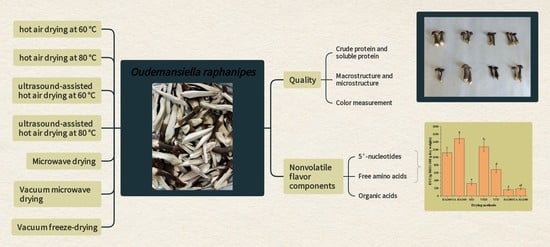
Effect of Different Drying Methods on the Quality and Nonvolatile Flavor Components of Oudemansiella raphanipes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Drying Methods
2.2.1. Hot Air Drying
2.2.2. Ultrasound-Assisted Hot Air Drying
2.2.3. Microwave Drying
2.2.4. Vacuum Microwave Drying
2.2.5. Vacuum Freeze-Drying
2.3. Crude Protein and Soluble Protein Analysis
2.4. Macrostructure and Microstructure Analyses
2.5. Color Measurement Analysis
2.6. Hydrophilic Flavor Component Analysis
2.7. Organic Acid Analysis
2.8. Statistical Analysis
3. Results and Discussion
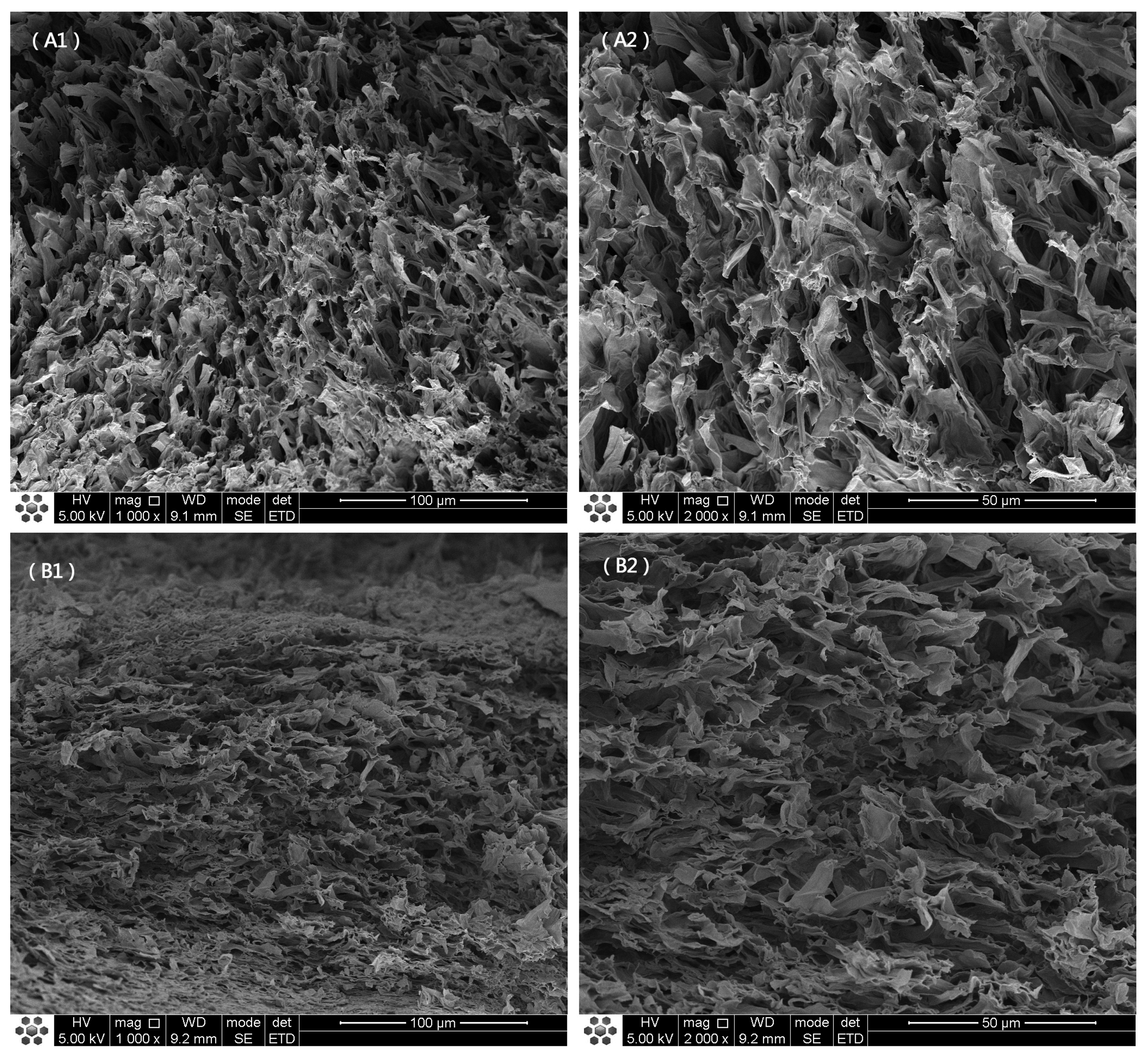
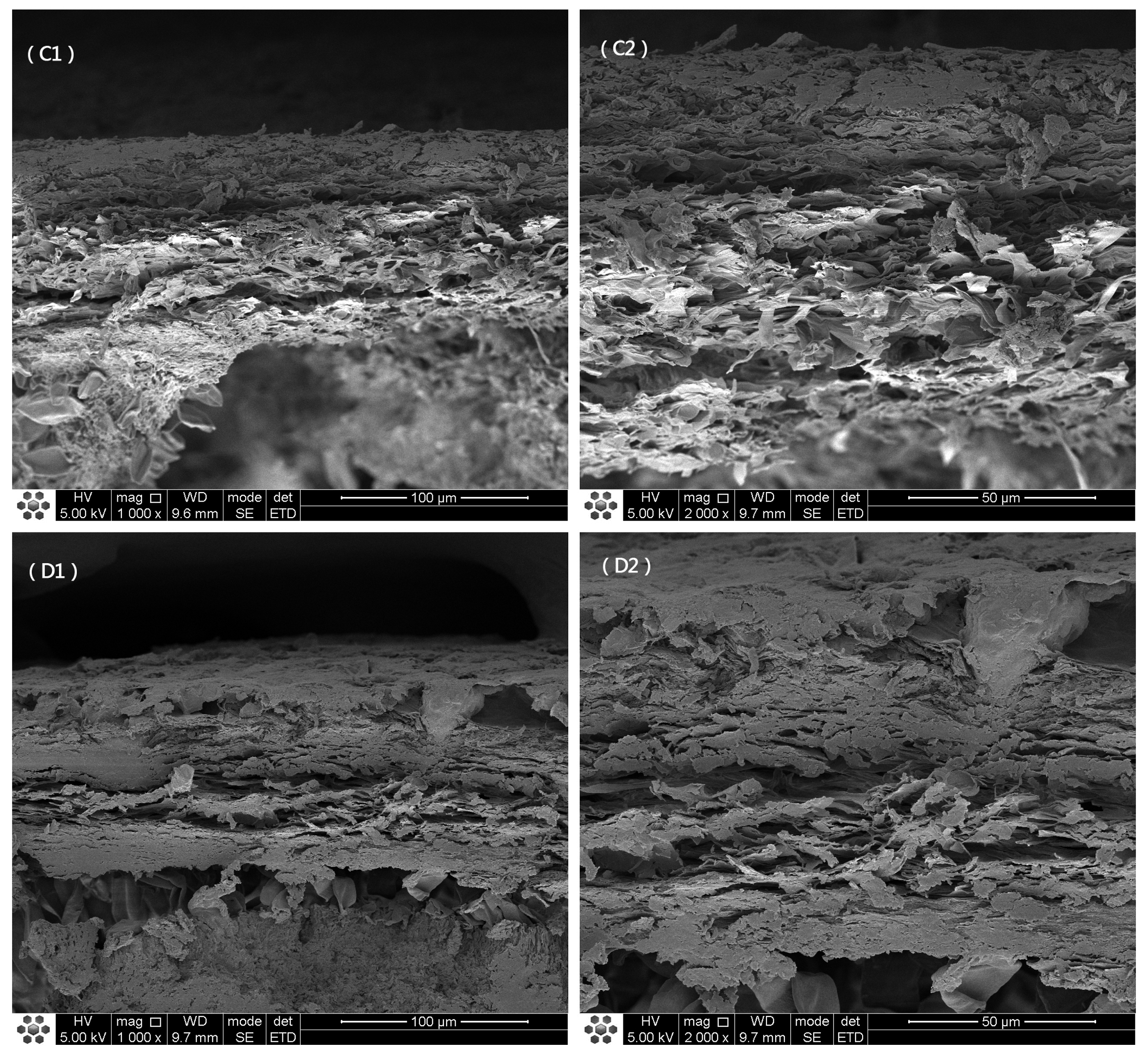
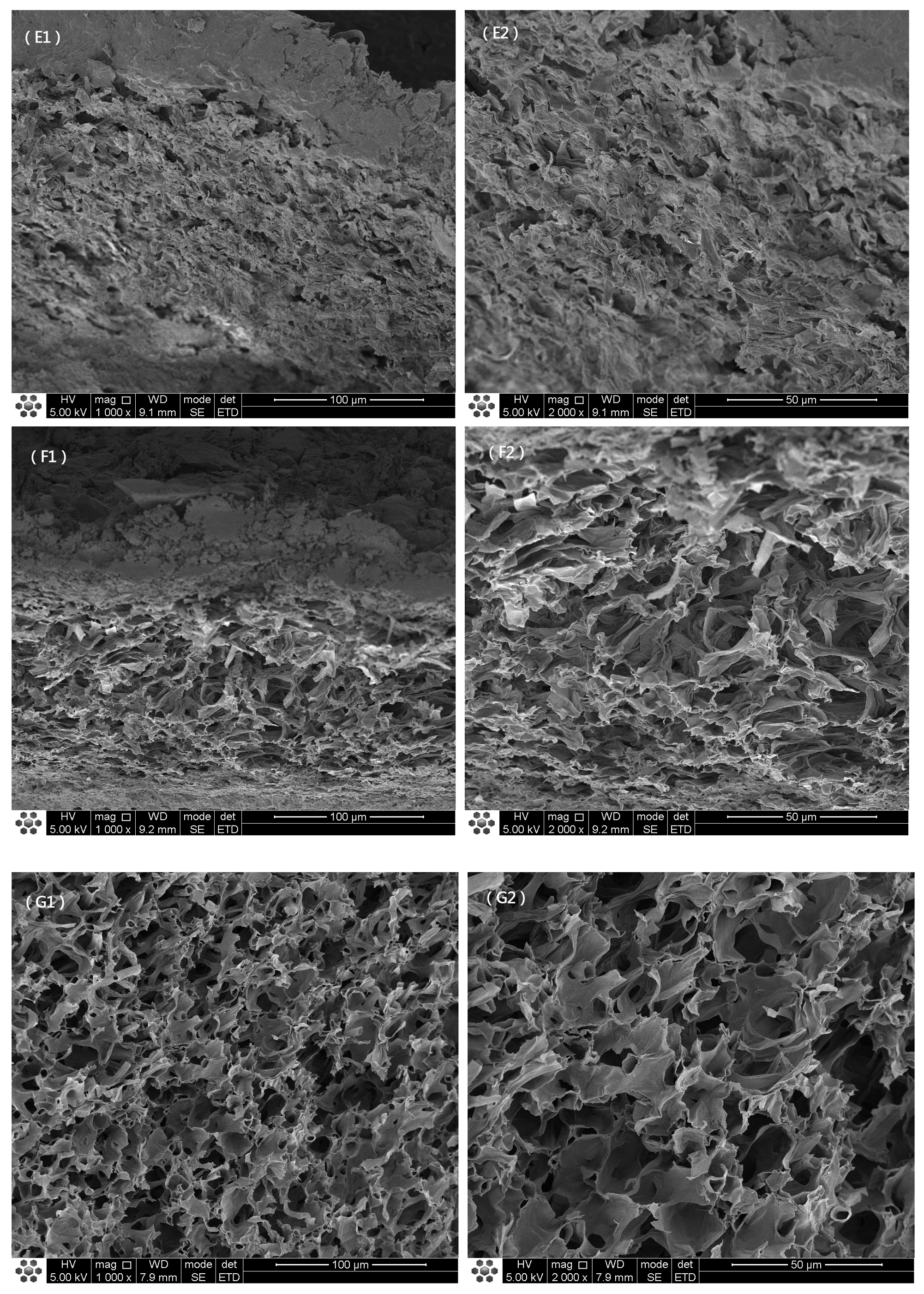
3.1. Macrostructure and Microstructure of O. raphanipes Mushrooms Subjected to Different Drying Methods
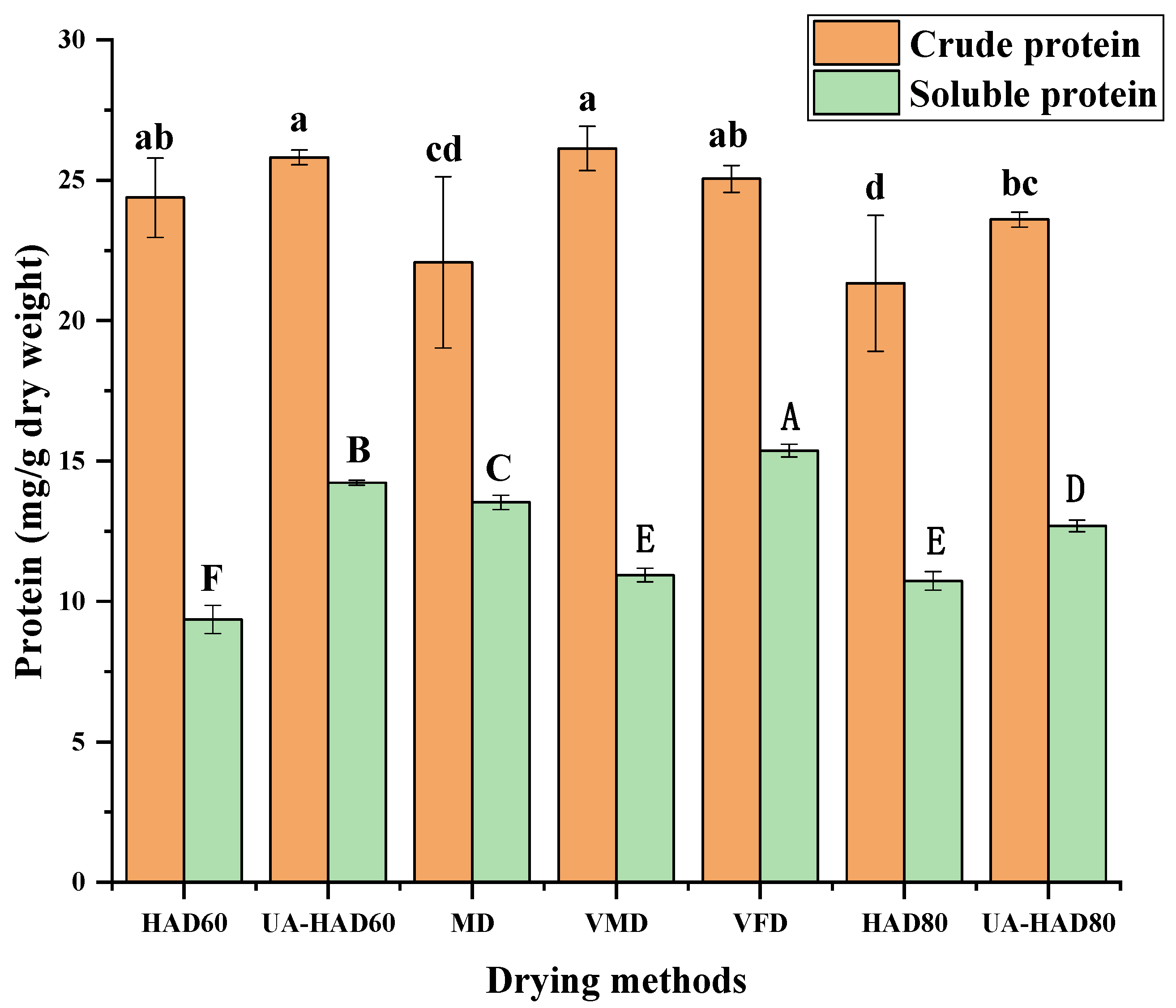
3.2. Protein Content of O. raphanipes Subjected to Different Drying Methods
3.3. Color Measurements of O. raphanipes Treated through Different Drying Methods
3.4. Free Amino Acids
3.5. Organic Acids
3.6. 5′-Nucleotides
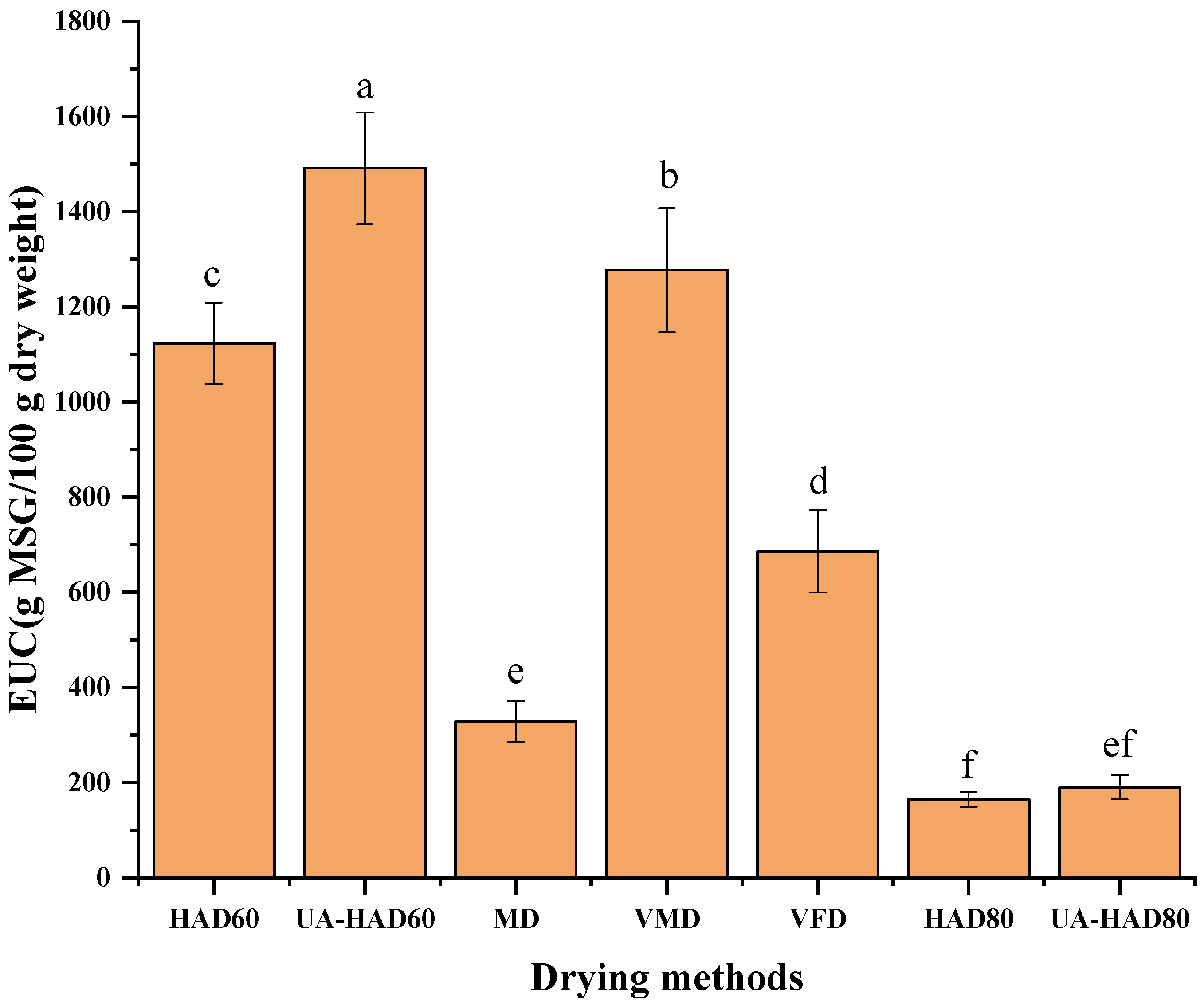
3.7. EUC Value
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, W.J.; Hu, R.S.; Chu, Z.; Zhao, J.P.; Tan, L.H. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Piskov, S.; Timchenko, L.; Grimm, W.-D.; Rzhepakovsky, I.; Avanesyan, S.; Sizonenko, M.; Kurchenko, V. Effects of Various Drying Methods on Some Physico-Chemical Properties and the Antioxidant Profile and ACE Inhibition Activity of Oyster Mushrooms (Pleurotus Ostreatus). Foods 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, S.M.; Sturm, B.; Nasirahmadi, A. Effects of hotair and hybrid hot air-microwave drying on drying kinetics and textural quality of nectarine slices. Heat Mass Transfer. 2018, 54, 915–927. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Somkane, S.; Nakagawa, K.; Mahayothee, B. Osmotic Dehydration, Drying Kinetics, and Quality Attributes of Osmotic Hot Air-Dried Mango as Affected by Initial Frozen Storage. Foods 2022, 11, 489. [Google Scholar] [CrossRef]
