Waste Orange Peels as a Feed Additive for the Enhancement of the Nutritional Value of Tenebrio molitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Insects
2.3. Feeding Trial
2.4. Larval Composition Analysis
2.4.1. Water Content Calculation
2.4.2. Crude Protein Content
2.4.3. Carbohydrates
2.4.4. Ash
2.4.5. Total Fat, Fatty Acids, and Calculated Oxidizability Value (COX)
2.4.6. β-Carotene–Vitamin A
2.4.7. Vitamin C
2.4.8. Total Polyphenol Content (TPC) Determination
2.4.9. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.5. Statistical Analysis
3. Results and Discussion
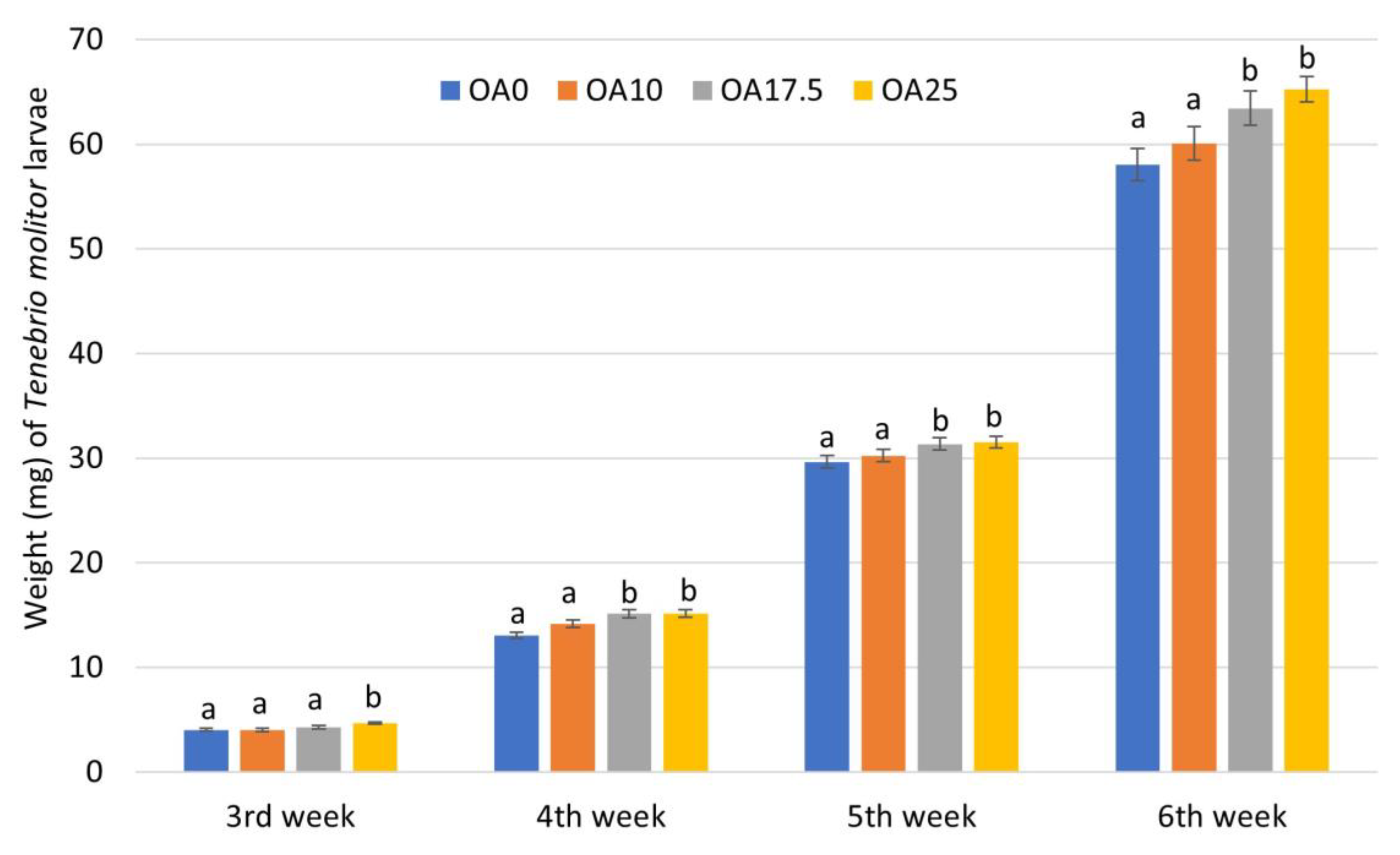
3.1. Survival and Growth of T. molitor Larvae
3.2. Evaluation of the Nutritional Value of the Larvae
3.2.1. Proximate Composition
3.2.2. Content of T. molitor Larvae in Vitamins A and C
3.2.3. Antioxidant Activity of the Larvae Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.M.; Fischer, A.R.H.; van Boekel, M.A.J.S.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Grasty, S.; FAO. Reducing Enteric Methane and Livelihoods Win-Win Opportunities for Farmers; FAO: Rome, Italy, 1999; Volume 14, ISBN 9789251079201. [Google Scholar]
- Patel, S.; Suleria, H.A.R.; Rauf, A. Edible insects as innovative foods: Nutritional and functional assessments. Trends Food Sci. Technol. 2019, 86, 352–359. [Google Scholar] [CrossRef]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-efficient food production to reduce global warming and ecodegradation: The use of edible insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a source of interesting natural compounds, their recovery processes, biological effects, and safety aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef] [PubMed]
- European Union Commission. Commission Implementing Regulation (EU) 2021/882 Authorising the Placing on the Market of Dried Tenebrio molitor Larva as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470 (Text with EEA Relevance); European Union Commission: Brussels, Belgium, 2021. [Google Scholar]
- Melo, V.; Garcia, M.; Sandoval, H.; Jiménez, H.D.; Calvo, C. Quality proteins from edible indigenous insect food of latin America and Asia. Emirates J. Food Agric. 2011, 23, 283–289. [Google Scholar]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 1–14. [Google Scholar] [CrossRef]
- Kröncke, N.; Benning, R. Self-Selection of Feeding Substrates by Tenebrio molitor Larvae of Different Ages to Determine Optimal Macronutrient Intake and the Influence on Larval Growth and Protein Content. Insects 2022, 13, 657. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Finke, M.D. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed. 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Punzo, F.; Mutchmor, J.A. Effects of Temperature, Relative Humidity and Period of Exposure on the Survival Capacity of Tenebrio molitor (Coleoptera: Tenebrionidae). Survival 1980, 53, 260–270. [Google Scholar]
