Cassava Starch Films Containing Quinoa Starch Nanocrystals: Physical and Surface Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
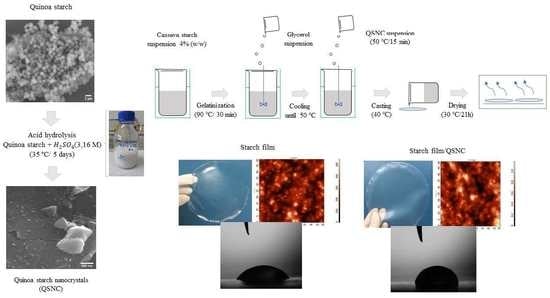
2.2. Starch Isolation and Nanoparticles Production
2.2.1. Quinoa Starch Isolation
2.2.2. Quinoa Starch Nanocrystal Production
2.3. CS, QS and QSNC Characterizations
2.3.1. CS, QS and QSNC Morphologies
2.3.2. CS, QS and QSNC X-ray Diffraction Patterns
2.4. Film Preparation
2.5. Film Characterizations
2.5.1. Film Thickness, Moisture Content and Solubility in Water
2.5.2. Film Water Vapor Permeability
2.5.3. Film Color and Opacity
2.5.4. Film Surface Microstructure
2.5.5. Film Gloss
2.5.6. Film Water Contact Angle
2.5.7. Film X-ray Diffraction Patterns
2.5.8. Film Mechanical Properties
2.5.9. Film Thermal Properties
2.5.10. Fourier Transform Infrared Spectroscopy
2.6. Statistical Analysis
3. Results
3.1. QS, CS and QSNC Characteristics
3.1.1. QS, CS and QSNC Morphologies
3.1.2. QS, CS and QSNC X-ray Diffraction Patterns
3.2. Film Characteristics
3.2.1. Film Thickness, Moisture Content and Solubility in Water
3.2.2. Film Water Vapor Permeability
3.2.3. Film Color and Opacity
3.2.4. Film Surface Microstructure
3.2.5. Film Gloss
3.2.6. Film Water Contact Angle
3.2.7. Film X-ray Diffraction Patterns
3.2.8. Film Mechanical Properties
3.2.9. Film Thermal Properties
3.2.10. Fourier Transform Infrared Spectroscopy (FTIR)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Martins, P.C.; Latorres, J.M.; Martins, V.G. Impact of starch nanocrystals on the physicochemical, thermal and structural characteristics of starch-based films. LWT Food Sci. Technol. 2022, 156, 113041. [Google Scholar] [CrossRef]
- Vicentini, N.M.; Dupuy, N.; Leitzelman, M.; Cereda, M.P.; Sobral, P.J.A. Prediction of cassava starch edible film properties by chemometric analysis of infrared spectra. Spectrosc. Lett. 2005, 38, 749–767. [Google Scholar] [CrossRef]
- Bonilla, J.; Vicentini, N.M.; Dos Santos, R.M.; Bittante, A.M.Q.; Sobral, P.J.A. Mechanical properties of cassava starch films as affected by different plasticizers and different relative humidity conditions. Int. J. Food Stud. 2015, 4, 116–125. [Google Scholar]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Valencia, G.A.; Luciano, C.G.; Lourenço, R.V.; Sobral, P.J.A. Microstructure and physical properties of nano-biocomposite films based on cassava starch and laponite. Int. J. Biol. Macromol. 2018, 107, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Sharma, S.; Mir, S.A.; Dar, B.N. Nanobiocomposite films: A “greener alternate” for food packaging. Food Bioprocess Technol. 2021, 14, 1013–1027. [Google Scholar] [CrossRef]
- Dai, L.; Qiu, C.; Xiong, L.; Sun, Q. Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem. 2015, 174, 82–88. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, C.; Wang, X.; Xiong, L.; Sun, Q. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT Food Sci. Technol. 2016, 69, 251–257. [Google Scholar] [CrossRef]
- González, K.; Iturriaga, L.; González, A.; Eceiza, A.; Gabilondo, N. Improving mechanical and barrier properties of thermoplastic starch and polysaccharide nanocrystals nanocomposites. Eur. Polym. J. 2020, 123, 109415. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, L.; Yi, J.; Zhou, L.; Cai, S. Chestnut starch nanocrystal combined with macadamia protein isolate to stabilize pickering emulsions with different oils. Foods 2022, 11, 3320. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Huang, J.; Chang, P.R.; Anderson, D.P.; Yu, J. Preparation, modification, and application of starch nanocrystals in nanomaterials: A review. J. Nanomater. 