Antioxidant Activity and Cell Protection of Glycosylated Products in Different Reducing Sugar Duck Liver Protein Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Duck Liver Protein
2.2. Preparation on Glycosylated Products of Duck Liver Protein
2.3. pH Measurement
2.4. Determination of Browning Value
2.5. Determination of DPPH Free Radical Scavenging Rate
2.6. Determination of ·OH Free Radical Scavenging Rate
2.7. Determination of ABTS+ Free Radical Scavenging Rate
2.8. Determination of the Total Antioxidant Capacity
2.9. Recovery of HepG2 Cells
2.10. Passaging Culture of HepG2 Cells
2.11. Cryopreservation of HepG2 Cells
2.12. Cytotoxicity Tests
2.13. Establishment of H2O2-Induced Oxidative Damage Model of HepG2 Cells
2.14. Protective Effect of Glycosylation Products on H2O2-Induced Oxidative Damage in HepG2 Cells
2.15. Determination of Oxidative Stress Factors (MDA, SOD, GSH-Px, CAT Activity)
2.16. Statistical Analysis
3. Results and Discussion
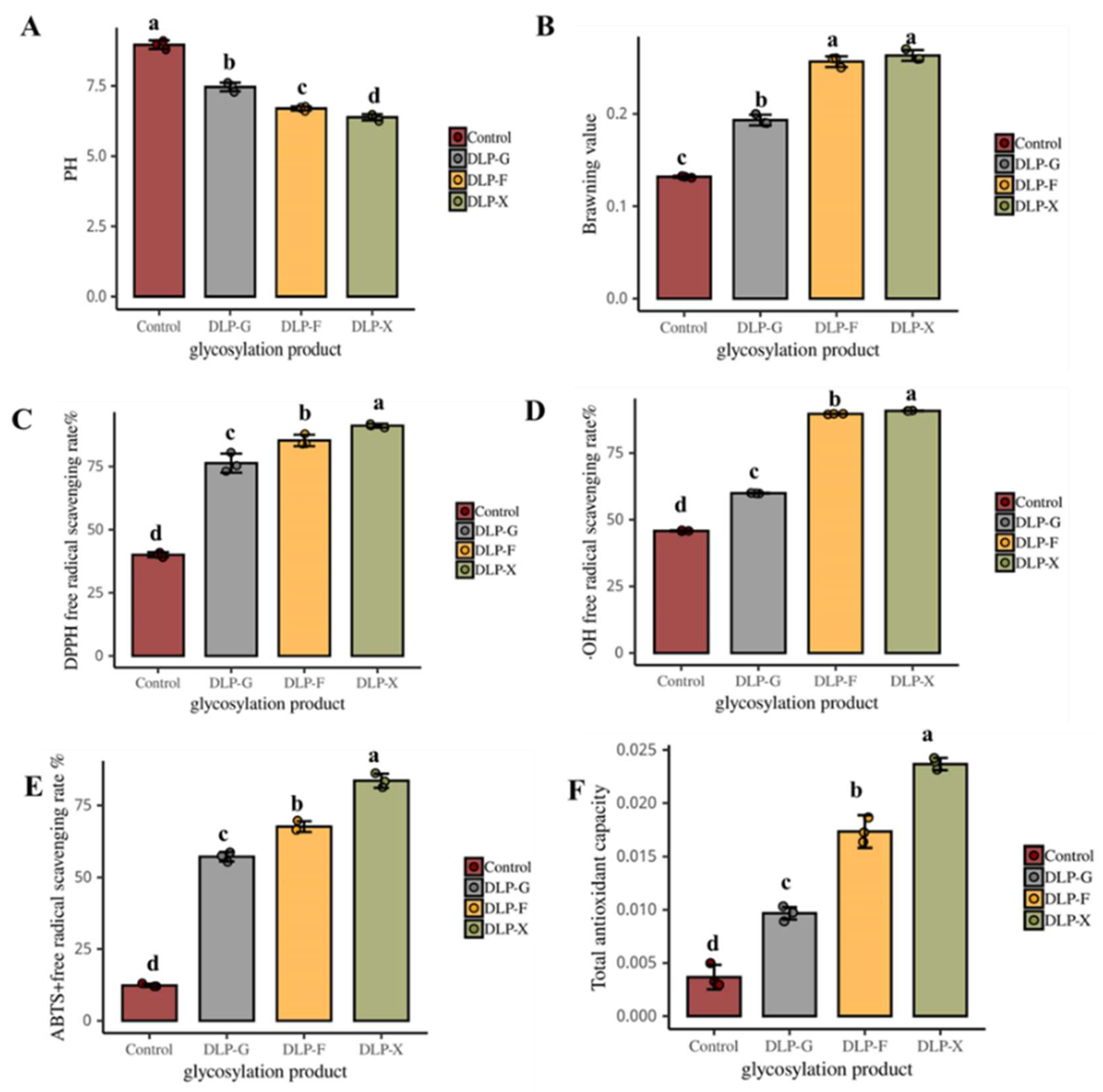
3.1. Changes in pH
3.2. The Effects on the Browning Value
3.3. DPPH Radical Scavenging
3.4. The Removal Rate of ·OH
3.5. ABTS+ Free Radical Scavenging Rate
3.6. Total Antioxidant Capacity
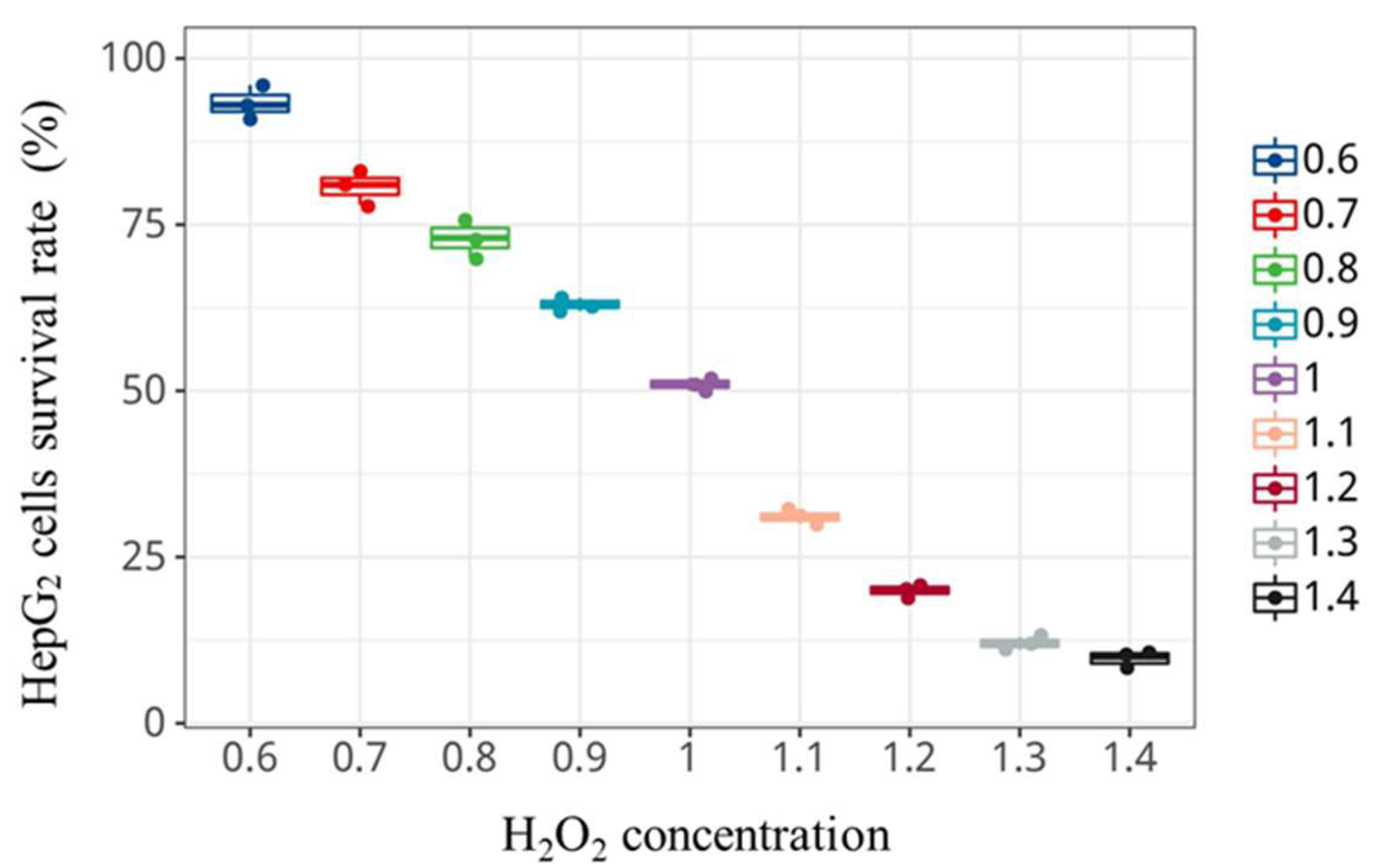
3.7. Cytotoxicity Assay
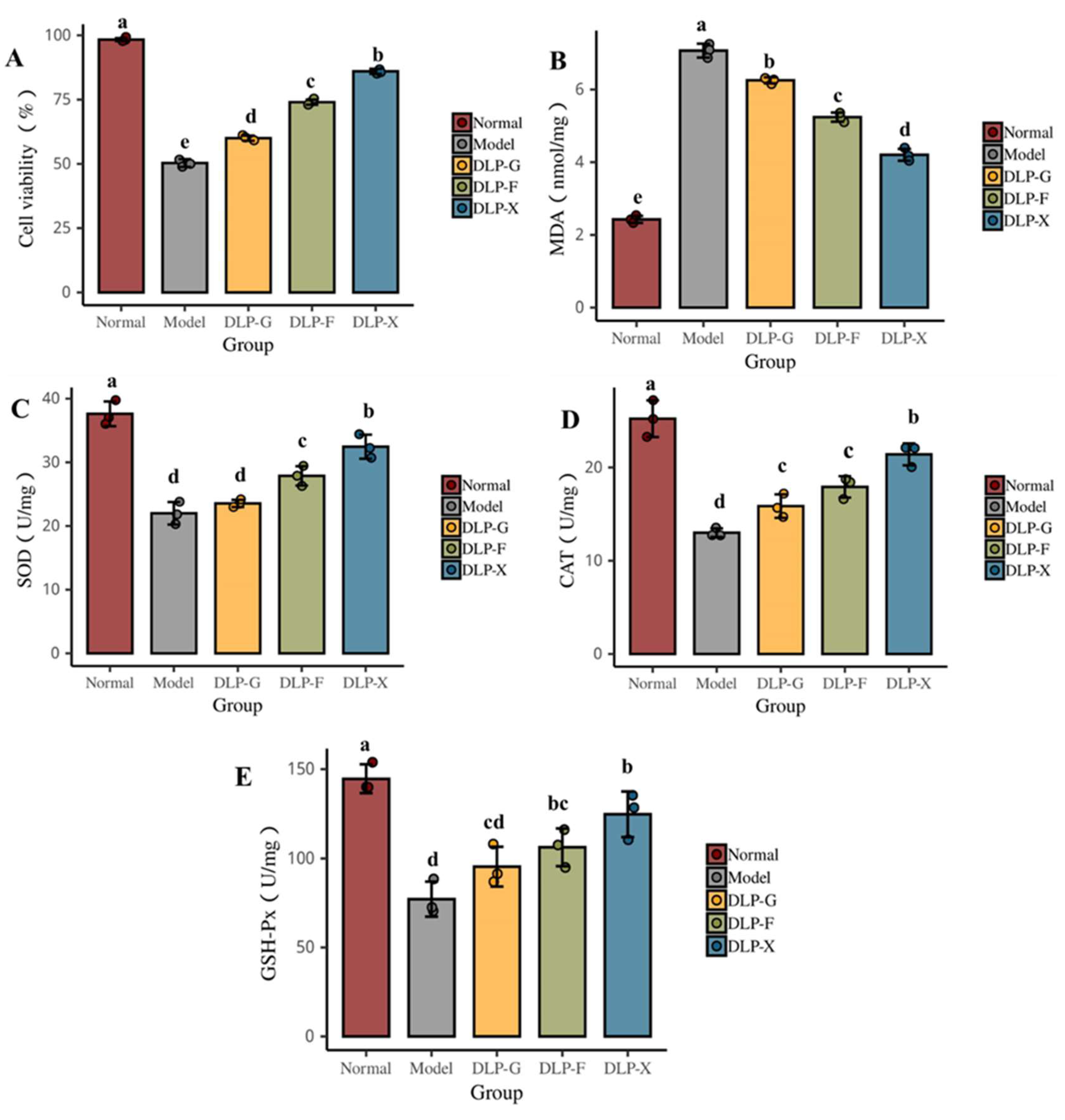
3.8. Protective Effect of Glycosylated Proteins on Oxidative Damage of Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, G.; Gao, X.; Wang, P.; Xu, X.; Zhou, G. Comparative study of extraction efficiency and composition of protein recovered from chicken liver by acid–alkaline treatment. Process Biochem. 2016, 51, 1629–1635. [Google Scholar] [CrossRef]
- Xu, L.; Xia, Q.; Cao, J.; He, J.; Zhou, C.; Guo, Y.; Pan, D. Ultrasonic effects on the headspace volatilome and protein isolate microstructure of duck liver, as well as their potential correlation mechanism. Ultrason. Sonochem. 2021, 71, 105358. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Shahidi, F.; Shi, H.; Wang, J.; Huang, Y.; Xu, W.; Wang, D. Values-added utilization of protein and hydrolysates from animal processing by-product livers: A review. Trends Food Sci. Technol. 