- Askari, G.R.; Emam-Djomeh, Z.; Mousavi, S.M. An Investigation of the Effects of Drying Methods and Conditions on Drying Characteristics and Quality Attributes of Agricultural Products during Hot Air and Hot Air/Microwave-Assisted Dehydration. Drying Technol. 2009, 27, 831–841. [Google Scholar] [CrossRef]
- Tan, S.D.; Xu, Y.; Zhu, L.C.; Geng, Z.H.; Zhang, Q.; Yang, X.H. Hot Air Drying of Seabuckthorn (Hippophae rhamnoides L.) Berries: Effects of Different Pretreatment Methods on Drying Characteristics and Quality Attributes. Foods 2022, 11, 3675. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perdoni, F.; Infantino, V.; Faliva, M.A.; Peroni, G.; Iannello, G.; Nichetti, M.; Alalwan, T.A.; Perna, S.; Cocuzza, C. Volatile organic compounds as biomarkers of gastrointestinal diseases and nutritional status. J. Anal. Methods Chem. 2019, 2019, 7247802. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, S.H.; Fu, C.C.; Fang, F.; Liu, J.; Kan, J.; Qian, C.L.; Chai, Q.Q.; Jin, C.H. Effects of Drying Methods on Taste Components and Flavor Characterization of Cordyceps militaris. Foods 2022, 11, 3933. [Google Scholar] [CrossRef]
- Fernandes, Â.; Barros, L.; Barreira, J.C.M.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effects of different processing technologies on chemical and antioxidant parameters of Macrolepiota procera wild mushroom. LWT-Food Sci. Technol. 2013, 54, 493–499. [Google Scholar] [CrossRef]
- Zhang, L.J.; Dong, X.B.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Effects of Drying Process on the Volatile and Non-Volatile Flavor Compounds of Lentinula edodes. Foods 2021, 10, 2836. [Google Scholar] [CrossRef]
- Hu, L.; Bi, J.F.; Jin, X.; Qiu, Y.; Sman, R.G.M.v.d. Study on the Rehydration Quality Improvement of shiitake Mushroom by Combined Drying Methods. Foods 2021, 10, 769. [Google Scholar] [CrossRef]
- Zheng, Q.R.; Gao, P.P.; Liu, T.T.; Gao, X.X.; Li, W.F.; Zhao, G.F. Effects of drying methods on colour, amino acids, phenolic profile, microstructure and volatile aroma components of Boletus aereus slices. Int. J. Food Sci. Technol. 2022, 57, 5164–5174. [Google Scholar] [CrossRef]