- Cotton, R.T.; Ashby, W. Insect pests of stored grains and seed. In Insects: The Yearbook of Agriculture; US Gov. Printing Office: Washington, DC, USA, 1952; pp. 629–639. [Google Scholar]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Van Peer, M.; Frooninckx, L.; Coudron, C.; Berrens, S.; Álvarez, C.; Deruytter, D.; Verheyen, G.; Van Miert, S. Valorisation potential of using organic side streams as feed for Tenebrio molitor, Acheta domesticus and Locusta migratoria. Insects 2021, 12, 796. [Google Scholar] [CrossRef] [PubMed]
- Pfaltzgraff, L.A.; De Bruyn, M.; Cooper, E.C.; Budarin, V.; Clark, J.H. Food waste biomass: A resource for high-value chemicals. Green Chem. 2013, 15, 307–314. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ramos, J.A.; Rojas, M.G.; Kelstrup, H.C.; Emery, V. Self-selection of agricultural by-products and food ingredients by Tenebrio molitor (Coleoptera: Tenebrionidae) and impact on food utilization and nutrient intake. Insects 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Rumbos, C.I.; Bliamplias, D.; Gourgouta, M.; Michail, V.; Athanassiou, C.G. Rearing Tenebrio molitor and Alphitobius diaperinus Larvae on Seed Cleaning Process Byproducts. Insects 2021, 12, 293. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.J.; Hwang, I.K.; Nho, C.W.; Kim, S.M.; Kim, S.H. Determination of carbohydrate composition in mealworm (Tenebrio molitor L.) larvae and characterization of mealworm chitin and chitosan. Foods 2021, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- European Union Commission. Commission Regulation (EC) No 796/2002 of 6 May 2002 amending Regulation (EEC) No 2568/91 on the Characteristics of Olive Oil and Olive-Pomace Oil and on the Relevant Methods of Analysis and the Additional Notes in the Annex to Council Regulation (EEC) No 2658/87 on the Tariff and Statistical Nomenclature and on the Common Customs Tariff; European Union Commission: Brussels, Belgium, 2002. [Google Scholar]
- Lalas, S.; Gortzi, O.; Athanasiadis, V.; Dourtoglou, E.; Dourtoglou, V. Full Characterisation of Crambe abyssinica Hochst. Seed Oil. J. Am. Oil Chem. Soc. 2012, 89, 2253–2258. [Google Scholar] [CrossRef]