2021, 110, 432–442. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Tomza-Marciniak, A.; Pilarczyk, R.; Udala, J.; Kruzhel, B.; Ligocki, M. Content of essential and non-essential elements in wild animals from western Ukraine and the health risks associated with meat and liver consumption. Chemosphere 2020, 244, 125506. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.A.; Mullen, A.M.; O’Neill, E.; Drummond, L.; Alvarez, C. Opportunities and perspectives for utilisation of co-products in the meat industry. Meat Sci. 2018, 144, 62–73. [Google Scholar] [CrossRef]
- Tan, B.; Sun, B.; Sun, N.; Li, C.; Zhang, J.; Yang, W. Structure, functional properties and iron bioavailability of Pneumatophorus japonicus myoglobin and its glycosylation products. Int. J. Biol. Macromol. 2021, 173, 524–531. [Google Scholar] [CrossRef]
- Zheng, Y.; Chang, Y.; Luo, B.; Teng, H.; Chen, L. Molecular structure modification of ovalbumin through controlled glycosylation with dextran for its emulsibility improvement. Int. J. Biol. Macromol. 2022, 194, 1–8. [Google Scholar] [CrossRef]
- Cui, H.; Yu, J.; Zhai, Y.; Feng, L.; Chen, P.; Hayat, K.; Xu, Y.; Zhang, X.; Ho, C.-T. Formation and fate of Amadori rearrangement products in Maillard reaction. Trends Food Sci. Technol. 2021, 115, 391–408. [Google Scholar] [CrossRef]
- Yang, W.; Tu, Z.; Li, Q.; Kaltashov, I.A.; McClements, D.J. Utilization of sonication-glycation to improve the functional properties of ovalbumin: A high-resolution mass spectrometry study. Food Hydrocoll. 2021, 119, 106822. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Duan, W.; Wang, Q.; An, F.; Luo, P.; Huang, Q. Ball-milling is an effective pretreatment of glycosylation modified the foaming and gel properties of egg white protein. J. Food Eng. 2022, 319, 110908. [Google Scholar] [CrossRef]
