Superfine Marigold Powder Improves the Quality of Sponge Cake: Lutein Fortification, Texture, and Sensory Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Determination of Particle Properties
2.4. Sponge Cake Preparation
2.5. Determination of the Cooking Properties of Sponge Cake
2.6. Quantification and Extraction of Lutein Content
2.7. Sensory Evaluation
2.8. Statistical Analysis
3. Results
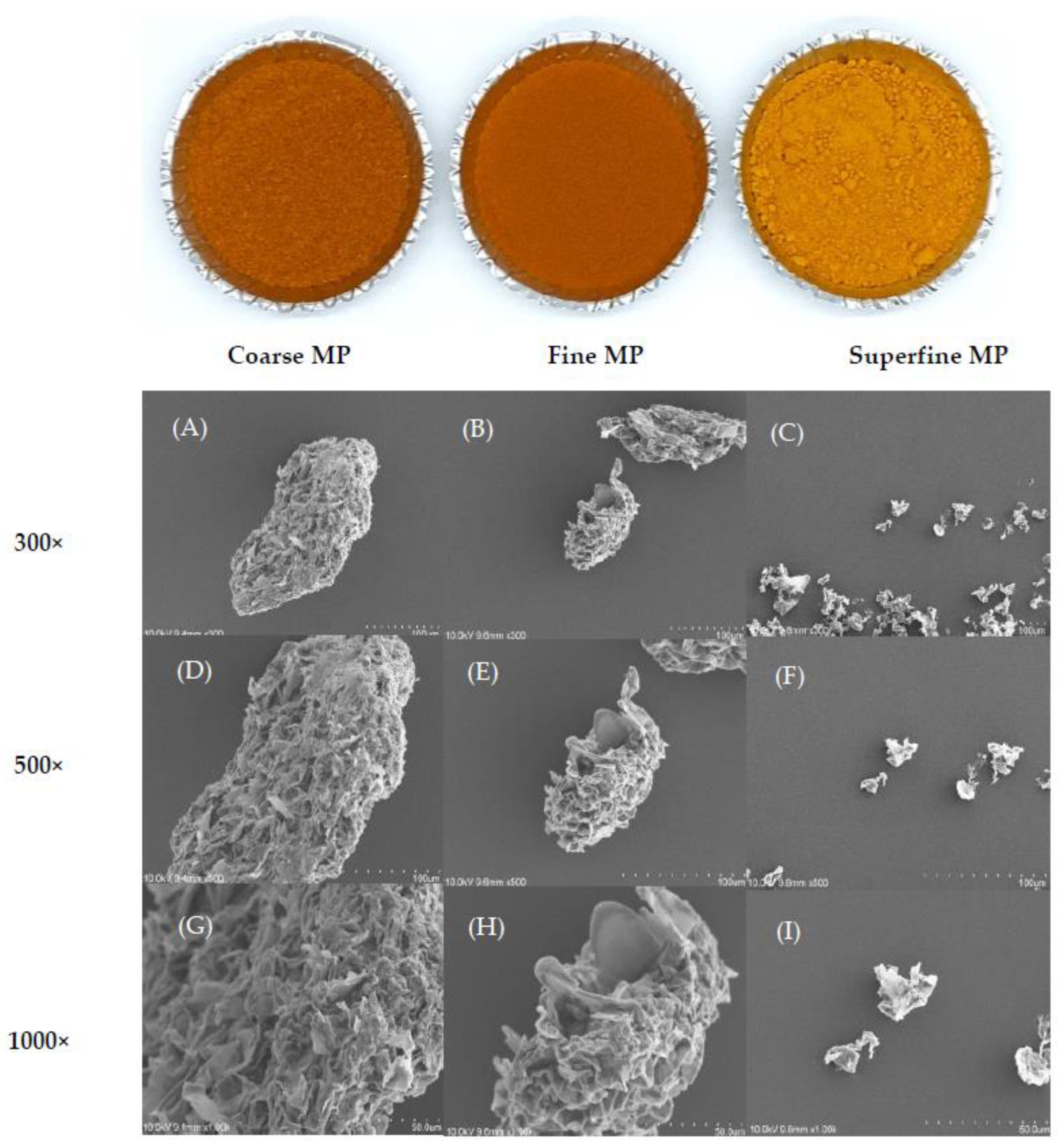
3.1. Particle Properties of Differently Sized MP
3.2. Hydration Properties and OHC of Differently Sized MP
3.3. Color and Microstructure of Differently Sized MP
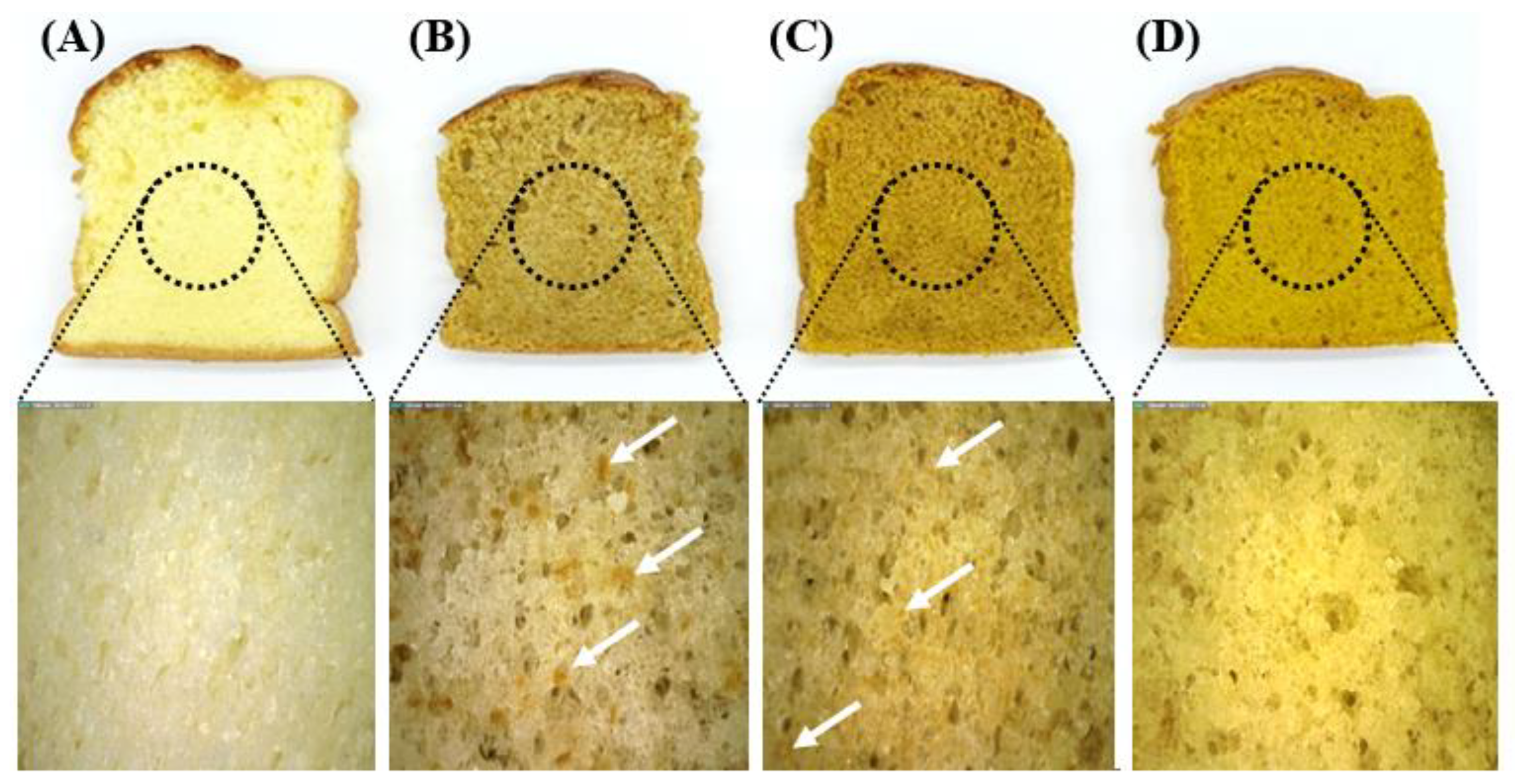
3.4. Quality Indicators and Color of the Sponge Cake
3.5. Texture Analysis of the Sponge Cake
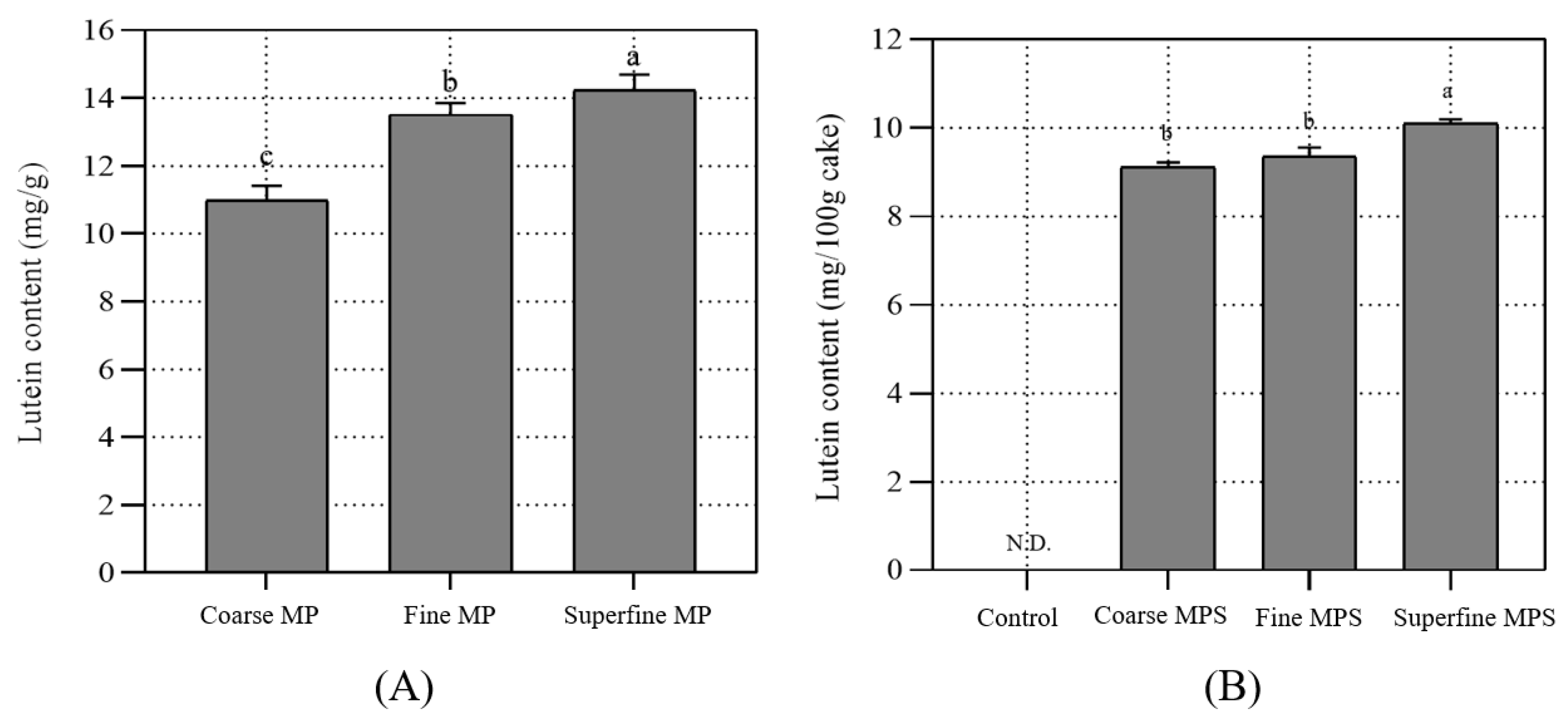
3.6. Determination of Lutein Content
3.7. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. UV/visible spectroscopy (Chapter 2). In Carotenoids. B: Spectroscopy; Birkhäuser Verlag: Basel, Switzerland, 1995; Volume 1. [Google Scholar]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.Q.; Welchowski, T.; Schmid, M.; Mauschitz, M.M.; Holz, F.G.; Finger, R.P. Prevalence and incidence of age-related macular degeneration in Europe: A systematic review and meta-analysis. Br. J. Ophthalmol. 2020, 104, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, M.M. Lutein: A valuable ingredient of fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2005, 45, 671–696. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Composition of lutein ester regioisomers in marigold flower, dietary supplement, and herbal tea. J. Agric. Food Chem. 2015, 63, 9740–9746. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, L.; Xing, Y.; Zhao, Y.; Wang, Z.; Han, J. Enhanced oral bioavailability of celecoxib nanocrystalline solid dispersion based on wet media milling technique: Formulation, optimization and in vitro/in vivo evaluation. Pharmaceutics 2019, 11, 328. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Bai, J.; Qian, X.; Yang, X.; Zhou, X.; Zhao, Y.; Dong, Y.; Xiao, X. Effect of superfine grinding on physical properties, bioaccessibility, and anti-obesity activities of bitter melon powders. J. Sci. Food Agric. 2020, 102, 4473–4483. [Google Scholar] [CrossRef]
- Street, N.O.M.C. A Healthier Food Supply: Public-private partnerships for Food and Nutrient Databases. In Proceedings of the 33rd National Nutrient Databank Conference, Bethesda, MD, USA, 17 April 2009. [Google Scholar]
- Bhattacharyya, S.; Datta, S.; Mallick, B.; Dhar, P.; Ghosh, S. Lutein content and in vitro antioxidant activity of different cultivars of Indian marigold flower (Tagetes patula L.) extracts. J. Agric. Food Chem. 2010, 58, 8259–8264. [Google Scholar] [CrossRef]
- Steiner, B.M.; McClements, D.J.; Davidov-Pardo, G. Encapsulation systems for lutein: A review. Trends Food Sci. Technol. 2018, 82, 71–81. [Google Scholar] [CrossRef]
- Rošul, M.; Đerić, N.; Mišan, A.; Pojić, M.; Šimurina, O.; Halimi, C.; Nowicki, M.; Cvetković, B.; Mandić, A.; Reboul, E. Bioaccessibility and uptake by Caco-2 cells of carotenoids from cereal-based products enriched with butternut squash (Cucurbita moschata L.). Food Chem. 2022, 385, 132595. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, H.; Peng, Z.; Luo, Q.; Ming, J.; Zhao, G. Characterization of stipe and cap powders of mushroom (Lentinus edodes) prepared by different grinding methods. J. Food Eng. 2012, 109, 406–413. [Google Scholar] [CrossRef]
- Choi, G.-L.; Hong, Y.-M.; Lee, K.-W.; Choi, Y.-J. Food functionalities of dried fish protein powder. J. Korean Soc. Food Sci. Nutr. 2006, 35, 1394–1398. [Google Scholar]