- Ourailoglou, D.; Athanasiadis, V.; Bozinou, E.; Salakidou, C.; Evmorfopoulos, E.; Lalas, S. Manufacturing Process and Physicochemical Analysis of Kariki: A Traditional Cheese from The Island of Tinos, Greece. Int. Food Res. J. 2021, 28, 262–268. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Hammond, E.G. Analysis of oleate, linoleate and linolenate hydroperoxides in oxidized ester mixtures. Lipids 1980, 15, 379–385. [Google Scholar] [CrossRef]
- Ocampo, E.T.M.; Libron, J.A.M.A.; Guevarra, M.L.D.; Mateo, J.M.C. Phytochemical screening, phenolic acid profiling and antioxidant activity analysis of peels from selected mango (Mangifera spp.) genotypes in the Philippines. Food Res. 2020, 4, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Palaiogiannis, D.; Poulianiti, K.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Extraction of Polyphenolic Antioxidants from Red Grape Pomace and Olive Leaves: Process Optimization Using a Tailor-Made Tertiary Deep Eutectic Solvent. Sustainability 2022, 14, 6864. [Google Scholar] [CrossRef]
- LeCato, G.; Flaherty, B. Description of eggs of selected species of stored-product insects (Coleoptera and Lepidoptera). J. Kansas Entomol. Soc. 1974, 47, 308–317. [Google Scholar]
- Abdelatti, Z.A.S.; Hartbauer, M. Plant oil mixtures as a novel botanical pesticide to control gregarious locusts. J. Pest Sci. (2004) 2020, 93, 341–353. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Zhao, C.; Tian, G.; Zhang, H.; Xiao, H.; He, L.; Zheng, J. Chemical Mapping of Essential Oils, Flavonoids and Carotenoids in Citrus Peels by Raman Microscopy. J. Food Sci. 2017, 82, 2840–2846. [Google Scholar] [CrossRef]
- Hazarika, U.; Gosztola, B. Lyophilization and its Effects on the Essential Oil Content and Composition of Herbs and Spices—A Review. Acta Sci. Pol. Technol. Aliment. 2020, 19, 467–473. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J. Bioconversion Potential of Agro-Industrial Byproducts by Tenebrio molitor—Long-Term Results. Insects 2022, 13, 810. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Costa, S.; Pedro, S.; Lourenço, H.; Batista, I.; Teixeira, B.; Bandarra, N.M.; Murta, D.; Nunes, R.; Pires, C. Evaluation of Tenebrio molitor larvae as an alternative food source. NFS J. 2020, 21, 57–64. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.E.; Alkoaik, F.N. The yellow mealworm as a novel source of protein. Am. J. Agric. Biol. Sci. 2009, 4, 319–331. [Google Scholar] [CrossRef]
- Ben-Shalom, N.; Pinto, R.; Berman, M. Polysaccharides and proteins in the orange albedo and in the aqueous extract of the tissue. Can. Inst. Food Sci. Technol. J. 1985, 18, 335–336. [Google Scholar] [CrossRef]