2011, 2011, 573687. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Chen, Y.; Li, Q.; Gao, Q. Preparation of starch nanocrystals through enzymatic pretreatment from waxy potato starch. Carbohydr. Polym. 2018, 184, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Castillo, L.E.; Leite, M.A.; Ditchfield, C.; Sobral, P.J.A.; Moraes, I.C.F. Quinoa starch nanocrystals production by acid hydrolysis: Kinetics and properties. Int. J. Biol. Macromol. 2020, 143, 93–101. [Google Scholar] [CrossRef]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic starch—Waxy maize starch nanocrystals nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef]
- Viguié, J.; Molina-Boisseau, S.; Dufresne, A. Processing and characterization of waxy maize starch films plasticized by sorbitol and reinforced with starch nanocrystals. Macromol. Biosci. 2007, 7, 1206–1216. [Google Scholar] [CrossRef]
- Piyada, K.; Waranyou, S.; Thawien, W. Mechanical, thermal and structural properties of rice starch films reinforced with rice starch nanocrystals. Int. Food Res. J. 2013, 20, 439–449. [Google Scholar]
- Li, X.; Qiu, C.; Ji, N.; Sun, C.; Xiong, L.; Sun, Q. Mechanical, barrier and morphological properties of starch nanocrystals-reinforced pea starch films. Carbohydr. Polym. 2015, 121, 155–162. [Google Scholar] [CrossRef]
- Mukurumbira, A.R.; Mellem, J.J.; Amonsou, E.O. Effects of amadumbe starch nanocrystals on the physicochemical properties of starch biocomposite films. Carbohydr. Polym. 2017, 165, 142–148. [Google Scholar] [CrossRef]
- LeCorre, D.; Bras, J.; Dufresne, A. Influence of botanic origin and amylose content on the morphology of starch nanocrystals. J. Nanopart. Res. 2011, 13, 7193–7208. [Google Scholar] [CrossRef]
- De la Concha, B.B.S.; Agama-Acevedo, E.; Nuñez-Santiago, M.C.; Bello-Perez, L.A.; Garcia, H.S.; Alvarez-Ramirez, J. Acid hydrolysis of waxy starches with different granule size for nanocrystal production. J. Cereal Sci. 2018, 79, 193–200. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Mariano, M.; Dufresne, A.; Mellem, J.J.; Amonsou, E.O. Microstructure, thermal properties and crystallinity of amadumbe starch nanocrystals. Int. J. Biol. Macromol. 2017, 102, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Zhu, F. Physicochemical properties of quinoa starch. Carbohydr. Polym. 2016, 137, 328–338. [Google Scholar] [CrossRef] [PubMed]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Physico-mechanical properties of biodegradable starch nanocomposites. Macromol. Mater. Eng. 2009, 294, 169–177. [Google Scholar] [CrossRef]
- Dai, L.; Yu, H.; Zhang, J.; Cheng, F. Preparation and characterization of cross-linked starch nanocrystals and self-reinforced starch-based nanocomposite films. Int. J. Biol. Macromol. 2021, 181, 868–876. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- ISO 6647:1987; Rice—Determination of Amylose Content. 1st ed. International Organization for Standardization: Geneva, Switzerland, 1987; pp. 1–4.
- Angellier, H.; Choisnard, L.; Molina-Boisseau, S.; Ozil, P.; Dufresne, A. Optimization of the preparation of aqueous suspensions of waxy maize starch nanocrystals using a response surface methodology. Biomacromolecules 2004, 5, 1545–1551. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T.J.S.S. Studies on the relationship between water-satured state and crystallinity by the diffraction method for moistened potato starch. Starch-Stärke 1983, 35, 407–410. [Google Scholar] [CrossRef]
- ASTM E96/E96M-10; Standard Test Method for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2010; pp. 1–8.