- Alotaibi, H.N.; Anderson, A.K.; Sidhu, J.S. Influence of lutein content of marigold flowers on functional properties of baked pan bread. Ann. Agric. Sci. 2021, 66, 162–168. [Google Scholar] [CrossRef]
- St. Paul. Approved Methods of the AACC, 10th ed.; American Association of Cereal Chemists, AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Liu, Y.; Perera, C.O.; Suresh, V. Comparison of three chosen vegetables with others from South East Asia for their lutein and zeaxanthin content. Food Chem. 2007, 101, 1533–1539. [Google Scholar] [CrossRef]
- Vechpanich, J.; Shotipruk, A. Recovery of free lutein from Tagetes erecta: Determination of suitable saponification and crystallization conditions. Sep. Sci. Technol. 2010, 46, 265–271. [Google Scholar] [CrossRef]
- Derrien, M.; Badr, A.; Gosselin, A.; Desjardins, Y.; Angers, P. Optimization of a sustainable purification protocol for lutein and chlorophyll from spinach by-products by a saponification procedure using Box Behnken design and desirability function. Food Bioprod. Process. 2019, 116, 54–62. [Google Scholar] [CrossRef]
- Muttakin, S.; Kim, M.S.; Lee, D.-U. Tailoring physicochemical and sensorial properties of defatted soybean flour using jet milling technology. Food Chem. 2015, 187, 106–111. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Wang, L.; Li, Z. Effect of particle size on the release behavior and functional properties of wheat bran phenolic compounds during in vitro gastrointestinal digestion. Food Chem. 2022, 367, 130751. [Google Scholar] [CrossRef]
- Heo, T.-Y.; Kim, Y.-N.; Park, I.B.; Lee, D.-U. Amplification of Vitamin D2 in the White Button Mushroom (Agaricus bisporus) by UV-B Irradiation and Jet milling for Its Potential Use as a Functional Ingredient. Foods 2020, 9, 1713. [Google Scholar] [CrossRef]
