Effect of Deep Dormancy Temperature Cultivation on Meat Quality of Crucian Carp (Carassius auratus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of DDT of Crucian Carp
2.3. Determination of Survival Time
2.4. Cooling Procedure
2.5. Determination of Blood Glucose of Crucian Carp
2.6. Determination of Blood Lactic Acid of Crucian Carp
2.7. Determination of pH
2.8. Determination of Water-Holding Capacity (WHC)
2.9. Determination of Cooking Loss
2.10. Determination of Color Difference
2.11. Determination of Muscle Fiber Diameter
2.12. Determination of Texture Profiles of Crucian Carp
2.13. Determination of Nucleotides
2.14. Sensory Evaluation
2.15. Data Analysis
3. Results and Discussion
3.1. DDT and Temporary Rearing Conditions of Crucian Carp
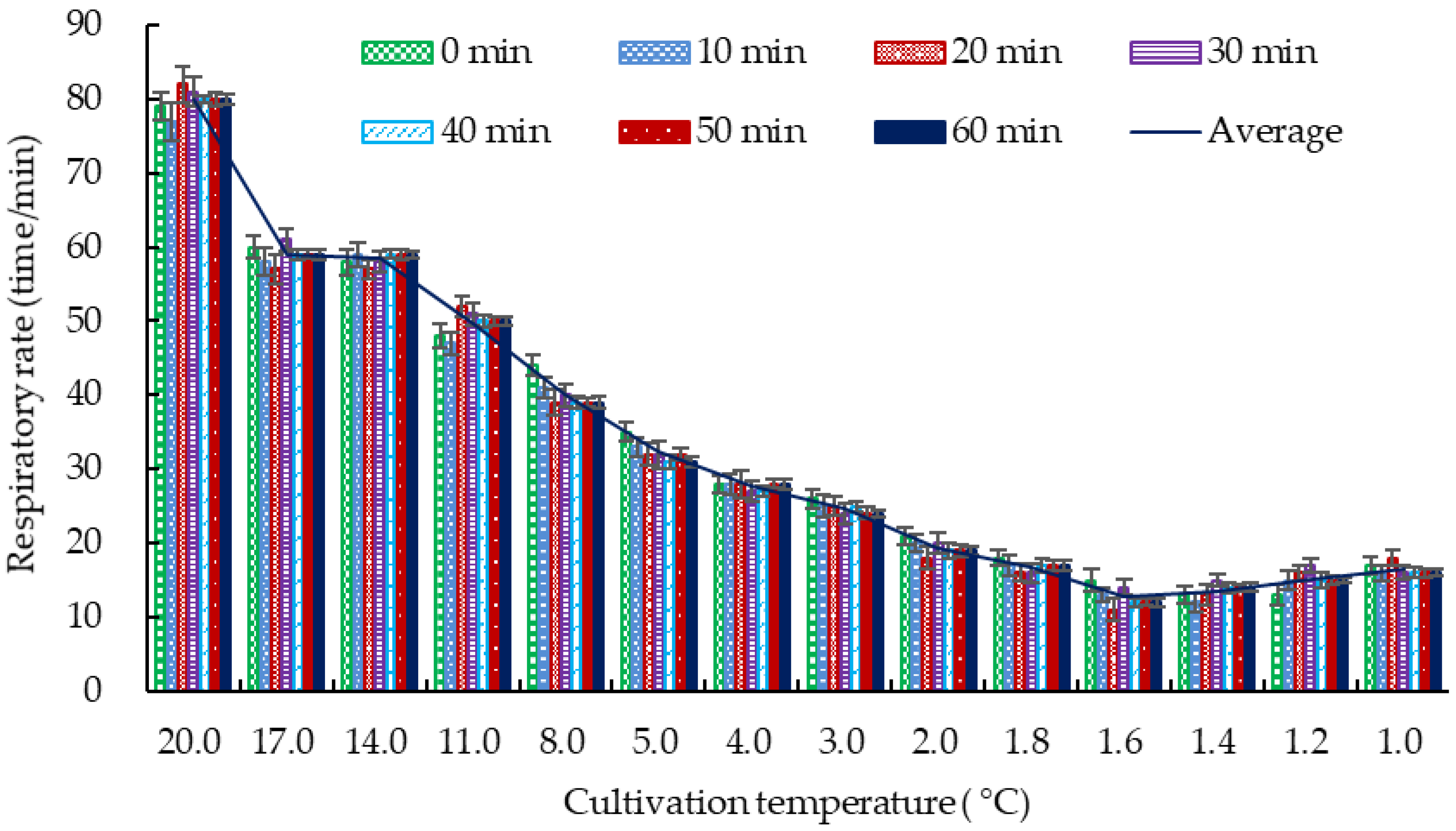
3.1.1. DDT of Crucian Carp
3.1.2. The Survival Time of Crucian Carp at Different Temperatures
3.1.3. Effects of Different Cooling Rates on Survival Time of Crucian Carp
3.2. Effects of Cultivation at DDT on the Edible Quality of Crucian Carp
3.2.1. The pH, WHC, and Cooking Loss of Crucian Carp Meat
3.2.2. The Color of Crucian Carp Meat
3.2.3. Texture Profile of Crucian Carp Meat
3.2.4. Umami-Enhancing Nucleotide Content of Crucian Carp Meat
3.2.5. Sensory Evaluation of Crucian Carp Meat
3.3. Effects of Cultivation at DDT on the Physiology and Biochemistry of Crucian Carp
3.3.1. Blood Glucose Content of Crucian Carp
3.3.2. Lactic Acid Content of Crucian Carp
3.3.3. Muscle Fiber Diameter of Crucian Carp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.P.; Mahanty, A.; Ganguly, S.; Mitra, T.; Karunakaran, D.; Anandan, R. Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem. 2019, 293, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Li, H.; Peng, H.; Qian, Q.; Zhang, Y.; Jianlin, J.; Zhang, P.; Wang, L.; Zhang, Y. Research progress of fish conservation transportation technology. Sichuan Agric. Sci. Technol. 2021, 12, 62–67. [Google Scholar]