- Li, X.B.; Feng, T.; Zhou, F.; Zhou, S.; Liu, Y.F.; Li, W.; Ye, R.; Yang, Y. Effects of drying methods on the tasty compounds of Pleurotus eryngii. Food Chem. 2015, 166, 358–364. [Google Scholar] [CrossRef]
- Tian, Y.T.; Zhao, Y.T.; Huang, J.J.; Zeng, H.L.; Zheng, B.D. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef]
- Hu, S.; Feng, X.; Huang, W.; Ibrahimc, S.A.; Liu, Y. Effects of drying methods on non-volatile taste components of Stropharia rugoso-annulata mushrooms. LWT-Food Sci. Technol. 2020, 127, 109428. [Google Scholar] [CrossRef]
- Yu, Y.S.; Jin, T.Z.; Fan, X.T.; Wu, J.J. Biochemical degradation and physical migration of polyphenolic compounds in osmotic dehydrated blueberries with pulsed electric field and thermal pretreatments. Food Chem. 2018, 239, 1219–1225. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.Y.; Xu, Y.J.; Wu, J.J.; Yu, Y.S.; Peng, J.; An, K.J.; Zou, B.; Yang, W.Y. Effect of ultrasound-assisted osmotic dehydration pretreatment on the drying characteristics and quality properties of Sanhua plum (Prunus salicina L.). LWT-Food Sci. Technol. 2021, 138, 110653. [Google Scholar] [CrossRef]
- Bozkir, H.; Ergün, A.; Serdar, E.; Metin, G.; Baysal, T. Influence of ultrasound and osmotic dehydration pretreatments on drying and quality properties of persimmon fruit. Ultrason. Sonochem. 2019, 54, 135–141. [Google Scholar] [CrossRef]
- Cakmak, R.S.; Tekeoglu, O.; Bozkir, H.; Ergun, A.R.; Baysal, T. Effects of electrical and sonication pretreatments on the drying rate and quality of mushrooms. LWT-Food Sci. Technol. 2016, 69, 197–202. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, F.F.; Cai, B.X.; Liu, S.R.; Zhang, W.R.; Chen, M.X. Extraction and Antioxidant Activities of Polysaccharides Produced by Submerged Mycelia Culture of Oudemansiella raphanipes. Curr. Top. Nutraceutical Res. 2022, 20, 484–491. [Google Scholar] [CrossRef]