- Caprez, A.; Arrigoni, E.; Amadò, R.; Neukom, H. Influence of different types of thermal treatment on the chemical composition and physical properties of wheat bran. J. Cereal Sci. 1986, 4, 233–239. [Google Scholar] [CrossRef]
- Nagashree, D.; Kulkarni, B.S. Evaluation of French marigold (Tagetes patula L.) genotypes for growth, flowering and yield related traits. J. Ornam. Hortic. 2020, 23, 130–135. [Google Scholar] [CrossRef]
- Auffret, A.; Ralet, M.; Guillon, F.; Barry, J.; Thibault, J. Effect of grinding and experimental conditions on the measurement of hydration properties of dietary fibres. LWT-Food Sci. Technol. 1994, 27, 166–172. [Google Scholar] [CrossRef]
- Jongaroontaprangsee, S.; Tritrong, W.; Chokanaporn, W.; Methacanon, P.; Devahastin, S.; Chiewchan, N. Effects of drying temperature and particle size on hydration properties of dietary fiber powder from lime and cabbage by-products. Int. J. Food Prop. 2007, 10, 887–897. [Google Scholar] [CrossRef]
- Chau, C.-F.; Wang, Y.-T.; Wen, Y.-L. Different micronization methods significantly improve the functionality of carrot insoluble fibre. Food Chem. 2007, 100, 1402–1408. [Google Scholar] [CrossRef]
- Yu, R.; Kim, Y. Trend of Ceramic Nano Pigments. Ceramist 2019, 22, 256–268. [Google Scholar] [CrossRef]
- Nykamp, G.; Carstensen, U.; Müller, B. Jet milling—A new technique for microparticle preparation. Int. J. Pharm. 2002, 242, 79–86. [Google Scholar] [CrossRef]
- De la Hera, E.; Martinez, M.; Oliete, B.; Gómez, M. Influence of flour particle size on quality of gluten-free rice cakes. Food Bioprocess Technol. 2013, 6, 2280–2288. [Google Scholar] [CrossRef]
- Lebesi, D.M.; Tzia, C. Effect of the addition of different dietary fiber and edible cereal bran sources on the baking and sensory characteristics of cupcakes. Food Bioprocess Technol. 2011, 4, 710–722. [Google Scholar] [CrossRef]
