Extraction Optimization and Characterization of Cellulose Nanocrystals from Apricot Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Purification of Cellulose and Cellulose Nanocrystals (CNCs) from AP
2.2.1. Alkaline Process
2.2.2. Bleaching
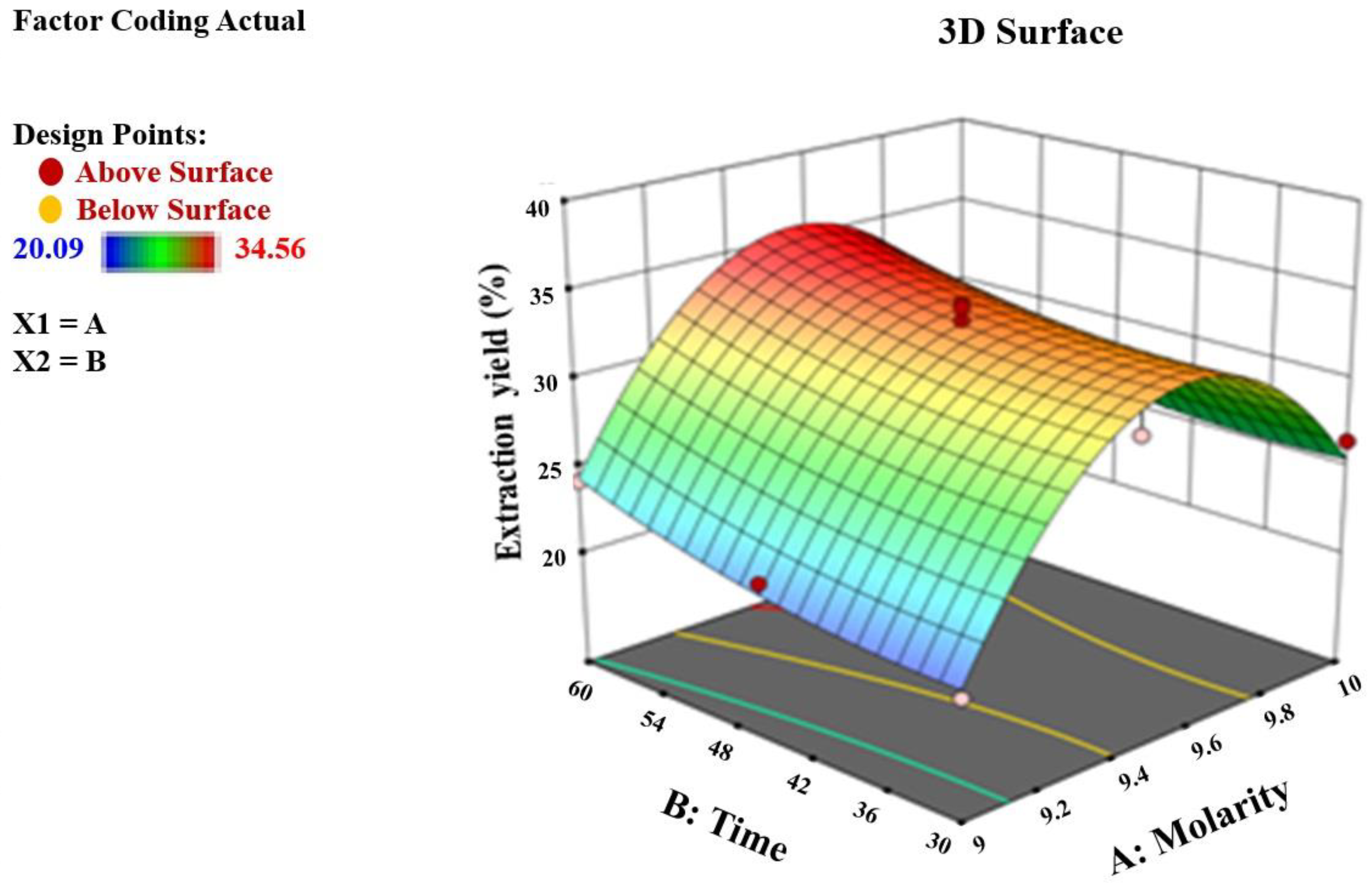
2.2.3. Experimental Design for Acid Hydrolysis
2.2.4. Determination of CNC Extraction Yield
2.3. Characterization of CNC
2.3.1. FTIR
2.3.2. TEM
2.3.3. SEM
2.3.4. TGA
2.3.5. XRD
3. Results and Discussion
3.1. Yield of Cellulose Nanocrystals from Apricot Pomace
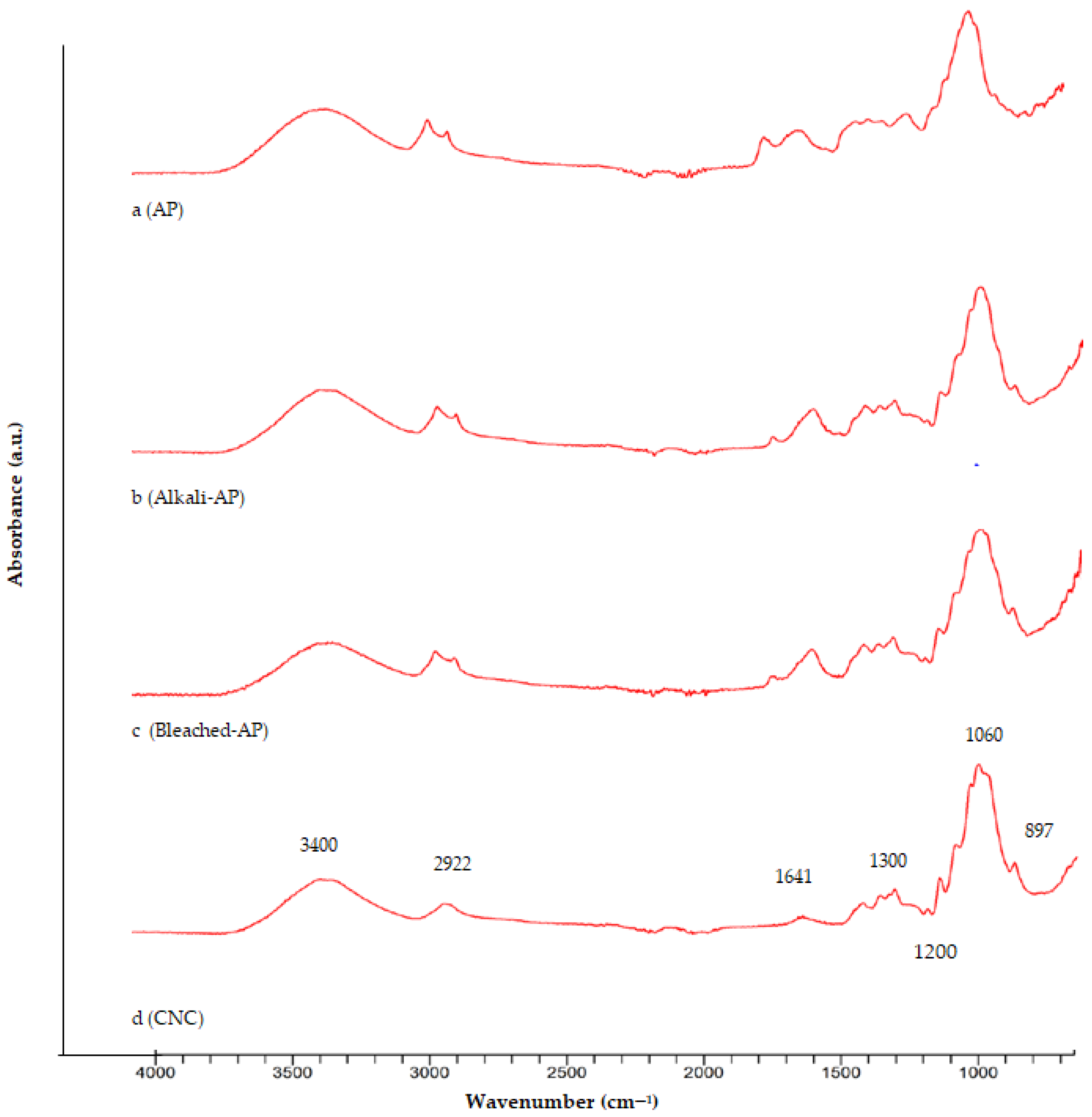
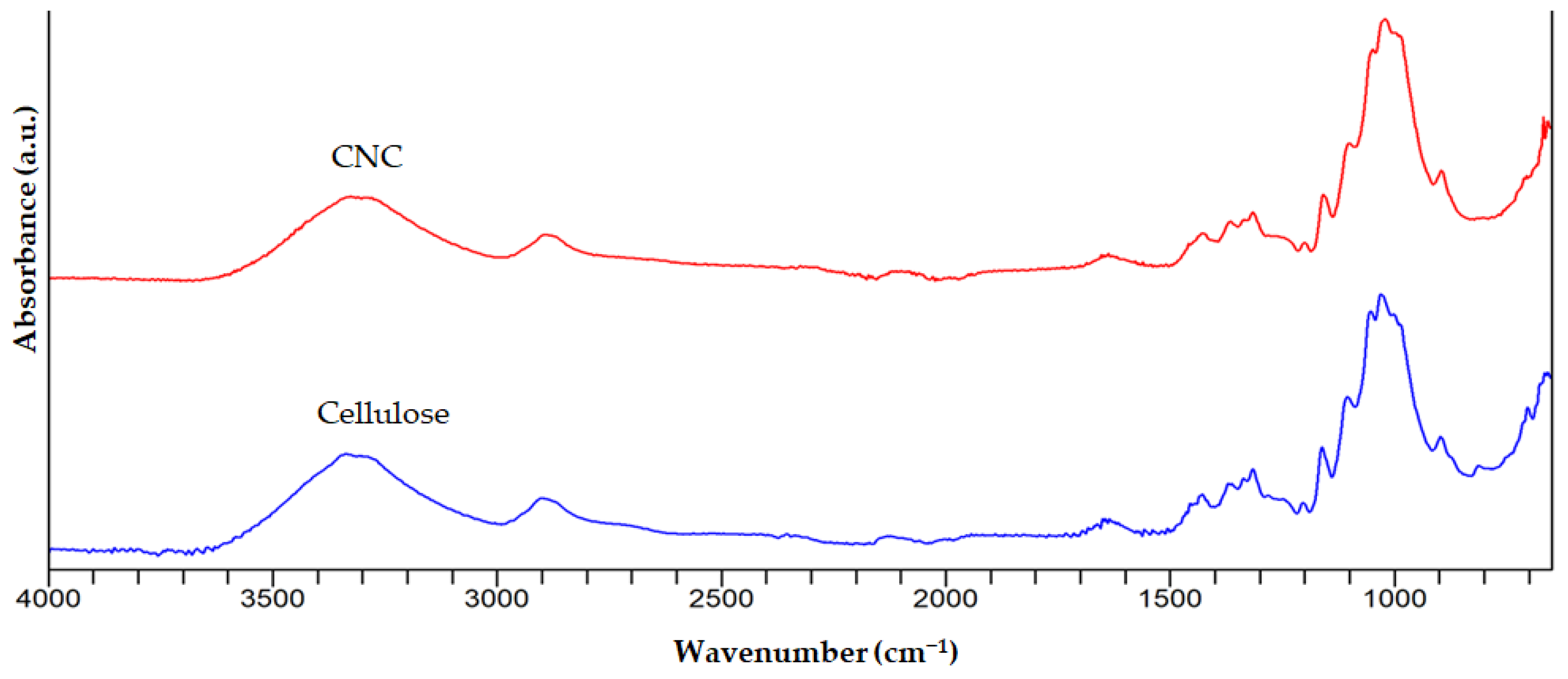
3.2. FTIR
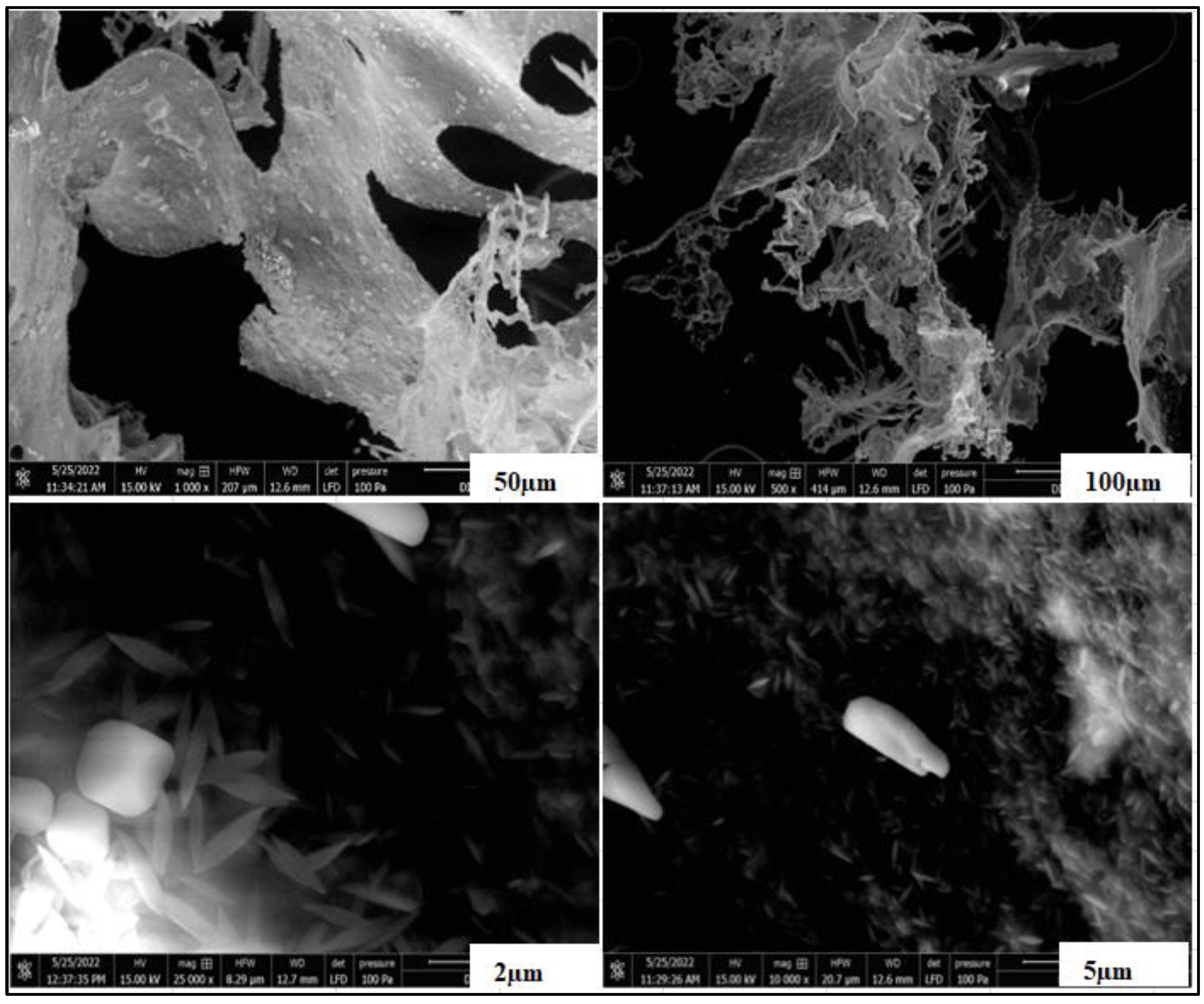
3.3. SEM
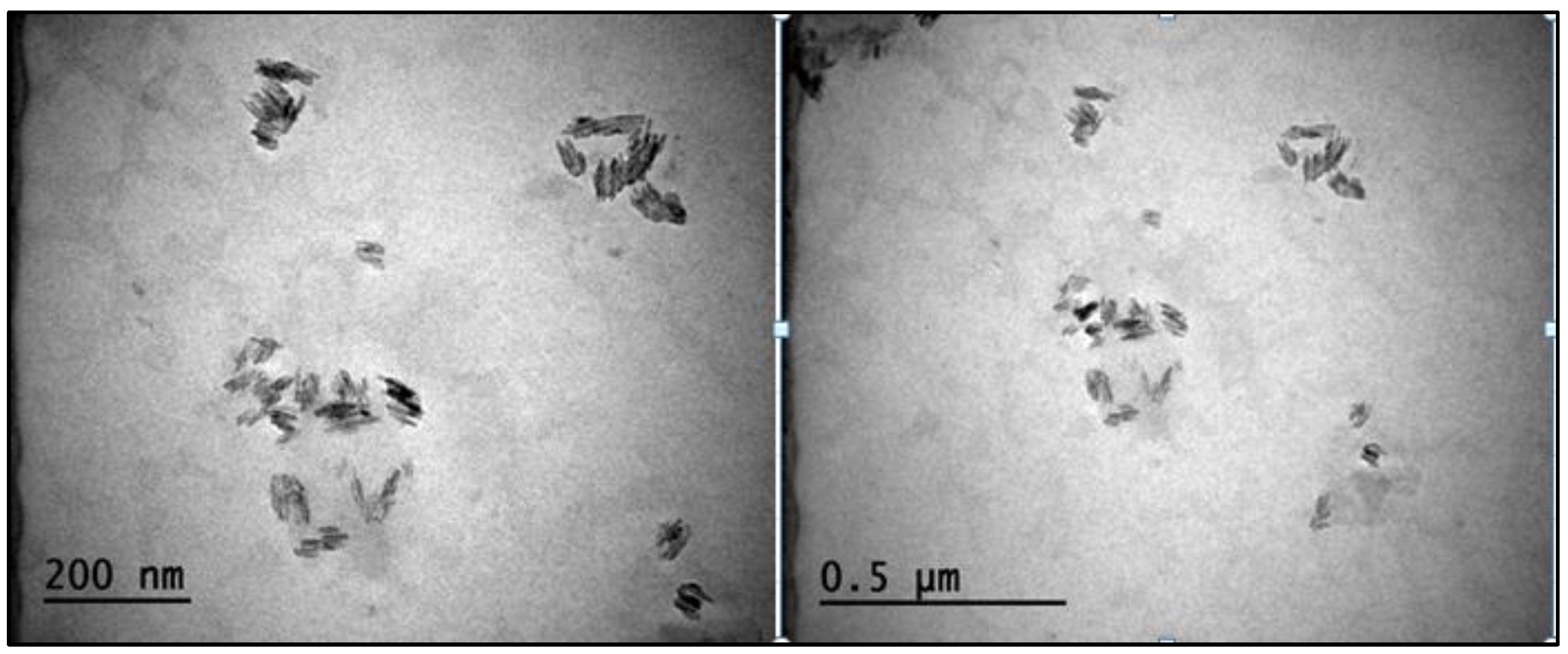
3.4. TEM
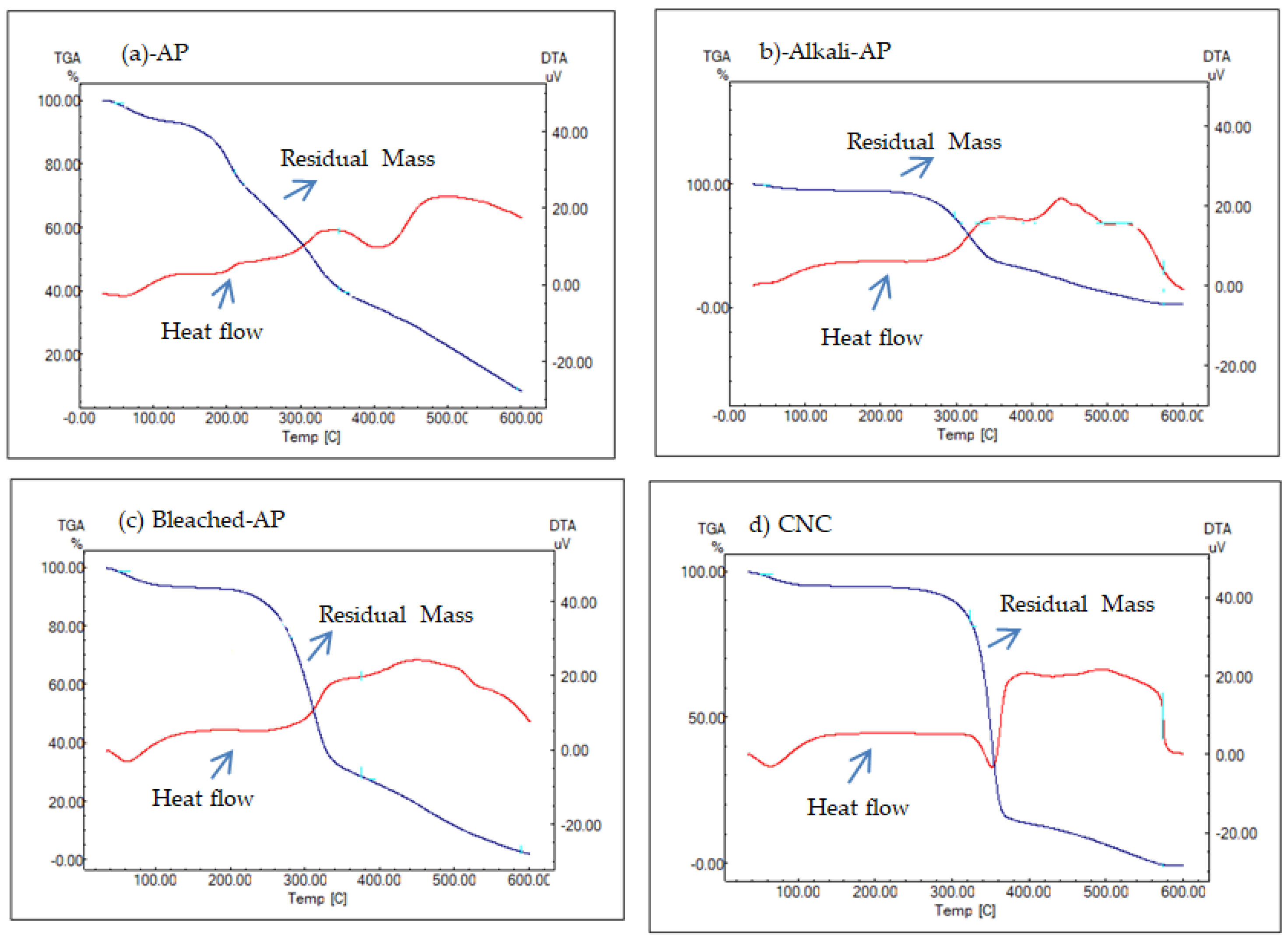
3.5. TGA
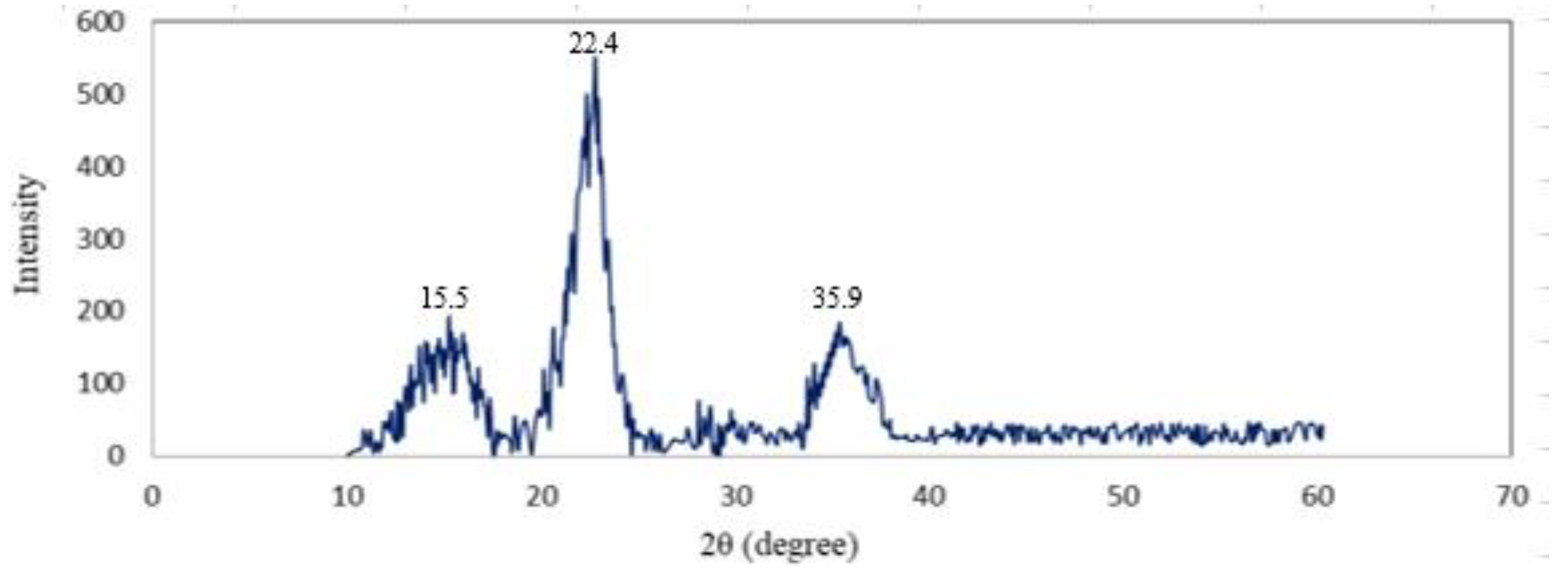
3.6. XRD
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mak, T.M.; Xiong, X.; Tsang, D.C.; Iris, K.M.; Poon, C.S. Sustainable food waste management towards circular bioeconomy: Policy review, limitations and opportunities. Bioresour. Technol. 2020, 297, 122497. [Google Scholar] [CrossRef] [PubMed]