- Xia, R.R.; Wang, Z.C.; Xu, H.R.; Hou, Z.S.; Li, Y.T.; Wang, Y.F.; Feng, Y.; Zhang, X.; Xin, G. Cutting root treatment combined with low-temperature storage regimes on non-volatile and volatile compounds of Oudemansiella raphanipes. LWT-Food Sci. Technol. 2022, 166, 113754. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, M.J.; Geng, X.R.; Wang, H.X.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the edible mushroom Oudemansiella radicata. Molecules 2017, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Li, H.F.; Chen, P.; Wang, Q. Chemical constituents and anticancer activity of the petroleum ether extract from the fruiting of Oudemansiella raphanipes. Chem. Nat. Compd. 2021, 57, 976–977. [Google Scholar] [CrossRef]
- Zhang, F.J.; Hu, A.Q.E.; Song, R.K.; Li, L.X. Optimization of hot-air microwave combined drying control system based on air outlet temperature and humidity monitoring. Int. J. Agric. Biol. Eng. 2021, 14, 255–261. [Google Scholar] [CrossRef]
- Zhang, F.J.; Shi, L.; Xin, L.; Li, L.; Tian, L.; Cui, X.; Gao, Y. Study on drying characteristics and moisture content prediction model of Panax notoginseng taproot by using segmented drying of microwave vacuum combined with hot air. J. Food Process. Eng. 2022, 45, e14179. [Google Scholar] [CrossRef]
- Wang, D.; Shi, L.J.; Fan, X.W.; Lou, H.Q.; Li, W.T.; Li, Y.L.; Ren, D.B.; Yi, L.Z. Development and validation of an efficient HILIC-QQQ-MS/MS method for quantitative and comparative profiling of 45 hydrophilic compounds in four types of tea (Camellia sentences). Food Chem. 2022, 371, 131201. [Google Scholar] [CrossRef]
- Shi, L.J.; Chen, N.; Wang, D.; Fan, X.W.; Hu, Y.D.; Yi, L.Z.; Ren, D.B. Quantitative and Comparative Studies on Phenolic Constituents in Different Types of Yunnan Large Leaf Tea Based on Validated Ultra-high Performance Liquid Chromatography-Triple Quadrupole-Tandem Mass Spectrometry. Food Sci. 2022, 43, 271–280. [Google Scholar]
- Zhang, Z.Y.; Liu, Z.B.; Liu, C.J.; Li, D.J.; Jiang, N.; Liu, C.Q. Effects of ultrasound pretreatment on drying kinetics and quality parameters of button mushroom slices. Drying Technol. 2016, 34, 1791–1800. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.J.; Ma, N.; Fang, Y.; Zhao, L.Y.; An, X.X.; Xin, Z.H.; Hu, Q.H. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC-MS and electronic nose. LWT-Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Rudy, S.; Dziki, D.; Krzykowski, A.; Gawlik-Dziki, U.; Polak, R.; Różyło, R.; Kulig, R. Influence of pre-treatments and freeze-drying temperature on the process kinetics and selected physico-chemical properties of cranberries (Vaccinium macrocarpon Ait.). LWT Food Sci. Technol. 2015, 63, 497–503. [Google Scholar] [CrossRef]
- Izli, N.; Isik, E. Effect of different drying methods on drying characteristics, colour and microstructure properties of mushroom. J. Food Nutr. Res. 2014, 53, 105–116. [Google Scholar]
- Chong, C.H.; Law, C.L.; Figiel, A.; Wojdylo, A.; Oziemblowski, M. Colour, phenolic content and antioxidant capacity of some fruits dehydrated by a combination of different methods. Food Chem. 2013, 141, 3889–3986. [Google Scholar] [CrossRef]
- Guo, L.; Lan, N.; Li, H.; Xiang, P.; Kan, H. Effect of hot air drying temperature on the quality and antioxidant activity of Boletus edulis Bull.: Fr. J. Food Process. Preserv. 2021, 45, 15540. [Google Scholar] [CrossRef]
- Yang, J.H.; Lin, H.C.; Mau, J.L. Non-volatile taste components of several commercial mushrooms. Food Chem. 2001, 72, 464–471. [Google Scholar] [CrossRef]
- Valentao, P.; Lopes, G.; Valente, M.; Barbosa, P.; Andrade, P.B.; Silva, B.M.; Baptista, P.; Seabra, R.M. Quantitation of nine organic acids in wild mushrooms. J. Agric. Food Chem. 2005, 53, 3626–3630. [Google Scholar] [CrossRef]
- Qiu, J.; Vuist, J.E.; Boom, R.M.; Schutyser, M.A.I. Formation and degradation kinetics of organic acids during heating and drying of concentrated tomato juice. LWT Food Sci. Technol. 2018, 87, 112–121. [Google Scholar] [CrossRef]
- Zhao, X.H.; Wei, Y.Y.; Gong, X.; Xu, H.R.; Xin, G. Evaluation of umami taste components of mushroom (Suillus granulatus) of different grades prepared by different drying methods. Food Sci. Hum. Well. 2020, 9, 192–198. [Google Scholar] [CrossRef]

| UA-HAD60 | UA-HAD80 | MD | VMD | HAD60 | HAD80 | VFD | |
|---|---|---|---|---|---|---|---|
| L | 47.92 ± 1.50 c | 45.04 ± 0.78 d | 30.55 ± 0.98 f | 62.04 ± 1.54 b | 47.71 ± 1.34 c | 39.45 ± 1.09 e | 67.09 ± 1.07 a |
