Olive Leaf Processing for Infusion Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
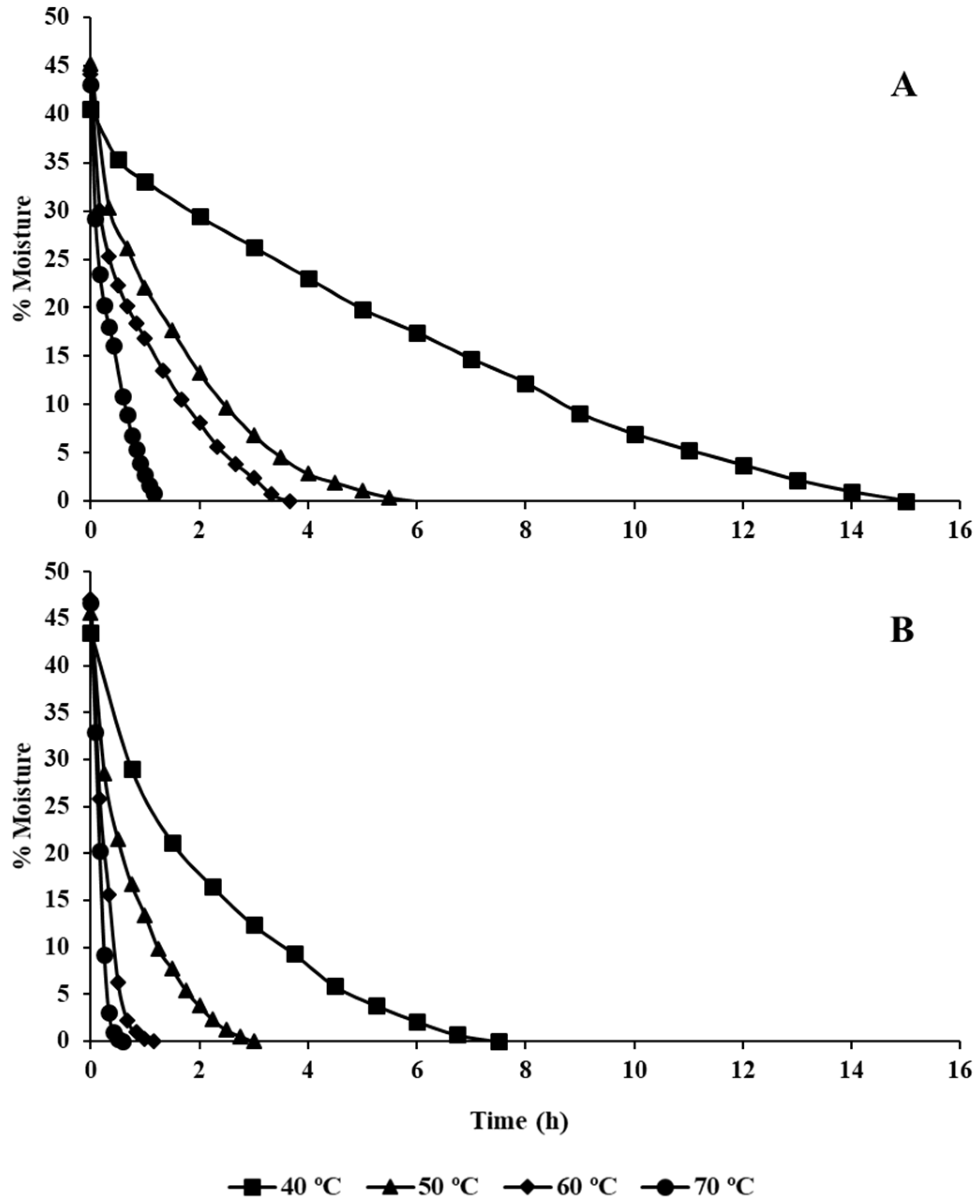
2.2. Drying Procedures
2.3. Grinding Procedures
2.4. Olive Tea Preparation
2.5. Phenolic Compound Analysis
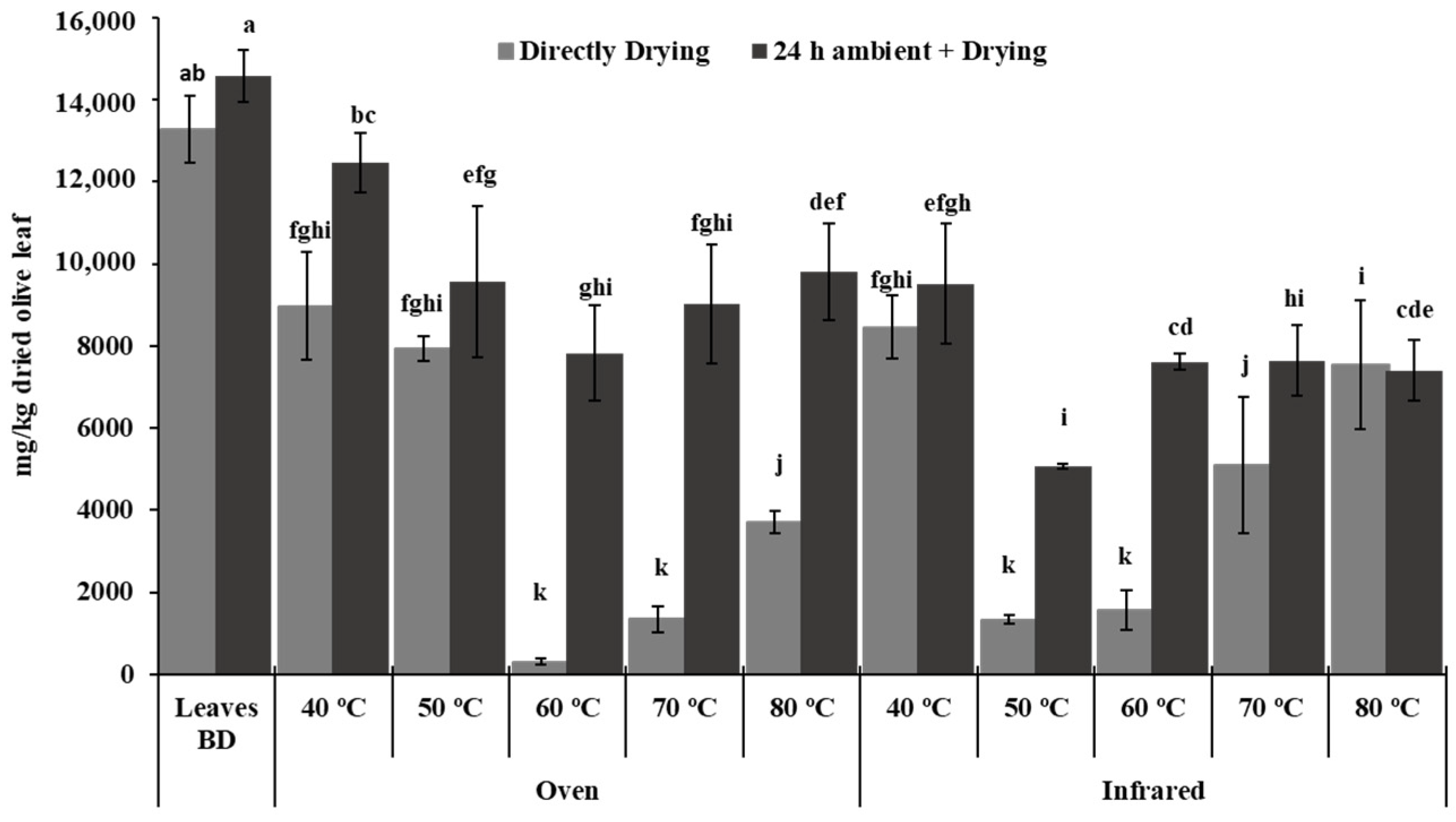
2.6. Triterpenic Acid Analysis
2.7. Color and Turbidity Analysis
2.8. Statistical Analysis
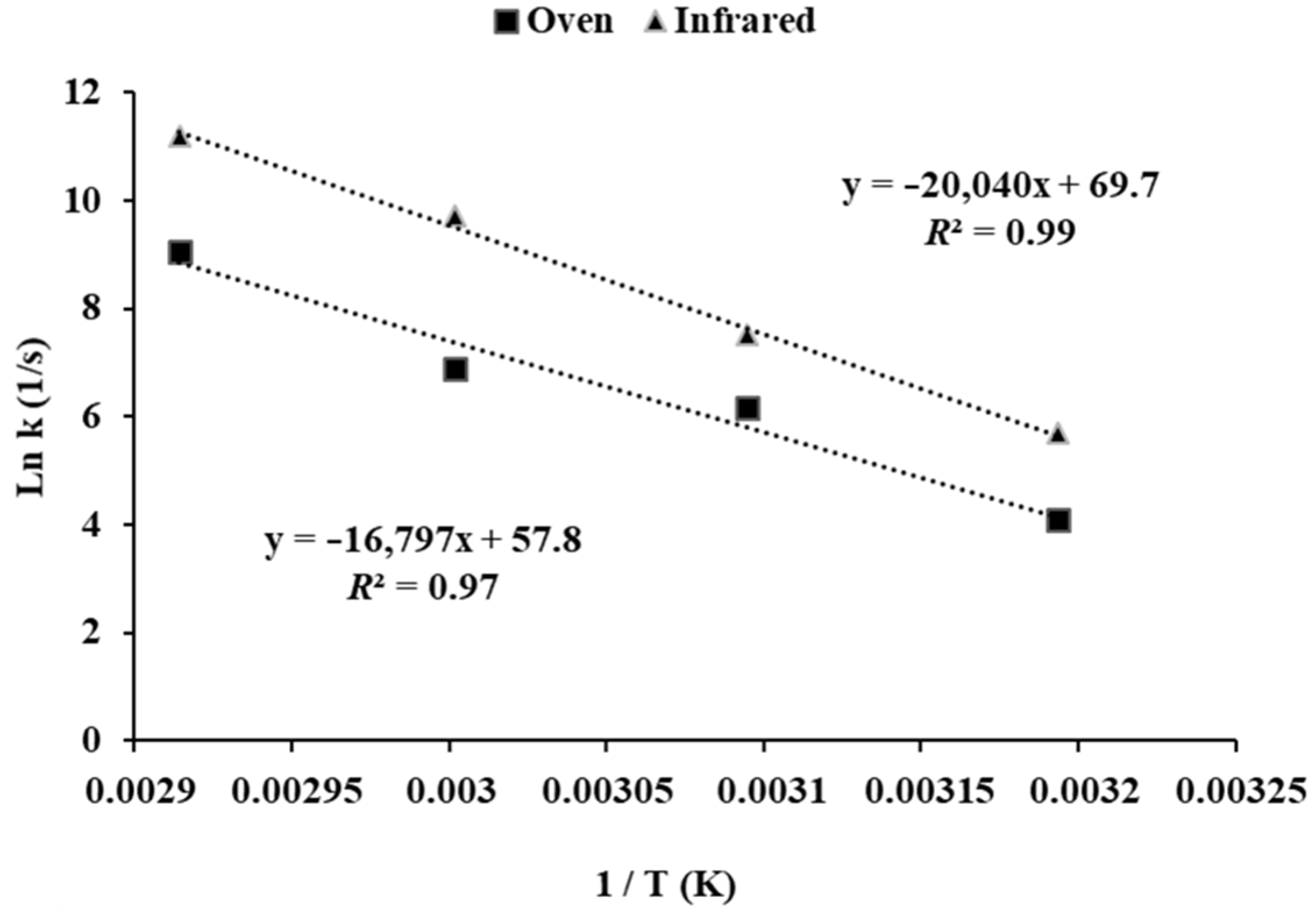
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romani, A.; Mulas, S.; Heimler, D. Polyphenols and secoiridoids in raw material (Olea europaea L. leaves) and commercial food supplements. Eur. Food Res. Technol. 2017, 243, 429–435. [Google Scholar] [CrossRef]
- Vergara-Barberán, M.; Lerma-García, M.J.; Herrero-Martínez, J.M.; Simó-Alfonso, E.F. Use of an enzyme-assisted method to improve protein extraction from olive leaves. Food Chem. 2015, 169, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Romero, C.; García, P.; Brenes, M. Characterization of bioactive compounds in commercial olive leaf extracts, and olive leaves and their infusions. Food Funct. 2019, 10, 4716–4724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorini, A.; Aranha, B.C.; Antunes, B.D.F.; Otero, D.M.; Jacques, A.C.; Zambiazi, R.C. Metabolic profile of olive leaves of different cultivars and collection times. Food Chem. 2021, 345, 128758. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Papoti, V.T.; Tsimidou, M.Z. Bioactive ingredients in olive leaves. In Olives and Olive Oil in Health and Disease Prevention. Part 1. General Aspects of Olives and Olive Oil, 2nd ed.; Preedy, V., Watson, R., Eds.; Academic Press: Oxford, UK, 2021; Chapter 5; pp. 65–78. [Google Scholar]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, A.