- Feng, J.; Berton-Carabin, C.C.; Fogliano, V.; Schroën, K. Maillard reaction products as functional components in oil-in-water emulsions: A review highlighting interfacial and antioxidant properties. Trends Food Sci. Technol. 2022, 121, 129–141. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Mu, D.-C.; Feng, Y.-Y.; Xu, Z.; Wen, L.; Chen, M.-L.; Ye, J. Glycosylation of rice protein with dextran via the Maillard reaction in a macromolecular crowding condition to improve solubility. J. Cereal Sci. 2022, 103, 103374. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.; Huang, Y.; Lin, H.; Chen, G.; Chen, Y.; Li, Z. Glycosylation reduces the allergenicity of turbot (Scophthalmus maximus) parvalbumin by regulating digestibility, cellular mediators release and Th1/Th2 immunobalance. Food Chem. 2022, 382, 132574. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Jiao, Y.; Li, D.J.; Liu, X.L.; Han, H. Glycosylated zein as a novel nanodelivery vehicle for lutein. Food Chem. 2021, 376, 131927. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, K.; Yang, Q.; Zhang, L.; Shi, C.; Tavakoli, S.; Tan, Y.; Luo, Y.; Hong, H. The antioxidant activities and flavor properties of glycated bighead carp meat hydrolysates produced with galactose and galacto-oligosaccharides. LWT 2022, 158, 113104. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Huang, Z.; Jia, S.; Zhang, Y.; Shi, J.; Hong, H.; Feng, L.; Luo, Y. Modification of gelatin hydrolysates from grass carp (Ctenopharyngodon idellus) scales by Maillard reaction: Antioxidant activity and volatile compounds. Food Chem. 2019, 295, 569–578. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Chen, N.; Xin, N.; Li, Q.; Ye, H.; Zhao, C.; Zhang, T. Glycated modification of the protein from Rana chensinensis eggs by Millard reaction and its stability analysis in curcumin encapsulated emulsion system. Food Chem. 2022, 382, 132299. [Google Scholar] [CrossRef]
- Wang, W.D.; Li, C.; Chen, C.; Fu, X.; Liu, R.H. Effect of chitosan oligosaccharide glycosylation on the emulsifying property of lactoferrin. Int. J. Biol. Macromol. 2022, 209, 93–106. [Google Scholar] [CrossRef]
- Wei, C.K.; Thakur, K.; Liu, D.H.; Zhang, J.G.; Wei, Z.J. Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products. Food Chem. 2018, 263, 186–193. [Google Scholar] [CrossRef]