| a | 0.74 ± 0.52 b | 0.51 ± 0.56 b | −4.40 ± 0.91 e | −2.56 ± 0.25 c | 2.97 ± 0.62 a | 2.47 ± 0.48 a | −4.49 ± 0.52 e |
| b | 16.65 ± 0.22 b | 17.91 ± 0.36 a | 17.31 ± 1.03 ab | 17.73 ± 0.55 a | 16.70 ± 0.30 b | 17.70 ± 0.44 a | 15.61 ± 0.28 c |
| HAD60 | UA-HAD60 | HAD80 | UA-HAD80 | MD | VMD | VFD | |
|---|---|---|---|---|---|---|---|
| Ala | 2.05 ± 0.04 c | 2.39 ± 0.12 b | 1.38 ± 0.20 d | 1.18 ± 0.11 e | 1.04 ± 0.03 e | 2.83 ± 0.07 a | 1.03 ± 0.11 e |
| Arg | 3.46 ± 0.07 c | 5.13 ± 0.03 a | 1.83 ± 0.06 e | 1.23 ± 0.18 f | 2.46 ± 0.13 d | 4.06 ± 0.09 b | 4.13 ± 0.10 b |
| Glu | 36.88 ± 2.44 a | 32.34 ± 2.66 b | 17.80 ± 0.51 d | 12.78 ± 1.86 e | 20.40 ± 0.78 d | 26.99 ± 2.06 c | 13.64 ± 1.14 e |
| Asp | 0.80 ± 0.03 d | 0.70 ± 0.01 e | 1.09 ± 0.06 b | 0.65 ± 0.04 e | 1.01 ± 0.09 c | 0.62 ± 0.04 e | 1.35 ± 0.04 a |
| His * | 1.82 ± 0.05 c | 2.76 ± 0.03 a | 1.11 ± 0.02 d | 1.19 ± 0.11 d | 1.23 ± 0.10 d | 2.11 ± 0.02 b | 1.85 ± 0.08 c |
| Val * | 2.57 ± 0.12 a | 2.80 ± 0.04 a | 1.86 ± 0.17 b | 1.44 ± 0.42 c | 1.51 ± 0.05 c | 2.12 ± 0.04 b | 1.51 ± 0.09 c |
| Thr * | 0.73 ± 0.02 b | 0.86 ± 0.03 a | 0.07 ± 0.01 e | 0.08 ± 0.01 e | 0.12 ± 0.01 e | 0.67 ± 0.04 c | 0.53 ± 0.05 d |
| Tyr | 1.18 ± 0.06 c | 1.68 ± 0.09 b | 0.84 ± 0.06 d | 0.79 ± 0.25 de | 0.60 ± 0.02 e | 2.31 ± 0.06 a | 2.13 ± 0.11 a |
| Hser | 1.36 ± 0.03 b | 1.72 ± 0.07 a | 1.04 ± 0.03 c | 0.89 ± 0.11 de | 0.84 ± 0.03 e | 1.32 ± 0.07 b | 0.98 ± 0.12 cd |
| GABA | 1.41 ± 0.01 b | 1.64 ± 0.15 a | 0.21 ± 0.00 de | 0.31 ± 0.03 d | 0.21 ± 0.02 de | 1.10 ± 0.08 c | 0.15 ± 0.02 e |
| Pro | 1.60 ± 0.11 a | 1.14 ± 0.02 b | 1.40 ± 0.04 a | 0.83 ± 0.29 c | 0.72 ± 0.04 c | 1.10 ± 0.05 b | 0.88 ± 0.02 c |
| Ile * | 1.87 ± 0.04 ab | 1.87 ± 0.01 ab | 2.29 ± 0.09 a | 1.18 ± 0.85 c | 0.80 ± 0.09 c | 1.37 ± 0.05 bc | 1.06 ± 0.08 c |
| Ser | 1.49 ± 0.04 b | 1.71 ± 0.06 a | 1.37 ± 0.06 c | 1.44 ± 0.06 bc | 1.49 ± 0.03 b | 1.40 ± 0.03 bc | 1.04 ± 0.07 d |
| Leu * | 3.52 ± 0.11 a | 3.49 ± 0.03 a | 2.34 ± 0.05 b | 1.31 ± 0.72 c | 1.12 ± 0.06 c | 3.91 ± 0.21 a | 3.66 ± 0.34 a |
| Lys * | 1.43 ± 0.05 e | 12.81 ± 0.08 a | 1.14 ± 0.02 e | 3.79 ± 0.19 d | 1.21 ± 0.09 e | 8.62 ± 0.43 c | 10.11 ± 0.38 b |