- Fisheries Administration of Ministry of Agriculture and Rural Affairs of China. China Fishery Statistics Yearbook (2021); China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Dobíková, R.; Svobodová, Z.; Bláhová, J.; Modrá, H.; Velíek, J. The effect of transport on biochemical and haematological indices of common carp (Cyprinus carpio L.). Czech J. Anim. Sci. 2009, 54, 510–518. [Google Scholar] [CrossRef]
- Mi, H.; Qian, C.; Mao, L. Quality and biochemical properties of artificially hibernated crucian carp for waterless preservation. Fish Physiol. Biochem. 2012, 38, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Ye, C.X.; Guo, Z.X.; Wang, A.L. Immune and physiological responses of pufferfish (Takifugu obscurus) under cold stress. Fish Shellfish Immunol. 2017, 64, 137–145. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, Y.; Adányi, N.; Zhang, X. Effects of waterless live transportation on survivability, physiological responses and flesh quality in Chinese farmed sturgeon (Acipenser schrenckii). Aquaculture 2020, 518, 734834. [Google Scholar] [CrossRef]
- He, R.; Su, Y.; Wang, A.; Lei, B.; Cui, K. Survival and serum biochemical responses of spotted sea bass Lateolabrax maculatus during simulated waterless live transportation. Aquac. Res. 2020, 51, 3495–3505. [Google Scholar] [CrossRef]
- Nie, X.; Lei, J.; Chen, S.; Zhang, Y.; Hong, W. Physiological, proteomic, and gene expression analysis of turbot (Scophthalmus maximus) in response to cold acclimation. Aquaculture 2018, 495, 281–287. [Google Scholar] [CrossRef]
- Yang, F.; Wu, H.; Wang, Y.; Li, N.; Shi, W.; Wang, J. Experiment of Macrobrachium rosenbergii in water preservation at low temperature. J. Hunan Agric. Univ. Nat. Sci. Ed. 2020, 46, 7–9. [Google Scholar]
- Shan, H. Carp pond culture technology. Friends Farmers Get. Rich 2017, 3, 54. [Google Scholar]
- National Bureau of Statistics of China. China Statistical Yearbook 2021; China Statistics Press: Beijing, China, 2021.