- ASTM D2457-08; Standard Test Method for Specular Gloss of Plastic Films and Solid Plastics. ASTM International: West Conshohocken, PA, USA, 2008; pp. 1–5.
- ASTM D7334-08; Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. ASTM International: West Conshohocken, PA, USA, 2008; pp. 1–3.
- ASTM D882-10; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2010; pp. 1–5.
- Araujo-Farro, P.C.; Podadera, G.; Sobral, P.J.A.; Menegalli, F.C. Development of films based on quinoa (Chenopodium quinoa, Willdenow) starch. Carbohydr. Polym. 2010, 81, 839–848. [Google Scholar] [CrossRef]
- Valencia, G.A.; Djabourov, M.; Sobral, P.J.A. Water desorption of cassava starch granules: A study based on thermogravimetric analysis of aqueous suspensions and humid powders. Carbohydr. Polym. 2016, 147, 533–541. [Google Scholar] [CrossRef]
- Condés, M.C.; Añón, M.C.; Mauri, A.N.; Dufresne, A. Amaranth protein films reinforced with maize starch nanocrystals. Food Hydrocoll. 2015, 47, 146–157. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Jiménez, B.; Torres-Vargas, O.L.; Rodríguez-García, M.E. Physicochemical characterization of quinoa (Chenopodium quinoa) flour and isolated starch. Food Chem. 2019, 298, 124982. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, F. Quinoa starch: Structure, properties, and applications. Carbohydr. Polym. 2018, 181, 851–861. [Google Scholar] [CrossRef]
- Costa, M.S.; Volanti, D.P.; Grossmann, M.V.E.; Franco, C.M.L. Structural, thermal, and morphological characteristics of cassava amylodextrins. J. Sci. Food Agric. 2018, 98, 2751–2760. [Google Scholar] [CrossRef] [PubMed]
- Tunc, S.; Angellier, H.; Cahyana, Y.; Chalier, P.; Gontard, N.; Gastaldi, E. Functional properties of wheat gluten/montmorillonite nanocomposite films processed by casting. J. Membr. Sci. 2007, 289, 159–168. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.A.; Menegalli, F.C. Nanocomposites based on banana starch reinforced with cellulose nanofibers isolated from banana peels. J. Colloid Interface Sci. 2017, 505, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of re-crystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll. 2012, 26, 302–310. [Google Scholar] [CrossRef]
- Villalobos, R.; Chanona, J.; Hernández, P.; Gutiérrez, G.; Chiralt, A. Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocoll. 2005, 19, 53–61. [Google Scholar] [CrossRef]
- Bergo, P.; Sobral, P.J.A.; Prison, J.M. Effect of glycerol on physical properties of cassava starch films. J. Food Process. Preserv. 2010, 34, 401–410. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef]