| Gly | 0.38 ± 0.01 a | 0.31 ± 0.02 b | 0.34 ± 0.01 b | 0.32 ± 0.02 b | 0.41 ± 0.01 a | 0.19 ± 0.02 c | 0.14 ± 0.03 d |
| Asn | 1.51 ± 0.02 c | 1.63 ± 0.03 b | 1.01 ± 0.03 e | 0.87 ± 0.09 f | 0.96 ± 0.07 e | 1.78 ± 0.02 a | 1.31 ± 0.05 d |
| Gln | 0.91 ± 0.04 d | 8.81 ± 0.11 a | 0.49 ± 0.06 e | 0.52 ± 0.14 e | 0.23 ± 0.06 e | 5.72 ± 0.25 c | 6.58 ± 0.37 b |
| Umami | 37.68 ± 2.46 a | 33.04 ± 2.67 b | 18.89 ± 0.56 d | 13.44 ± 1.89 e | 21.41 ± 0.87 d | 27.61 ± 2.06 c | 14.98 ± 1.15 e |
| Sweetness | 6.24 ± 0.08 a | 6.40 ± 0.12 a | 4.56 ± 0.25 b | 3.86 ± 0.43 c | 3.78 ± 0.10 c | 6.20 ± 0.02 a | 3.62 ± 0.25 c |
| Bitterness | 13.24 ± 0.07 b | 16.05 ± 0.03 a | 9.43 ± 0.24 c | 6.34 ± 1.91 d | 7.12 ± 0.35 d | 13.57 ± 0.29 b | 12.21 ± 0.62 b |
| Total | 64.98 ± 2.48 b | 83.78 ± 2.27 a | 37.62 ± 0.62 d | 30.80 ± 2.51 e | 36.36 ± 1.46 d | 68.22 ± 2.99 b | 52.08 ± 2.64 c |
| HAD60 | UA-HAD60 | HAD80 | UA-HAD80 | MD | VMD | VFD | |
|---|---|---|---|---|---|---|---|
| Quinic acid | 10.03 ± 0.01 a | 0.42 ± 0.01 e | 1.11 ± 0.01 b | 0.05 ± 0.01 f | 0.03 ± 0.00 f | 0.75 ± 0.03 d | 0.96 ± 0.09 c |
| Succinic anhydride | 4.73 ± 0.05 a | 0.53 ± 0.06 d | 1.09 ± 0.04 c | 0.14 ± 0.00 e | 0.05 ± 0.01 e | 4.49 ± 0.10 b | 4.67 ± 0.07 a |
| Succinic acid | 3.87 ± 0.02 b | 0.37 ± 0.02 cd | 0.69 ± 0.02 c | 0.09 ± 0.00 d | 0.05 ± 0.01 d | 4.37 ± 0.14 a | 3.98 ± 0.48 b |
| Citric acid monohydrate | 3.78 ± 0.04 a | 0.20 ± 0.01 e | 0.38 ± 0.01 c | 0.16 ± 0.00 e | 0.16 ± 0.00 e | 0.30 ± 0.00 d | 0.43 ± 0.06 b |
| Fumaric acid | 0.75 ± 0.00 d | 0.87 ± 0.02 b | 1.79 ± 0.01 a | 0.23 ± 0.03 f | nd | 0.81 ± 0.02 c | 0.68 ± 0.01 e |
| Maleic acid | 0.91 ± 0.00 d | 1.67 ± 0.02 a | 1.19 ± 0.04 b | 0.38 ± 0.03 f | 0.22 ± 0.03 g | 1.02 ± 0.06 c | 0.83 ± 0.03 e |
| Citric acid | 3.01 ± 0.19 a | 1.62 ± 0.02 c | 2.11 ± 0.05 b | 0.42 ± 0.02 e | nd | 0.97 ± 0.01 d | 1.56 ± 0.18 c |
| Pyruvic acid | 3.61 ± 0.01 a | 0.44 ± 0.00 d | 0.75 ± 0.00 b | nd | nd | nd | 0.67 ± 0.02 c |
| Tartaric acid | 4.61 ± 0.01 a | 0.28 ± 0.01 c | 0.64 ± 0.01 b | 0.10 ± 0.00 e | 0.08 ± 0.00 f | 0.10 ± 0.00 e | 0.11 ± 0.00 d |