- Zeng, P.; Shen, J.; Chen, T. Effects of dormancy mode, temperature and time on physiological indexes of Crucian carp stored out of water. J. Aquat. Sci. 2019, 38, 492–497. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Li, H.; Bai, T.; Qian, Q.; Peng, H.; Mu, Y.; Wang, L.; Liu, B.; Chen, J. Effect of 4 °C and ice temperature on umami-enhancing nucleotides of conditioned pork. Food Chem. 2023, 401, 134146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Zhang, Y.; Wang, L.; Zhang, P.; Jia, J.; Peng, H.; Qian, Q.; Zhang, J.; Pan, Z. Storage Stability and Flavor Change of Marinated Pork. Foods 2022, 11, 1825. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, T.; Shen, J. Effects of short-term continuous cooling on some physiological indexes of juvenile crucian carp. J. Fish. 2015, 28, 1–6. [Google Scholar]
- Ha, H.J.; Min, J.; Lee, J.D.; Goo, J.S.; Patk, T.I. Optimal Conditions for Long-distance Transportation of Live Black Rockfish (Sebastes schlegeli) and Changes in their Characteristics during Transport. J. Aquat. Food Prod. Technol. 2019, 28, 762–771. [Google Scholar] [CrossRef]
- Annamalai, J.; Lakshmi, N.M.; Sivam, V.; Rao, N.C.R. A Comparative Study on the Quality Changes of Croaker (Johnius dussumieri) Fish Stored in Slurry Ice and Flake Ice. J. Aquat. Food Prod. Technol. 2018, 27, 508–517. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, Y.; Wang, Y.; Chen, J. Study on prediction model of freshness of refrigerated grass carp meat based on pH change. Food Res. Dev. 2021, 42, 14–17. [Google Scholar]
- Ocano-Higuera, V.M.; Maeda-Martínez, A.N.; Marquez-Ríos, E.; Canizales-Rodríguez, D.F.; Castillo-Yánez, F.J.; Ruíz-Bustos, E.; Graciano-Verdugo, A.Z.; Plascencia-Jatomea, M. Freshness assessment of ray fish stored in ice by biochemical, chemical and physical methods. Food Chem. 2011, 125, 49–54. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Zhao, L.; Yuan, M.; Jiang, Y. Effects of different thawing methods on the quality characteristics of crispy grass carp. J. Henan Univ. Technol. Nat. Sci. Ed. 2019, 40, 7–11. [Google Scholar]
- Rodríguez, A.; Maier, L.; Paseiro-Losada, P.; Aubourg, S.P. Changes in Sensory and Physical Parameters in Chill-Stored Farmed Coho Salmon (Oncorhynchus kisutch). J. Aquat. Food Prod. Technol. 2015, 25, 633–643. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, M.; Zhang, L.; Chen, B.; Zhang, X. Evaluation of dynamic stress level and physiological change for live salmon in waterless and low-temperature transportation. Aquaculture 2021, 544, 737128. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zhang, P.; Li, Y.; Tian, M.; Wang, X.; Ke, H.; Xiong, W. Effect of carbon monoxide on the meat quality of white mullet during cold storage. Mod. Food Sci. Technol. 2019, 35, 6–9. [Google Scholar]
- Hou, C. Histological characteristics of muscle fibers in rainbow trout. Anhui Agric. Sci. 2013, 41, 3902–3904. [Google Scholar]
- Zhang, Y.; Zhang, Y.; Li, H.; Guo, T.; Jia, J.; Zhang, P.; Wang, L.; Xia, N.; Qian, Q.; Peng, H. Comparison of Nutrition and Flavor Characteristics of Five Breeds of Pork in China. Foods 2022, 11, 2704. [Google Scholar] [CrossRef]
- Que, T. Effects of Different Low Temperature Preservation Methods on Physicochemical Properties and Tissue Structure of Snakehead. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2014. [Google Scholar]
- ISO8586-1:1993; Sensory Analysis—General Guidance for the Selection, Training and Monitoring of Assessors—Part 1: Selected Assessors. Distributed through British Standards Institute (BSI): London, UK, 1993.