- Lu, Y.; Pan, D.; Xia, Q.; Cao, J.; Zhou, C.; He, J.; Sun, Y.; Xu, S. Impact of pH-dependent succinylation on the structural features and emulsifying properties of chicken liver protein. Food Chem. 2021, 358, 129868. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, J.; Shi, X.; Abdul, Q.; Jiang, Z. Characterization and Antioxidant Activity of Products Derived from Xylose-Bovine Casein Hydrolysate Maillard Reaction: Impact of Reaction Time. Foods 2019, 8, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vhangani, L.N.; Van Wyk, J. Antioxidant activity of Maillard reaction products (MRPs) derived from fructose-lysine and ribose-lysine model systems. Food Chem. 2013, 137, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bonvehí, J.S.; Gutiérrez, A.L. Antioxidant Activity and Total Phenolics of Propolis from the Basque Country (Northeastern Spain). J. Am. Oil Chem. Soc. 2011, 88, 1387–1395. [Google Scholar] [CrossRef]
- Hsu, S.S.; Lin, Y.S.; Chio, L.M.; Liang, W.Z. Evaluation of the mycotoxin patulin on cytotoxicity and oxidative stress in human glioblastoma cells and investigation of protective effect of the antioxidant N-acetylcysteine (NAC). Toxicon 2022, 221, 106957. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Biological activities of Maillard reaction products (MRPs) in a sugar–amino acid model system. Food Chem. 2011, 126, 221–227. [Google Scholar] [CrossRef]
- Liu, S.C.; Yang, D.J.; Jin, S.Y.; Hsu, C.H.; Chen, S.L. Kinetics of color development, pH decreasing, and anti-oxidative activity reduction of Maillard reaction in galactose/glycine model systems. Food Chem. 2008, 108, 533–541. [Google Scholar] [CrossRef]
- Lertittikul, W.; Benjakul, S.; Tanaka, M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein–glucose model system as influenced by pH. Food Chem. 2007, 100, 669–677. [Google Scholar] [CrossRef]
- Arribas-Lorenzo, G.; Morales, F.J. Analysis, distribution, and dietary exposure of glyoxal and methylglyoxal in cookies and their relationship with other heat-induced contaminants. J. Agric. Food Chem. 2010, 58, 2966–2972. [Google Scholar] [CrossRef] [Green Version]