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. olive leaves through the evaluation of their extracts: Antioxidant and antimicrobial activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Sahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical role of polyphenols and triterpenes present in the extracts of fruits and leaves of Olea europaea as antioxidants, anti- infectives and anticancer agents on healthy growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Cognigni, A.; de Faria, E.L.P.; Silvestre, A.J.D.; Zirbs, R.; Freire, M.G.; Bica, K. Valorization of olive tree leaves: Extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids. Sep. Purif. Technol. 2018, 204, 30–37. [Google Scholar] [CrossRef]
- Mohamed, M.B.; Guasmi, F.; Ben Ali, S.; Radhouani, F.; Faghim, J.; Triki, T.; Kammoun, N.G.; Baffi, C.; Lucini, L.; Benincasa, C. The LC-MS/MS characterization of phenolic compounds in leaves allows classifying olive cultivars grown in South Tunisia. Biochem. Syst. Ecol. 2018, 78, 84–90. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: Extraction with the Soxhlet method and pressurized liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Romero, C.; Medina, E.; Mateo, M.A.; Brenes, M. Quantification of bioactive compounds in Picual and Arbequina olive leaves and fruit. J. Sci. Food Agric. 2017, 97, 1725–1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinda, A.; Castellano, J.M.; Santos-Lozano, J.M.; Delgado-Hervás, T.; Gutiérrez-Adánez, P.; Rada, M. Determination of major bioactive compounds from olive leaf. LWT Food Sci. Technol. 