tef, Corn and Quinoa Flours" Foods 12, no. 3: 484. https://doi.org/10.3390/foods12030484