- Karnjanapratum, S.; Benjakul, S.; O’Brien, N. Production of Antioxidative Maillard Reaction Products from Gelatin Hydrolysate of Unicorn Leatherjacket Skin. J. Aquat. Food Prod. Technol. 2017, 26, 148–162. [Google Scholar] [CrossRef]
- Tan, J.e.; Liu, T.; Yao, Y.; Wu, N.; Du, H.; Xu, M.; Liao, M.; Zhao, Y.; Tu, Y. Changes in physicochemical and antioxidant properties of egg white during the Maillard reaction induced by alkali. LWT 2021, 143, 111151. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Phongkanpai, V.; Tanaka, M. Antioxidative activity of caramelisation products and their preventive effect on lipid oxidation in fish mince. Food Chem. 2005, 90, 231–239. [Google Scholar] [CrossRef]
- Limsuwanmanee, J.; Chaijan, M.; Manurakchinakorn, S.; Panpipat, W.; Klomklao, S.; Benjakul, S. Antioxidant activity of Maillard reaction products derived from stingray (Himantura signifier) non-protein nitrogenous fraction and sugar model systems. LWT—Food Sci. Technol. 2014, 57, 718–724. [Google Scholar] [CrossRef]
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chem. 2018, 243, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Chawla, S.P.; Chander, R.; Sharma, A. Antioxidant potential of Maillard reaction products formed by irradiation of chitosan–glucose solution. Carbohydr. Polym. 2011, 83, 714–719. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Bioactive peptides from shrimp shell processing discards: Antioxidant and biological activities. J. Funct. Foods 2017, 34, 7–17. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Hayase, F.; Usui, T.; Watanabe, H. Chemistry and some biological effects of model melanoidins and pigments as Maillard intermediates. Mol. Nutr. Food Res. 2006, 50, 1171–1179. [Google Scholar] [CrossRef]
- Wang, W.Q.; Bao, Y.H.; Chen, Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013, 139, 355–361. [Google Scholar] [CrossRef]
- Cao, J.; Yan, H.; Liu, L. Optimized preparation and antioxidant activity of glucose-lysine Maillard reaction products. LWT 2022, 161, 113343. [Google Scholar] [CrossRef]