- ISO8586-2:1994; Sensory Analysis—General Guidance for the Selection, Training and Monitoring of Assessors—Part 2: Experts. Distributed through British Standards Institute (BSI): London, UK, 1994.
- Marcos, B.; Gou, P.; Arnau, J.; Guardia, M.D.; Comaposada, J. Co-extruded alginate as an alternative to collagen casings in the production of dry-fermented sausages: Impact of coating composition. Meat Sci. 2020, 169, 108184. [Google Scholar] [CrossRef]
- Wang, C.; Bai, C.; Li, N.; Lei, X.; Wang, J.; Xiong, G.; Liao, T. Comparison of water free survival quality of California perch dormant at different cooling rates. Mod. Food Sci. Technol. 2020, 36, 129–137. [Google Scholar]
- Chen, K.; Wang, C.Q.; Fan, Y.Q.; Xie, Y.S.; Yin, Z.F.; Xu, Z.J.; Zhang, H.L.; Cao, J.T.; Han, Z.H.; Wang, Y. The Evaluation of Rapid Cooling As an Anesthetic Method for the Zebrafish. Zebrafish 2014, 11, 71–75. [Google Scholar] [CrossRef]
- Bai, Y.; Tan, Z.; Di, X.; Shi, W.; Wang, Z. Study on the technique of keeping Pelteobagrus fulvidraco alive without water. Food Ind. Sci. Technol. 2013, 34, 4–7. [Google Scholar]
- Santurtun, E.; Broom, D.M.; Cjc, P. A review of factors affecting the welfare of Atlantic salmon (Salmo salar). Anim. Welf. 2018, 27, 193–204. [Google Scholar] [CrossRef]
- Qin, X. Study on Low Temperature Dormancy and Survival Transportation Technology of Pengze Crucian Carp. Ph.D. Thesis, Nanchang University, Nanchang, China, 2018. [Google Scholar]
- Liu, J.; Arner, A.; Puolanne, E.; Ertbjerg, P. On the water-holding of myofibrils: Effect of sarcoplasmic protein denaturation. Meat Sci. 2016, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Refaey, M.M.; Tian, X.; Tang, R.; Li, D. Changes in physiological responses, muscular composition and flesh quality of channel catfish Ictalurus punctatus suffering from transport stress. Aquaculture 2017, 478, 9–15. [Google Scholar] [CrossRef]
- Li, B. Study on the Key Technology of Cold Fresh Sturgeon Meat Preservation. Ph.D. Thesis, Wuhan University of Light Industry, Wuhan, China, 2018. [Google Scholar]
- Zhang, Y.; And, S.L.; Wang, W. Stability and Sensory Quality of Fresh Fruit and Vegetable Juices: Evaluated with Chromatic Aberration. J. Food Process. Preserv. 2014, 38, 1259–1268. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Zhang, H.; Zhang, J. Meat Sensory Color Grade: Mathematical Simulation and Its Application in Quality Analysis of Chilled Pork. J. Food Process. Preserv. 2014, 38, 1957–1964. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Yang, Q.; Dong, H.; Zhang, X. Changes of physiological stress and meat quality of Megalobrama amblycephala under waterless condition. J. Agric. Eng. 2016, 32, 295–300. [Google Scholar]
- Zhang, Y.; Zhang, L.Y.; Venkitasamy, C.; Pan, Z.L.; Ke, H.; Guo, S.Y.; Wu, D.; Wu, W.X.; Zhao, L.M. Potential effects of umami ingredients on human health: Pros and cons. Crit. Rev. Food Sci. Nutr. 2020, 60, 2294–2302. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.L.; Wang, W. Recent developments on umami ingredients of edible mushrooms—A review. Trends Food Sci. Technol. 2013, 33, 78–92. [Google Scholar] [CrossRef]