- Yu, X.; He, Q.; Wang, D. Dynamic Analysis of Major Components in the Different Developmental Stages of Tenebrio molitor. Front. Nutr. 2021, 8, 689746. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jędras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. Agric. Sci. 2013, 04, 287–291. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods- a review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Baioumy, A.A.; Abedelmaksoud, T.G. Quality properties and storage stability of beef burger as influenced by addition of orange peels (albedo). Theory Pract. Meat Process. 2021, 6, 33–38. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.-H.; Choi, W.-H.; Hong, S.-J.; Kim, N.-J. Nutritional Value of Mealworm, Tenebrio molitor as Food Source. Int. J. Ind. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Ghosh, S.; Lee, S.M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia. Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Poelaert, C.; Ernens, M.; Liotta, M.; Blecker, C.; Danthine, S.; Tyteca, E.; Haubruge, É.; Alabi, T.; Bindelle, J.; et al. Effect of household cooking techniques on the microbiological load and the nutritional quality of mealworms (Tenebrio molitor L. 1758). Food Res. Int. 2018, 106, 503–508. [Google Scholar] [CrossRef]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- Burr, M.; Miller, S. Fatty Acids in Nutrition. J. Biol 1932, 97, 1–9. [Google Scholar]
- Nutter, M.K.; Lockhart, E.E.; Harris, R.S. The chemical composition of depot fats in chickens and turkeys. Oil Soap 1943, 20, 231–234. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Huth, E.J. A Library for Internists VI Recommended by the American College of Physicians. Ann. Intern. Med. 1988, 108, 497–498. [Google Scholar] [CrossRef]
- Solomons, N.W.; Orozco, M. Alleviation of vitamin A deficiency with palm fruit and its products. Asia Pac. J. Clin. Nutr. 2003, 12, 373–384. [Google Scholar] [PubMed]
- Sommer, A. Vitamin A deficiency and clinical disease: An historical overview. J. Nutr. 2008, 138, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yi, P.; Wang, X.; Zhang, B.; Jie, Z.; Soong, L.; Sun, J. Retinoic Acid Modulates Hyperactive T Cell Responses and Protects Vitamin A–Deficient Mice against Persistent Lymphocytic Choriomeningitis Virus Infection. J. Immunol. 2020, 204, 2984–2994. [Google Scholar] [CrossRef]
- Ross, S.A.; McCaffery, P.J.; Drager, U.C.; De Luca, L.M. Retinoids in embryonal development. Physiol. Rev. 2000, 80, 1021–1054. [Google Scholar] [CrossRef]