- Akhone, M.A.; Bains, A.; Tosif, M.M.; Chawla, P.; Fogarasi, M.; Fogarasi, S. Apricot Kernel: Bioactivity, Characterization, Applications, and Health Attributes. Foods 2022, 11, 2184. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Kasapoglu, E.D.; Kahraman, S.; Tornuk, F. Optimization of ultrasound assisted antioxidant extraction from apricot pomace using response surface methodology. Food Meas. Charact. 2021, 15, 5277–5287. [Google Scholar] [CrossRef]
- Haciseferogullari, H.; Gezer, I.; Özcan, M.M.; MuratAsma, B. Post-harvest chemical and physical–mechanical properties of some apricot varieties cultivated in Turkey. J. Food Eng. 2007, 79, 364–373. [Google Scholar] [CrossRef]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Tyagi, S.M.; Singh, D. Pectinolytic liquefaction of apricot, plum, and mango pulps for juice extraction. Int. J. Food Prop. 2001, 4, 103–109. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic bionanocomposites: A review of preparation, properties and applications. Polymers. 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Kalia, S.; Dufresne, A.; Cherian, B.M.; Kaith, B.S.; Avérous, L.; Njuguna, J.; Nassiopoulos, E. Cellulose-based bio-and nanocomposites: A review. Int. J. Polym. Sci. 2011, 2011, 1–35. [Google Scholar] [CrossRef]