- Yin, Z.; Venkitasamy, C.; Pan, Z.; Liu, W.; Zhao, L. Novel Umami Ingredients: Umami Peptides and Their Taste. J. Food Sci. 2016, 82, 16–23. [Google Scholar]
- Nan, Q. Meat Industry Handbook; China Architecture & Building Press: Beijing, China, 2003. [Google Scholar]
- Barido, F.H.; Lee, S.K. Changes in proteolytic enzyme activities, tenderness-related traits, and quality properties of spent hen meat affected by adenosine 5 ′-monophosphate during cold storage. Poult. Sci. 2021, 100, 10. [Google Scholar] [CrossRef]
- Wang, K.; Zhuang, H.; Bing, F.; Chen, D.; Feng, T.; Xu, Z. Evaluation of eight kinds of flavor enhancer of umami taste by an electronic tongue. Food Sci. Nutr. 2021, 9, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Qin, L.; Ma, L.X.; Zhao, Z.Y.; Du, M.; Kunihiko, K.; Zhu, B.W. Postmortem nucleotide degradation in turbot mince during chill and partial freezing storage. Food Chem. 2020, 311, 125900. [Google Scholar] [CrossRef]
- Fan, X.; Qin, X.; Zhang, C.; Chen, J.; Zhu, Q.; Chen, P. Effects of temperature on metabolism and meat quality of water preserved grouper. J. Agric. Eng. 2018, 34, 8–11. [Google Scholar]
- Vanlandeghem, M.M.; Wahl, D.H.; Suski, C.D. Physiological responses of largemouth bass to acute temperature and oxygen stressors. Fish. Manag. Ecol. 2010, 17, 414–425. [Google Scholar] [CrossRef]
- Samaras, A.; Papandroulakis, N.; Costari, M.; Pavlidis, M. Stress and metabolic indicators in a relatively high (European sea bass, Dicentrarchus labrax) and a low (meagre, Argyrosomus regius) cortisol responsive species, in different water temperatures. Aquac. Res. 2016, 47, 3501–3515. [Google Scholar] [CrossRef]
- Yin, L.; Su, L.; Jin, Y. Research progress on the relationship between muscle fiber characteristics and fish quality and its regulation techniques. Food Ind. 2015, 36, 4–7. [Google Scholar]
- Hamrick, M.; Arounleut, P.; Bowser, M.; Fulzele, S.; Pollock, N.; Isales, C.M.; Erion, J.; Stranahan, A. Reduction of muscle fiber size, muscle IGF-1, and increased myostatin in the leptin receptor-deficient POUND mouse. FASEB J. 2012, 26, 730. [Google Scholar] [CrossRef]




| Items | Definition | Score |
|---|---|---|
| Appearance | The fish has a boiled, fresh meat appearance | 0–10 score (0 is the worst; 10 is the best) |
| Odor | Aroma intensity of boiled fish meat | |
| Taste | Intensity of meaty taste | |
| Sensory texture | Restitution ability after pressing and chewing hardness |
| Temperature (°C) | Respiratory Rate (Breaths/min) | Crucian Carp Behavior |
|---|---|---|
| 20.0 | 82.0 ± 1.0 a | Swimming around with normal breathing |
| 17.0 | 59.0 ± 2.0 b | Slow breathing with normal swimming |
| 14.0 | 58.0 ± 1.0 b | |
| 11.0 | 50.0 ± 2.0 c | Decreased vitality, slow swimming, unresponsiveness |
| 8.0 | 39.0 ± 1.0 d | |
| 5.0 | 31.0 ± 2.0 e | Irregular breathing, more vigorous swimming, head swaying |
| 4.0 | 27.0 ± 1.0 f | Wandering around, distressed |
| 3.0 | 24.0 ± 1.0 f | Slow breathing, sticking to the bottom of the tank, basically not swimming |