- Benjakul, S.; Lertittikul, W.; Bauer, F. Antioxidant activity of Maillard reaction products from a porcine plasma protein–sugar model system. Food Chem. 2005, 93, 189–196. [Google Scholar] [CrossRef]
- Jia, X.; Li, L.; Teng, J.; Li, M.; Long, H.; Xia, N. Glycation of rice protein and d-xylose pretreated through hydrothermal cooking-assisted high hydrostatic pressure: Focus on the structural and functional properties. LWT 2022, 160, 113194. [Google Scholar] [CrossRef]
- Zhang, J.; Che, C.; Cai, M.; Hu, Y. Taurine improves health of juvenile rice field eel (Monopterus albus) fed with oxidized fish oil: Involvement of lipid metabolism, antioxidant capacity, inflammatory response. Aquac. Rep. 2022, 27, 101388. [Google Scholar] [CrossRef]
- Ye, J.; Han, Y.; Wang, C.; Yu, W. Cytoprotective effect of polypeptide from Chlamys farreri on neuroblastoma (SH-SY5Y) cells following HO exposure involves scavenging ROS and inhibition JNK phosphorylation. J. Neurochem. 2010, 111, 441–451. [Google Scholar] [CrossRef]
- Young, I.C.; Chuang, S.T.; Hsu, C.H.; Sun, Y.J.; Lin, F.H. C-phycocyanin alleviates osteoarthritic injury in chondrocytes stimulated with H2O2 and compressive stress. Int. J. Biol. Macromol. 2016, 93, 852–859. [Google Scholar] [CrossRef]
- Zhao, X.C.; Zhang, L.; Yu, H.X.; Sun, Z.; Lin, X.F.; Tan, C.; Lu, R.R. Curcumin protects mouse neuroblastoma Neuro-2A cells against hydrogen-peroxide-induced oxidative stress. Food Chem. 2011, 129, 387–394. [Google Scholar] [CrossRef]
- Guo, X.; Xiong, Y.L. Characteristics and functional properties of buckwheat protein–sugar Schiff base complexes. LWT—Food Sci. Technol. 2013, 51, 397–404. [Google Scholar] [CrossRef]
- Chailangka, A.; Seesuriyachan, P.; Wangtueai, S.; Ruksiriwanich, W.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R.; Leksawasdi, N.; Barba, F.J.; Phimolsiripol, Y. Cricket protein conjugated with different degrees of polymerization saccharides by Maillard reaction as a novel functional ingredient. Food Chem. 2022, 395, 133594. [Google Scholar] [CrossRef]