- Bastos Maia, S.; Rolland Souza, A.S.; Costa Caminha, M.d.F.; Lins da Silva, S.; Callou Cruz, R.d.S.B.L.; Carvalho dos Santos, C.; Batista Filho, M. Vitamin A and Pregnancy: A Narrative Review. Nutrients 2019, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; Van Der Poel, A.F.B. Effects of diet on the chemical composition of migratory locusts (Locusta migratoria). Zoo Biol. 2011, 30, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Finke, M.D. Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, E.; Panteli, N.; Feidantsis, K.; Mastoraki, M.; Koutsogeorgiou, E.; Grivaki, E.; Papagrigoriou, T.; Christias, S.; Chatzifotis, S.; Lazari, D.; et al. Carob (Ceratonia siliqua) as Functional Feed Is Beneficial in Yellow Mealworm (Tenebrio molitor) Rearing: Evidence from Growth, Antioxidant Status and Cellular Responses. Antioxidants 2022, 11, 1840. [Google Scholar] [CrossRef]
- Keil, C.; Grebenteuch, S.; Kröncke, N.; Kulow, F.; Pfeif, S.; Kanzler, C.; Rohn, S.; Boeck, G.; Benning, R.; Haase, H. Systematic Studies on the Antioxidant Capacity and Volatile Compound Profile of Yellow Mealworm Larvae (Tenebrio molitor L.) under Different Drying Regimes. Insects 2022, 13, 166. [Google Scholar] [CrossRef]
- Sánchez-Muros, M.J.; de Haro, C.; Sanz, A.; Trenzado, C.E.; Villareces, S.; Barroso, F.G. Nutritional evaluation of Tenebrio molitor meal as fishmeal substitute for tilapia (Oreochromis niloticus) diet. Aquac. Nutr. 2016, 22, 943–955. [Google Scholar] [CrossRef]
| Diets | Survival (%) | |||
|---|---|---|---|---|
| Third Week | Fourth Week | Fifth Week | Sixth Week | |
| OA0 | 86.64 ± 2.15 | 86.36 ± 2.96 | 82.24 ± 2.50 | 81.16 ± 2.79 |
| OA10 | 84.48 ± 2.02 | 81.44 ± 2.56 | 80.80 ± 2.94 | 79.88 ± 2.14 |
| OA17.5 | 85.16 ± 2.23 | 83.84 ± 2.21 | 82.44 ± 2.76 | 82.16 ± 2.21 |
| OA25 | 94.32 ± 3.68 | 90.84 ± 4.21 | 88.60 ± 2.14 | 88.04 ± 2.98 |
| % Composition of Dry Weight | OA0 | OA10 | OA17.5 | OA25 |
|---|---|---|---|---|
| Crude protein | 32.25 ± 0.94 a,* | 36.5 ± 1.28 b | 40.18 ± 1.65 c | 44.2 ± 2.08 d |
| Crude fat | 42.16 ± 1.12 a | 41.97 ± 1.98 a | 41.12 ± 1.03 a | 38.84 ± 0.81 b |
| Carbohydrates | 22.8 ± 0.89 a | 19.28 ± 0.75 b | 15.91 ± 0.46 c | 15.67 ± 0.56 c |
| Crude ash | 1.06 ± 0.03 a | 1.17 ± 0.03 b | 1.29 ± 0.05 c | 1.34 ± 0.06 c |
| Energy (kcal/100 g) | 599.64 ± 23.07 a | 600.85 ± 22.58 a | 594.44 ± 24.45 a | 590.24 ± 15.04 a |
| Fatty Acid (%) | Diets | |||
|---|---|---|---|---|
| OA0 | OA10 | OA17.5 | OA25 | |
| C12:0 | 0.16 ± 0.01 b,c,* | 0.15 ± 0.01 c | 0.19 ± 0.01 a | 0.17 ± 0.01 b |
| C14:0 | 2.32 ± 0.11 b | 2.71 ± 0.08 a | 2.68 ± 0.06 a | 2.70 ± 0.06 a |
| C16:0 | 21.39 ± 0.98 a | 23.27 ± 1.16 a | 23.03 ± 0.71 a | 23.22 ± 1.46 a |
| C16:1 | 0.12 ± 0.01 a,b | 0.10 ± 0.01 c | 0.11 ± 0.01 b,c | 0.12 ± 0.01 a |
| C18:0 | 0.13 ± 0.01 d | 0.17 ± 0.01 c | 0.19 ± 0.01 b | 0.20 ± 0.01 a |