2015, 64, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Peragón, J. Time course of pentacyclic triterpenoids from fruits and leaves of olive tree (Olea europaea L.) cv. Picual and cv. Cornezuelo during ripening. J. Agric. Food Chem. 2013, 61, 6671–6678. [Google Scholar] [CrossRef]
- Ramírez, E.M.; Brenes, M.; Romero, C.; Medina, E. Chemical and enzymatic characterization of leaves from Spanish table olive cultivars. Foods 2022, 11, 3879. [Google Scholar] [CrossRef]
- Ramírez, E.; Medina, E.; Brenes, M.; Romero, C. Endogenous enzymes involved in the transformation of oleuropein in Spanish table olive varieties. J. Agric. Food Chem. 2014, 62, 9569–9575. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Povedano, M.; Priego-Capote, F.; Luque-De Castro, M.D. Selective ultrasound-enhanced enzymatic hydrolysis of oleuropein to its aglycon in olive (Olea europea L.) leaf extracts. Food Chem. 2017, 220, 282–288. [Google Scholar] [CrossRef]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-Bustillos, R.J.; Olaizola, M.; Arboleya, J.C. Olive leaf waste management. Front. Sustain. Food Syst. 2021, 5, 660582. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef]
- Coppa, C.F.S.C.; Gonçalves, B.L.; Lee, S.H.; Nunes, V.M.R.; Gonçalves, C.B.; Rodrigues, C.E.C.; Oliveira, C.A.F. Extraction of oleuropein from olive leaves and applicability in foods. Qual. Assur. Saf. Crops Food 2020, 12, 50–62. [Google Scholar] [CrossRef]
- Demir, V.; Gunhan, T.; Yagcioglu, A.K. Mathematical modelling of convection drying of green table olives. Biosyst. Eng. 2007, 98, 47–53. [Google Scholar] [CrossRef]