- de Oliveira Junior, S.D.; Asevedo, E.A.; de Araujo, J.S.; Brito, P.B.; dos Santos Cruz Costa, C.L.; de Macedo, G.R.; dos Santos, E.S. Enzymatic extract of Aspergillus fumigatus CCT 7873 for hydrolysis of sugarcane bagasse and generation of cellulose nanocrystals (CNC). Biomass Convers. Biorefin. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Malainine, M.E.; Dufresne, A.; Dupeyre, D.; Mahrouz, M.; Vuong, R.; Vignon, M.R. Structure and morphology of cladodes and spines of Opuntia ficus-indica. Cellulose extraction and characterisation. Carbohydr. Polym. 2003, 51, 77–83. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose nanocrystals and related nanocomposites: Review of some properties and challenges. J. Polym. Sci. B. Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Silvério, H.A.; Neto, W.P.F.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from corncob for application as reinforcing agent in nanocomposites. Ind. Crops Prod. 2013, 44, 427–436. [Google Scholar] [CrossRef]
- Morais, J.P.S.; de Freitas Rosa, M.; Nascimento, L.D.; do Nascimento, D.M.; Cassales, A.R. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr. Polym. 2013, 91, 229–235. [Google Scholar] [CrossRef]
- Pasquini, D.; de Morais Teixeira, E.; da Silva Curvelo, A.A.; Belgacem, M.N.; Dufresne, A. Extraction of cellulose whiskers from cassava bagasse and their applications as reinforcing agent in natural rubber. Ind. Crops Prod. 2010, 32, 486–490. [Google Scholar] [CrossRef]