- Arayaphan, J.; Maijan, P.; Boonsuk, P.; Chantarak, S. Synthesis of photodegradable cassava starch-based double network hydrogel with high mechanical stability for effective removal of methylene blue. Int. J. Biol. Macromol. 2021, 168, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Orsuwan, A.; Sothornvit, R. Development and characterization of banana flour film incorporated with montmorillonite and banana starch nanoparticles. Carbohydr. Polym. 2017, 174, 235–242. [Google Scholar] [CrossRef] [PubMed]







| QSNC Concentration (%) | ||||
|---|---|---|---|---|
| Properties | 0 | 2.5 | 5.0 | 7.5 |
| Thickness (mm) | 0.103 ± 0.002 a | 0.102 ± 0.003 a | 0.100 ± 0.003 a | 0.106 ± 0.002 a |
| Moisture content (%) | 11.8 ± 0.4 a | 11.0 ± 0.6 a | 10.5 ± 0.3 a | 10.7 ± 0.4 a |
| Solubility in water (%) | 25.1 ± 1.4 a | 24.3 ± 0.7 a | 22.9 ± 1.0 a | 23.9 ± 0.7 a |
| WVP (g.mm/m2·h·kPa) | 0.54 ± 0.01 a | 0.50 ± 0.01 a,b | 0.45 ± 0.03 b | 0.52 ± 0.03 a,b |
| L* | 91.3 ± 0.0 b | 91.3 ± 0.2 b | 91.7 ± 0.1 b | 92.2 ± 0.0 a |
| a* | −0.67 ± 0.01 a,b | −0.67 ± 0.01 b | −0.66 ± 0.01 b | −0.69 ± 0.01 a |
| b* | 2.4 ± 0.0 b | 2.5 ± 0.0 b | 2.5 ± 0.1 b | 2.8 ± 0.1 a |
| ∆E* | 3.2 ± 0.0 a | 3.1 ±0.2 a | 2.9 ±0.1 a | 2.4 ± 0.1 b |
| Opacity | 0.51 ± 0.08 d | 1.19 ± 0.18 c | 2.05 ± 0.09 b | 3.29 ± 0.08 a |
| Ra (nm) | 85 ± 1 b | 102 ± 6 b | 205 ± 8 a | 247 ± 33 a |
| Rq (nm) | 108 ± 2 c | 129 ± 7 b | 261 ± 17 a | 309 ± 39 a |
| Gloss (GU) | 58.3 ± 5.0 a | 35.2 ± 2.6 b | 19.5 ± 0.8 c | 15.6 ± 0.3 c |
| Contact angle (°) | 47.9 ± 2.3 b | 57.8 ± 2.8 b | 75.3 ± 2.8 a | 73.5 ± 1.4 a |
| QSNC (%) | TS (MPa) | EB (%) | M (MPa/%) |
|---|---|---|---|
| 0 | 6.5 ± 0.5 c | 10.2 ± 1.0 a | 2.8 ± 0.2 c |
| 2.5 | 8.4 ± 0.5 b,c | 7.4 ± 0.9 b | 3.7 ± 0.2 b,c |
| 5.0 | 16.5 ± 2.5 a | 8.7 ± 0.6 a,b | 6.6 ± 0.8 a |
| 7.5 | 11.8 ± 0.3 b | 7.1 ± 0.7 b | 4.8 ± 0.1 b |
| QSNC (%) | TgG (°C) | TgS (°C) | Tm (°C) | ∆Hm (J/g) |
|---|---|---|---|---|
| 0 | −70.1 ± 3.0 a | 15.0 ± 2.1 b | 212.8 ± 2.3 a | 56.2 ± 7.0 b |
| 2.5 | −71.7 ± 2.1 a | 13.6 ± 1.4 b | 208.1 ± 5.5 a | 60.9 ± 3.7 b |
| 5.0 | −71.6 ± 1.1 a | 17.7 ± 0.9 a,b | 214.5 ± 2.4 a | 161.2 ± 14.6 a |
| 7.5 | −72.2 ± 2.1 a | 23.1 ± 3.5 a | 215.8 ± 2.5 a | 147.2 ± 5.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-Castillo, L.E.; Leite, M.A.; Tisnado, V.J.A.; Ditchfield, C.; Sobral, P.J.d.A.; Moraes, I.C.F. Cassava Starch Films Containing Quinoa Starch Nanocrystals: Physical and Surface Properties. Foods 2023, 12, 576. https://doi.org/10.3390/foods12030576
Velásquez-Castillo LE, Leite MA, Tisnado VJA, Ditchfield C, Sobral PJdA, Moraes ICF. Cassava Starch Films Containing Quinoa Starch Nanocrystals: Physical and Surface Properties. Foods. 2023; 12(3):576. https://doi.org/10.3390/foods12030576
Chicago/Turabian StyleVelásquez-Castillo, Lía Ethel, Mariani Agostinetto Leite, Victor Jesús Aredo Tisnado, Cynthia Ditchfield, Paulo José do Amaral Sobral, and Izabel Cristina Freitas Moraes. 2023. "Cassava Starch Films Containing Quinoa Starch Nanocrystals: Physical and Surface Properties" Foods 12, no. 3: 576. https://doi.org/10.3390/foods12030576