- An, H.K.; Hong, G.J.; Lee, E.J. Properties of sponge cake with added saltwort (Salicorniaherbacea L.). J. Korean Soc. Food Cult. 2010, 25, 47–53. [Google Scholar]
- Lee, J.-S.; Seong, Y.-B.; Jeong, B.-Y.; Yoon, S.-J.; Lee, I.-S.; Jeong, Y.-H. Quality characteristics of sponge cake with black garlic powder added. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1222–1228. [Google Scholar] [CrossRef]
- Choi, G.Y.; Bae, J.H.; Han, G.J. The quality characteristics of sponge cake containing a functional and natural product (1. mulberry leaf powder). J. East Asian Soc. Diet. Life 2007, 17, 703–709. [Google Scholar]
- Qv, X.-Y.; Zeng, Z.-P.; Jiang, J.-G. Preparation of lutein microencapsulation by complex coacervation method and its physicochemical properties and stability. Food Hydrocoll. 2011, 25, 1596–1603. [Google Scholar] [CrossRef]
- Muktiningrum, T.A.; Fauza, G.; Ariviani, S.; Muhammad, D.R.; Affandi, D.R. Sensory Profile Analysis of chocolate Drinks Using Quantitative Descriptive Analysis (QDA); E3S Web of Conferences: Les Ulis, France, 2022. [Google Scholar]

| Descriptor | Definition | References |
|---|---|---|
| Roughness | The mouthfeel of the particles | Weak: plain yogurt Strong: corn flour |
| Hardness | Force required to deform the sample | Weak: cream cheese Strong: chocolate |
| Sweetness | The fundamental taste sensation produced by the aqueous sucrose solution | Weak: 0 Strong: 10% sucrose solution |
| Flavor of marigold | Flavor imparted from the added marigold powder in the cake | Weak: 0 Strong: cooked marigold (Flavor) |
| Overall acceptability | How much the participant liked or did not like the product based on all the sensorial attributes tested | - |
| Samples | Particle Size Distribution Properties | Hydration Properties and Oil Holding Capacity | Color | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dv10 (μm) | Dv50 (μm) | Dv90 (μm) | D[4,3] (μm) | Specific Surface Area (m2/kg) | WHC (g/g) | WSI (%) | SC (mL/g) | OHC (g/g) | L* | a* | b* | ΔE | |