- Zuluaga, R.; Putaux, J.L.; Cruz, J.; Vélez, J.; Mondragon, I.; Gañán, P. Cellulose microfibrils from banana rachis: Effect of alkaline treatments on structural and morphological features. Carbohydr. Polym. 2009, 76, 51–59. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose whiskers extracted from mulberry: A novel biomass production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M. Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos. Sci. Technol. 2007, 67, 2521–2527. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Compos. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Structure and properties of high quality natural cellulose fibers from cornstalks. Polymer 2005, 46, 5494–5500. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymer 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Sheltami, R.; Ahmad, I. Acid Hydrolysis Process of Mengkuang Cellulose. Malays. J. Anal. Sci. 2021, 1, 1–12. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ismadji, S. Cellulose nanocrystals from passion fruit peels waste as antibiotic drug carrier. Carbohydr. Polym. 2017, 175, 370–376. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.L. Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef]
- Rhim, J.W.; Reddy, J.P.; Luo, X. Isolation of cellulose nanocrystals from onion skin and their utilization for the preparation of agar-based bio-nanocomposites films. Cellulose 2015, 22, 407–420. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Tang, J.; Xie, K.; Hou, A. Efficient extraction of cellulose nanocrystals from waste Calotropis gigantea fiber by SO42-/TiO2 nano-solid superacid catalyst combined with ball milling exfoliation. Ind. Crops Prod. 2020, 152, 112524. [Google Scholar] [CrossRef]
- Marett, J.; Aning, A.; Foster, E.J. The isolation of cellulose nanocrystals from pistachio shells via acid hydrolysis. Ind. Crops Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Ismadji, S.; Aparamarta, H.W.; Gunawan, S. Optimization of cellulose nanocrystals from bamboo shoots using Response Surface Methodology. Heliyon 2019, 5, e02807. [Google Scholar] [CrossRef]
- Henrique, M.A.; Silvério, H.A.; Neto, W.P.F.; Pasquini, D. Valorization of an agro-industrial waste, mango seed, by the extraction and characterization of its cellulose nanocrystals. J. Environ. Manag. 2013, 121, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef]