- Boudhrioua, N.; Bahloul, N.; Ben Slimen, I.; Kechaou, N. Comparison on the total phenol contents and the color of fresh and infrared dried olive leaves. Ind. Crops Prod. 2009, 29, 412–419. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, C.; Liu, L.; Xu, Z.; Chen, T.; Zhou, L.; Yuan, M.; Li, T.; Ding, C. Comparison of phenolic compounds in olive leaves by different drying and storage methods. Separations 2021, 8, 156. [Google Scholar] [CrossRef]
- Helvaci, H.U.; Menon, A.; Aydemir, L.Y.; Korel, F.; Akkurt, G.G. Drying of olive leaves in a geothermal dryer and determination of quality parameters of dried product. Energy Procedia 2019, 161, 108–114. [Google Scholar] [CrossRef]
- Malik, N.S.A.; Bradford, J.M. Recovery and stability of oleuropein and other phenolic compounds during extraction and processing of olive (Olea europaea L.) leaves. J. Food Agric. Env. 2008, 6, 8–13. [Google Scholar]
- Filgueira-Garro, I.; González-Ferrero, C.; Mendiola, D.; Marín-Arroyo, M.R. Effect of cultivar and drying methods on phenolic compounds and antioxidant capacity in olive (Olea europaea L.) leaves. AIMS Agric. Food 2022, 7, 250–264. [Google Scholar] [CrossRef]
- Islam, M.Z.; Cho, D.-K.; Lee, Y.-T. Bioactive compounds and antioxidant capacity of tea infusion prepared from whole and ground medicinal herb parts. CyTA J. Food 2020, 18, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Alsaud, N.; Farid, M. Insight into the influence of grinding on the extraction efficiency of selected bioactive compounds from various plant leaves. Appl. Sci. 2020, 10, 6362. [Google Scholar] [CrossRef]
- Zhang, J.; Donga, Y.; Zhongxiang, C.; Wang, Z.C.; Guo, Y. Effect of superfine-grinding on the physicochemical and antioxidant properties of Lycium ruthenicum Murray powders. Powder Technol. 2020, 372, 68–75. [Google Scholar] [CrossRef]