| 2.0 | 19.0 ± 2.0 g | Slow breathing, keeping balance, staying still but can swim with stimulation |
| 1.0 | 17.0 ± 1.0 g | Weak breathing, rolling over, sedate, but can swim with stimulation |
| 0.5 | 6.0 ± 1.0 h | Weak breathing, rolling over, no response to stimulation |
| 0.0 | 3.0 ± 2.0 h | Bending into an arched shape, very weak breathing, dead within a short time |
| Project | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| Hardness (g) | 1976.87 ± 127.43 a | 2031.25 ± 138.02 a | 2065.25 ± 152.43 a | 2124.32 ± 154.84 a |
| Gumminess (g·s) | −3.94 ± 0.12 c | −3.79 ± 0.18 bc | −3.54 ± 0.13 ab | −3.24 ± 0.17 a |
| Springiness | 0.73 ± 0.01 b | 0.74 ± 0.01 ab | 0.75 ± 0.01 ab | 0.77 ± 0.02 a |
| Cohesiveness | 0.48 ± 0.03 b | 0.51 ± 0.02 ab | 0.52 ± 0.03 ab | 0.54 ± 0.02 a |
| Stickiness (g) | 862.53 ± 27.53 b | 883.74 ± 32.53 ab | 896.53 ± 34.03 ab | 929.68 ± 33.53 a |
| Chewiness (g) | 769.59 ± 15.99 c | 794.15 ± 17.29 bc | 813.25 ± 22.99 ab | 845.36 ± 19.59 a |
| Resilience | 0.28 ± 0.01 b | 0.29 ± 0.02 ab | 0.30 ± 0.02 ab | 0.33 ± 0.02 a |
| Project | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| GMP (mg/100 g) | 1.41 ± 0.06 a | 1.46 ± 0.09 a | 1.34 ± 0.11 a | 1.26 ± 0.10 a |
| IMP (mg/100 g) | 184.38 ± 6.57 a | 179.95 ± 3.17 a | 175.86 ± 7.77 a | 169.77 ± 4.77 a |
| CMP (mg/100 g) | 2.89 ± 0.18 b | 3.06 ± 0.11 ab | 3.13 ± 0.17 ab | 3.46 ± 0.27 a |
| UMP (mg/100 g) | 1.95 ± 0.08 b | 2.09 ± 0.13 ab | 2.18 ± 0.07 ab | 2.31 ± 0.10 a |
| AMP (mg/100 g) | 13.34 ± 0.28 a | 12.91 ± 0.47 ab | 12.75 ± 0.19 ab | 12.05 ± 0.38 b |
| Item | Group | Score |
|---|---|---|
| Appearance | S1 | 8.5 ± 0.1 b |
| S2 | 9.1 ± 0.1 a | |
| S3 | 9.1 ± 0.1 a | |
| S4 | 9.2 ± 0.1 a | |
| Odor | S1 | 8.4 ± 0.2 c |
| S2 | 8.6 ± 0.2 bc | |
| S3 | 8.8 ± 0.2 ab | |
| S4 | 9.1 ± 0.1 a | |
| Taste | S1 | 8.7 ± 0.2 b |
| S2 | 9.1 ± 0.2 b | |
| S3 | 9.2 ± 0.2 a | |
| S4 | 9.2 ± 0.1 a | |
| Sensory texture | S1 | 8.8 ± 0.2 b |
| S2 | 8.8 ± 0.1 b | |
| S3 | 9.2 ± 0.1 a | |
| S4 | 9.3 ± 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, L.; Mu, Y.; Zeng, Q.; Jia, J.; Zhang, P.; Pan, Z. Effect of Deep Dormancy Temperature Cultivation on Meat Quality of Crucian Carp (Carassius auratus). Foods 2023, 12, 792. https://doi.org/10.3390/foods12040792
Zhang Y, Wang L, Mu Y, Zeng Q, Jia J, Zhang P, Pan Z. Effect of Deep Dormancy Temperature Cultivation on Meat Quality of Crucian Carp (Carassius auratus). Foods. 2023; 12(4):792. https://doi.org/10.3390/foods12040792
Chicago/Turabian StyleZhang, Yin, Linguo Wang, Yunlong Mu, Qing Zeng, Jianlin Jia, Pengcheng Zhang, and Zhongli Pan. 2023. "Effect of Deep Dormancy Temperature Cultivation on Meat Quality of Crucian Carp (Carassius auratus)" Foods 12, no. 4: 792. https://doi.org/10.3390/foods12040792
APA StyleZhang, Y., Wang, L., Mu, Y., Zeng, Q., Jia, J., Zhang, P., & Pan, Z. (2023). Effect of Deep Dormancy Temperature Cultivation on Meat Quality of Crucian Carp (Carassius auratus). Foods, 12(4), 792. https://doi.org/10.3390/foods12040792