- Sheltami, R.M.; Abdullah, I.; Ahmad, I.; Dufresne, A.; Kargarzadeh, H. Extraction of cellulose nanocrystals from mengkuang leaves (Pandanus tectorius). Carbohydr. Polym. 2012, 88, 772–779. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Chen, Y.; Hu, S.; Ren, Z.; Zhu, M. Efficient extraction of cellulose nanocrystals through hydrochloric acid hydrolysis catalyzed by inorganic chlorides under hydrothermal conditions. ACS Sustain. Chem. Eng. 2017, 5, 4656–4664. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Basirun, W.J.; Rahman, N.M.M.A.; Salleh, N.M. The effect of particle size of almond shell powders, temperature and time on the extraction of cellulose. J. Nat. Fibers. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Agustin, M.B.; Ahmmad, B.; De Leon, E.R.P.; Buenaobra, J.L.; Salazar, J.R.; Hirose, F. Starch-based biocomposite films reinforced with cellulose nanocrystals from garlic stalks. Polym. Compos. 2013, 34, 1325–1332. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, M.; Chen, W.C.; van de Ven, T.G.; Moores, A. An improved methodology for imaging cellulose nanocrystals by transmission electron microscopy. Nord. Pulp. Pap. Res. J. 2014, 29, 77–84. [Google Scholar] [CrossRef]
- Coelho, C.C.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue–Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Martínez-Sanz, E.; Ossipov, D.A.; Hilborn, J.; Larsson, S.; Jonsson, K.B.; Varghese, O.P. Bone reservoir: Injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J. Control Release 2011, 152, 232–240. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- de Morais Teixeira, E.; Bondancia, T.J.; Teodoro, K.B.R.; Corrêa, A.C.; Marconcini, J.M.; Mattoso, L.H.C. Sugarcane bagasse whiskers: Extraction and characterizations. Ind. Crops. Prod. 2011, 33, 63–66. [Google Scholar] [CrossRef]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Hou, Q.; Liu, R.; Wu, T.; Si, C. Further characterization of cellulose nanocrystal (CNC) preparation from sulfuric acid hydrolysis of cotton fibers. Cellulose 2016, 23, 439–450. [Google Scholar] [CrossRef]

| Independent Variables | Units | Codes | Coded Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Acid concentration | M | X1 | 9 | 9.5 | 10 |
| Time | Min | X2 | 30 | 45 | 60 |
| Factor 1 | Factor 2 | Response 1 | ||
|---|---|---|---|---|
| Std | Run | Acid Concentration (M) | Time (min) | Extraction Yield (%) |
| 7 | 1 | 9.5 | 30 | 32.09 |
| 11 | 2 | 9.5 | 45 | 33.4 |
| 10 | 3 | 9.5 | 45 | 34.32 |
| 9 | 4 | 9.5 | 45 | 32.55 |
| 2 | 5 | 10 | 30 | 26.5 |
| 5 | 6 | 9 | 45 | 22.1 |
| 3 | 7 | 9 | 60 | 24.15 |
| 12 | 8 | 9.5 | 45 | 34.1 |
| 8 | 9 | 9.5 | 60 | 34.56 |
| 6 | 10 | 10 | 45 | 23.4 |
| 1 | 11 | 9 | 30 | 20.09 |
| 4 | 12 | 10 | 60 | 29.2 |
| Source | CNC Yield (%) | |||||