| C18:1 | 39.07 ± 2.89 b | 44.1 ± 2.82 a | 43.79 ± 0.18 a | 43.68 ± 2.10 a |
| C18:2 (ω-6) | 27.34 ± 0.57 b | 29.28 ± 0.73 a | 29.84 ± 1.1 a | 29.68 ± 0.65 a |
| C20:0 | 1.36 ± 0.05 | nd ** | nd | nd |
| C18:3 (ω-3) | 0.08 ± 0.01 b | 0.11 ± 0.01 a | 0.09 ± 0.01 b | 0.11 ± 0.01 a |
| C22:0 | 6.04 ± 0.31 a | 0.03 ± 0.01 b | 0.02 ± 0.01 b | 0.03 ± 0.01 b |
| C22:1 | 1.82 ± 0.13 a | 0.09 ± 0.01 b | 0.07 ± 0.01 b | 0.08 ± 0.01 b |
| C24:0 | 0.16 ± 0.01 | nd | nd | nd |
| ∑ SFA 1 | 31.57 ± 1.48 a | 26.32 ± 1.26 b | 26.11 ± 0.78 b | 26.32 ± 1.54 b |
| ∑ MUFA 2 | 41.01 ± 3.02 a | 44.29 ± 2.83 a | 43.97 ± 0.18 a | 43.88 ± 2.11 a |
| ∑ PUFA 3 | 27.42 ± 0.58 b | 29.39 ± 0.74 a | 29.93 ± 1.11 a | 29.79 ± 0.66 a |
| PUFA: SFA ratio | 0.87 ± 0.02 b | 1.12 ± 0.03 a | 1.15 ± 0.01 a | 1.13 ± 0.04 a |
| MUFA: PUFA ratio | 1.49 ± 0.08 a | 1.51 ± 0.06 a | 1.47 ± 0.05 a | 1.47 ± 0.04 a |
| ω-6: ω-3 ratio | 331.29 ± 6.96 a | 261.62 ± 8.92 b | 344.68 ± 9.69 a | 277.82 ± 3.62 b |
| COX 4 | 3.22 ± 0.09 b | 3.48 ± 0.11 a | 3.53 ± 0.12 a | 3.52 ± 0.09 a |
| Diets | β-Carotene (µg/g) | Vitamin A (μg RAE/100 g) | Vitamin C (μg/g) |
|---|---|---|---|
| OA0 | 3.83 ± 0.06 a,* | 11.41 ± 0.19 a | 200.37 ± 6.44 a |
| OA10 | 7.10 ± 0.29 b | 21.17 ± 0.86 b | 251.44 ± 4.63 b |
| OA17.5 | 9.45 ± 0.01 c | 28.19 ± 0.04 c | 282.50 ± 2.88 c |
| OA25 | 11.43 ± 0.1 d | 34.08 ± 0.29 d | 292.90 ± 1.42 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Adamaki-Sotiraki, C.; Rumbos, C.I.; Athanassiou, C.G.; Lalas, S.I. Waste Orange Peels as a Feed Additive for the Enhancement of the Nutritional Value of Tenebrio molitor. Foods 2023, 12, 783. https://doi.org/10.3390/foods12040783
Kotsou K, Chatzimitakos T, Athanasiadis V, Bozinou E, Adamaki-Sotiraki C, Rumbos CI, Athanassiou CG, Lalas SI. Waste Orange Peels as a Feed Additive for the Enhancement of the Nutritional Value of Tenebrio molitor. Foods. 2023; 12(4):783. https://doi.org/10.3390/foods12040783
Chicago/Turabian StyleKotsou, Konstantina, Theodoros Chatzimitakos, Vassilis Athanasiadis, Eleni Bozinou, Christina Adamaki-Sotiraki, Christos I. Rumbos, Christos G. Athanassiou, and Stavros I. Lalas. 2023. "Waste Orange Peels as a Feed Additive for the Enhancement of the Nutritional Value of Tenebrio molitor" Foods 12, no. 4: 783. https://doi.org/10.3390/foods12040783
APA StyleKotsou, K., Chatzimitakos, T., Athanasiadis, V., Bozinou, E., Adamaki-Sotiraki, C., Rumbos, C. I., Athanassiou, C. G., & Lalas, S. I. (2023). Waste Orange Peels as a Feed Additive for the Enhancement of the Nutritional Value of Tenebrio molitor. Foods, 12(4), 783. https://doi.org/10.3390/foods12040783