| Coarse MP (2) | 175.67 ± 0.58 a(1) | 315.33 ± 5.77 a | 505.33 ± 7.51 a | 326.33 ± 4.93 a | 95.67 ± 1.26 b | 10.35 ± 0.55 a | 34.67 ± 1.15 c | 11.47 ± 0.76 b | 3.41 ± 0.03 a | 53.37 ± 0.21 c | 25.23 ± 0.41 c | 32.96 ± 0.61 c | - |
| Fine MP | 68.60 ± 0.17 b | 119.33 ± 0.58 b | 205.00 ± 0.00 b | 128.67 ± 0.58 b | 210.43 ± 1.31 b | 10.22 ± 0.70 a | 38.67 ± 1.15 b | 12.53 ± 0.12 b | 2.96 ± 0.04 b | 55.20 ± 0.04 b | 26.39 ± 0.02 b | 35.34 ± 0.24 b | 3.22 |
| Superfine MP | 2.59 ± 0.08 c | 10.40 ± 0.26 c | 22.17 ± 0.74 c | 11.53 ± 0.32 c | 5084.00 ± 168.88 a | 5.47 ± 0.44 b | 41.33 ± 1.15 a | 14.20 ± 1.00 a | 2.58 ± 0.06 c | 66.14 ± 0.02 a | 27.59 ± 0.04 a | 57.48 ± 0.18 a | 27.75 |
| Samples (3) | Moisture Content (%) | pH | Baking Loss (%) | Specific Volume (L/kg) | Color | |||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | |||||
| Control | 36.77 ± 0.68 NS(1) | 8.10 ± 0.05 a(2) | 5.15 ± 0.53 b | 2.88 ± 0.20 a | 83.36 ± 0.41 a | 0.21 ± 0.05 d | 31.07 ± 0.27 c | |
| Coarse MPS (2) | 37.09 ± 0.62 | 7.81 ± 0.02 b | 6.14 ± 0.49 a | 2.50 ± 0.09 b | 57.83 ± 0.85 b | 6.15 ± 0.30 c | 30.48 ± 0.80 c | 26.32 |
| Fine MPS | 37.69 ± 0.27 | 7.70 ± 0.03 c | 5.56 ± 0.60 ab | 2.68 ± 0.04 ab | 54.04 ± 0.85 c | 8.65 ± 0.37 b | 36.12 ± 0.73 b | 31.04 |
| Superfine MPS | 36.96 ± 0.12 | 7.65 ± 0.02 d | 4.94 ± 0.19 b | 2.89 ± 0.20 a | 53.23 ± 0.66 d | 9.90 ± 0.22 a | 51.58 ± 2.18 a | 37.82 |
| Samples | Hardness (N) | Springiness | Cohesiveness | Chewiness | Gumminess (N) |
|---|---|---|---|---|---|
| Control (3) | 9.31 ± 1.06 b(2) | 0.88 ± 0.01 NS(1) | 0.63 ± 0.00 a | 5.76 ± 0.61 NS | 6.55 ± 0.74 ab |
| Coarse MPS | 11.28 ± 1.26 a | 0.87 ± 0.01 | 0.60 ± 0.01 b | 6.75 ± 0.72 | 7.71 ± 0.76 a |