| Lactic acid | 7.67 ± 0.41 a | 0.63 ± 0.01 c | 1.39 ± 0.01 b | 0.20 ± 0.01 d | 0.14 ± 0.02 d | 0.13 ± 0.02 d | 0.10 ± 0.01 d |
| Total | 42.97 ± 0.42 a | 7.01 ± 0.04 e | 11.14 ± 0.07 d | 1.76 ± 0.03 f | 0.72 ± 0.05 g | 12.93 ± 0.14 c | 13.97 ± 0.24 b |
| HAD60 | UA-HAD60 | HAD80 | UA-HAD80 | MD | VMD | VFD | |
|---|---|---|---|---|---|---|---|
| 5′-AMP | 0.42 ± 0.02 c | 0.65 ± 0.03 b | 0.19 ± 0.00 e | 0.30 ± 0.03 d | 0.33 ± 0.04 d | 0.62 ± 0.03 b | 1.16 ± 0.02 a |
| 5′-GMP | 0.38 ± 0.01 c | 0.56 ± 0.03 ab | 0.24 ± 0.02 d | 0.40 ± 0.03 c | 0.43 ± 0.05 c | 0.59 ± 0.03 a | 0.50 ± 0.04 b |
| 5′-CMP | 0.17 ± 0.01 b | 0.26 ± 0.02 a | 0.12 ± 0.02 c | 0.09 ± 0.01 d | 0.09 ± 0.00 d | 0.24 ± 0.02 a | 0.24 ± 0.01 a |
| 5′-IMP | 0.02 ± 0.00 d | 0.04 ± 0.01 b | 0.01 ± 0.00 e | 0.02 ± 0.00 d | 0.02 ± 0.01 d | 0.03 ± 0.00 c | 0.07 ± 0.01 a |
| 5′-XMP | 0.35 ± 0.02 d | 0.54 ± 0.03 a | 0.25 ± 0.03 e | 0.37 ± 0.03 cd | 0.41 ± 0.04 bc | 0.57 ± 0.03 a | 0.46 ± 0.06 b |
| Flavor 5′-nucleotide | 0.75 ± 0.03 d | 1.13 ± 0.04 a | 0.50 ± 0.06 e | 0.79 ± 0.06 cd | 0.86 ± 0.05 c | 1.19 ± 0.06 a | 1.03 ± 0.09 b |
| Total | 1.33 ± 0.06 c | 2.04 ± 0.08 b | 0.81 ± 0.07 d | 1.18 ± 0.09 c | 1.29 ± 0.09 c | 2.05 ± 0.09 b | 2.44 ± 0.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Q.; He, Z.; Ding, Y.; Sun, L. Effect of Different Drying Methods on the Quality and Nonvolatile Flavor Components of Oudemansiella raphanipes. Foods 2023, 12, 676. https://doi.org/10.3390/foods12030676
Shen Q, He Z, Ding Y, Sun L. Effect of Different Drying Methods on the Quality and Nonvolatile Flavor Components of Oudemansiella raphanipes. Foods. 2023; 12(3):676. https://doi.org/10.3390/foods12030676
Chicago/Turabian StyleShen, Qiulian, Zedong He, Yangyue Ding, and Liping Sun. 2023. "Effect of Different Drying Methods on the Quality and Nonvolatile Flavor Components of Oudemansiella raphanipes" Foods 12, no. 3: 676. https://doi.org/10.3390/foods12030676
APA StyleShen, Q., He, Z., Ding, Y., & Sun, L. (2023). Effect of Different Drying Methods on the Quality and Nonvolatile Flavor Components of Oudemansiella raphanipes. Foods, 12(3), 676. https://doi.org/10.3390/foods12030676