- Shu, Y.; Li, J.; Yang, X.; Dong, X.; Wang, X. Effect of particle size on the bioaccessibility of polyphenols and polysaccharides in green tea powder and its antioxidant activity after simulated human digestion. J. Food Sci. Technol. 2019, 56, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Baccouri, B.; Rajhi, I.; Theresa, S.; Najjar, Y.; Mohamed, S.N.; Willenberg, I. The potential of wild olive leaves (Olea europaea L. subsp. oleaster) addition as a functional additive in olive oil production: The effects on bioactive and nutraceutical compounds using LC–ESI–QTOF/MS. Eur. Food Res. Technol. 2022, 248, 2809–2823. [Google Scholar] [CrossRef] [PubMed]
| Equipment | Temperature (°C) | L* | a* | b* | Chromaticity | Hue Angle |
|---|---|---|---|---|---|---|
| Oven | 40 | 98.3 (0.4) b | 1.4 (0.0) f | 9.3 (0.1) e | 9.5 (0.1) ef | 81.7 (0.1) c |
| 50 | 97.4 (0.2) d | 2.5 (0.0) ab | 15.5 (0.2) ab | 15.7 (0.2) ab | 80.8 (0.1) fg | |
| 60 | 97.8 (0.3) cd | 2.3 (0.1) bc | 14.4 (0.7) bc | 14.6 (0.7) bc | 81.0 (0.1) ef | |
| 70 | 97.6 (0.8) cd | 2.1 (0.3) cd | 13.3 (1.3) cd | 13.5 (1.4) cd | 81.2 (0.4) ef | |
| 80 | 98.0 (0.2) bc | 1.7 (0.0) e | 12.8 (0.0) d | 12.9 (0.0) d | 82.5 (0.0) b | |
| Infrared | 40 | 99.0 (0.1) a | 1.0 (0.1) g | 7.7 (0.3) f | 7.8 (0.3) fg | 82.8 (0.1) a |
| 50 | 98.1 (0.1) bc | 1.9 (0.1) de | 13.5 (0.5) cd | 13.6 (0.5) cd | 81.9 (0.0) c | |
| 60 | 97.6 (0.2) cd | 2.4 (0.2) b | 14.4 (0.1) bc | 14.6 (1.0) bc | 80.7 (0.0) g | |
| 70 | 97.3 (0.1) d | 2.7 (0.2) a | 16.7 (0.9) a | 16.9 (1.0) a | 80.8 (0.0) fg | |