- Ercoşkun, H.; Özkal, S.G. Kinetics of traditional Turkish sausage quality aspects during fermentation. Food Control 2011, 22, 165–172. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, L.; Deng, K.; Xu, G.; Wang, Y.; Ni, Q.; Zhang, Y. Investigation of isoflavone constituents from tuber of Apios americana Medik and its protective effect against oxidative damage on RIN-m5F cells. Food Chem. 2023, 405, 134655. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Inhibitory effect of polyphenol cyanidin on TNF-alpha-induced apoptosis through multiple signaling pathways in endothelial cells. Atherosclerosis 2007, 193, 299–308. [Google Scholar] [CrossRef] [PubMed]
| Concentration (g/L) | Reducing Sugar Type | ||
|---|---|---|---|
| DLP-G | DLP-F | DLP-X | |
| 0.5 | 112.9 ± 11.04 a | 110.54 ± 13.23 a | 125.76 ± 4.54 a |
| 1 | 104.68 ± 8.54 ab | 105.75 ± 5.60 a | 115.68 ± 13.32 b |
| 1.5 | 98.67 ± 5.42 b | 96.27 ± 1.25 b | 93.67 ± 1.81 c |
| 2 | 95.86 ± 3.76 b | 94.12 ± 4.60 b | 90.84 ± 3.72 c |
| 2.5 | 97.87 ± 4.09 b | 79.83 ± 9.18 c | 81.93 ± 3.85 d |
| 5 | 82.32 ± 8.87 c | 71.13 ± 8.75 c | 55.44 ± 5.41 e |
| 10 | 57.20 ± 7.49 d | 32.75 ± 4.12 d | 22.67 ± 0.77 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, F.; Luo, J.; Sun, Y.; Hu, Y.; Fan, X.; Pan, D. Antioxidant Activity and Cell Protection of Glycosylated Products in Different Reducing Sugar Duck Liver Protein Systems. Foods 2023, 12, 540. https://doi.org/10.3390/foods12030540
Zhan F, Luo J, Sun Y, Hu Y, Fan X, Pan D. Antioxidant Activity and Cell Protection of Glycosylated Products in Different Reducing Sugar Duck Liver Protein Systems. Foods. 2023; 12(3):540. https://doi.org/10.3390/foods12030540
Chicago/Turabian StyleZhan, Feili, Jiafeng Luo, Yangying Sun, Yangyang Hu, Xiankang Fan, and Daodong Pan. 2023. "Antioxidant Activity and Cell Protection of Glycosylated Products in Different Reducing Sugar Duck Liver Protein Systems" Foods 12, no. 3: 540. https://doi.org/10.3390/foods12030540
APA StyleZhan, F., Luo, J., Sun, Y., Hu, Y., Fan, X., & Pan, D. (2023). Antioxidant Activity and Cell Protection of Glycosylated Products in Different Reducing Sugar Duck Liver Protein Systems. Foods, 12(3), 540. https://doi.org/10.3390/foods12030540