| Fine MPS | 10.75 ± 0.55 ab | 0.90 ± 0.01 | 0.63 ± 0.00 a | 6.72 ± 0.44 | 7.53 ± 0.38 ab |
| Superfine MPS | 9.01 ± 0.72 b | 0.87 ± 0.02 | 0.64 ± 0.00 a | 5.59 ± 0.53 | 6.43 ± 0.49 b |
| Samples | Roughness | Hardness | Sweetness | Flavor of Marigold | Overall Acceptability |
|---|---|---|---|---|---|
| Control (2) | 1.30 ± 0.73 d(1) | 3.65 ± 0.99 c | 7.55 ± 1.05 a | 1.00 ± 0.00 d | 7.20 ± 1.44 a |
| Coarse MPS | 7.85 ± 0.75 a | 5.70 ± 0.66 a | 6.30 ± 0.86 b | 3.90 ± 1.02 c | 3.90 ± 1.17 c |
| Fine MPS | 5.55 ± 0.83 b | 4.60 ± 0.88 b | 5.25 ± 0.72 c | 5.40 ± 0.94 b | 5.65 ± 1.18 b |
| Superfine MPS | 2.80 ± 0.77 c | 3.80 ± 0.70 c | 3.75 ± 0.85 d | 7.90 ± 0.97 a | 7.55 ± 0.76 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Hong, S.-Y.; Choi, H.-S.; Kim, J.-H.; Jeong, S.-H.; Lee, S.-Y.; Kim, S.-H.; Lee, D.-U. Superfine Marigold Powder Improves the Quality of Sponge Cake: Lutein Fortification, Texture, and Sensory Properties. Foods 2023, 12, 508. https://doi.org/10.3390/foods12030508
Kim S-Y, Hong S-Y, Choi H-S, Kim J-H, Jeong S-H, Lee S-Y, Kim S-H, Lee D-U. Superfine Marigold Powder Improves the Quality of Sponge Cake: Lutein Fortification, Texture, and Sensory Properties. Foods. 2023; 12(3):508. https://doi.org/10.3390/foods12030508
Chicago/Turabian StyleKim, Si-Yeon, Seok-Young Hong, Hyun-Su Choi, Jong-Hun Kim, Se-Ho Jeong, Su-Yong Lee, Sung-Hun Kim, and Dong-Un Lee. 2023. "Superfine Marigold Powder Improves the Quality of Sponge Cake: Lutein Fortification, Texture, and Sensory Properties" Foods 12, no. 3: 508. https://doi.org/10.3390/foods12030508
APA StyleKim, S.-Y., Hong, S.-Y., Choi, H.-S., Kim, J.-H., Jeong, S.-H., Lee, S.-Y., Kim, S.-H., & Lee, D.-U. (2023). Superfine Marigold Powder Improves the Quality of Sponge Cake: Lutein Fortification, Texture, and Sensory Properties. Foods, 12(3), 508. https://doi.org/10.3390/foods12030508