| 80 | 97.3 (0.3) d | 2.5 (0.3) ab | 16.7 (2.0) b | 16.9 (2.1) a | 81.5 (0.1) d |
| Sample | Grinding Grade (mm) | Oleuropein (mg/kg) | Oleanolic Acid (mg/kg) | Maslinic Acid (mg/kg) | Color Parameters | Turbidity (NTA) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | Chromaticity | Hue Angle | ||||||
| Dried leaves | - | 9419 (955) a | 28,264 (2547) a | 5755 (206) a | - | - | - | - | - | - |
| Leaves infusions | >5 | 42 (12) f | ND | ND | 98.7 (0.4) a | −0.8 (0.0) e | −1.3 (0.1) f | 1.5 (0.1) d | 237.8 (1.0) a | 2.0 f |
| 5–3.15 | 80 (2) f | ND | ND | 98.6 (0.3) a | −0.7 (0.0) e | −0.7 (0.2) e | 1.0 (0.2) e | 221.2 (8.9) b | 2.5 e | |
| 3.15–2 | 159 (3) e | ND | ND | 97.9 (0.3) ab | −0.6 (0.0) d | 0.7 (0.2) d | 0.9 (0.1) e | 129.4 (9.1) c | 6.5 d | |
| 2–1 | 358 (0) d | ND | ND | 97.6 (0.9) bc | −0.2 (0.1) c | 3.5 (0.2) c | 3.5 (0.2) c | 93.9 (1.6) d | 9.0 c | |
| 1–0.5 | 727 (24) c | 1 (0) b | 1 (0) b | 97.1 (0.7) c | 0.3 (0.1) b | 9.0 (0.7) b | 9.0 (0.7) b | 88.4 (0.3) d | 15.0 b | |
| <0.5 | 906 (30) b | 14 (0) b | 4 (0) b | 95.8 (0.3) d | 0.8 (0.0) a | 14.5 (0.2) a | 14.5 (0.2) a | 86.8 (0.0) d | 35.0 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, E.M.; Brenes, M.; Romero, C.; Medina, E. Olive Leaf Processing for Infusion Purposes. Foods 2023, 12, 591. https://doi.org/10.3390/foods12030591
Ramírez EM, Brenes M, Romero C, Medina E. Olive Leaf Processing for Infusion Purposes. Foods. 2023; 12(3):591. https://doi.org/10.3390/foods12030591
Chicago/Turabian StyleRamírez, Eva María, Manuel Brenes, Concepción Romero, and Eduardo Medina. 2023. "Olive Leaf Processing for Infusion Purposes" Foods 12, no. 3: 591. https://doi.org/10.3390/foods12030591