|---|---|---|---|---|---|---|
| SS | DF | MS | F-Value | p-Value | ||
| Model | 301.81 | 5 | 60.36 | 28.07 | 0.0004 | Significant |
| Residual | 12.9 | 6 | 2.15 | |||
| Lack of Fit | 10.99 | 3 | 3.66 | 5.75 | 0.0923 | Not significant |
| Pure Error | 1.91 | 3 | 0.6369 | |||
| Core Total | 314.71 | 11 | ||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| X1-Acid concentration | 27.14 | 1 | 27.14 | 12.62 | 0.012 |
| X2-Time | 14.2 | 1 | 14.2 | 6.6 | 0.0424 |
| X1 X2 | 0.4624 | 1 | 0.4624 | 0.215 | 0.6592 |
| X12 | 245.31 | 1 | 245.31 | 114.07 | <0.0001 |
| X22 | 2.58 | 1 | 2.58 | 1.2 | 0.3153 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinçel Kasapoğlu, E.; Kahraman, S.; Tornuk, F. Extraction Optimization and Characterization of Cellulose Nanocrystals from Apricot Pomace. Foods 2023, 12, 746. https://doi.org/10.3390/foods12040746
Dinçel Kasapoğlu E, Kahraman S, Tornuk F. Extraction Optimization and Characterization of Cellulose Nanocrystals from Apricot Pomace. Foods. 2023; 12(4):746. https://doi.org/10.3390/foods12040746
Chicago/Turabian StyleDinçel Kasapoğlu, Ekin, Sibel Kahraman, and Fatih Tornuk. 2023. "Extraction Optimization and Characterization of Cellulose Nanocrystals from Apricot Pomace" Foods 12, no. 4: 746. https://doi.org/10.3390/foods12040746






