Simulation and Control of the Formation of Ethyl Carbamate during the Fermentation and Distillation Processes of Chinese Baijiu
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. The Simulation of Baijiu Fermentation
2.3. The Simulation of Aqueous Reactions between Urea and Ethanol or Cyanide and Ethanol during Distillation
2.3.1. The Effect of Temperature on the Formation of EC and on Urea and Cyanide Residue
2.3.2. The Effect of pH Value on the Formation of EC and on Urea and Cyanide Residue
2.3.3. The Effect of Ethanol Concentration on the Formation of EC and on Urea and Cyanide Residue
2.3.4. The Effect of Metal Ions on the Formation of EC and on Urea and Cyanide Residue
2.4. The Simulation of Gaseous Reactions between Urea and Ethanol or Cyanide and Ethanol during Distillation
2.4.1. The Effect of Different Concentrations of Urea and Cyanide on the Formation of EC
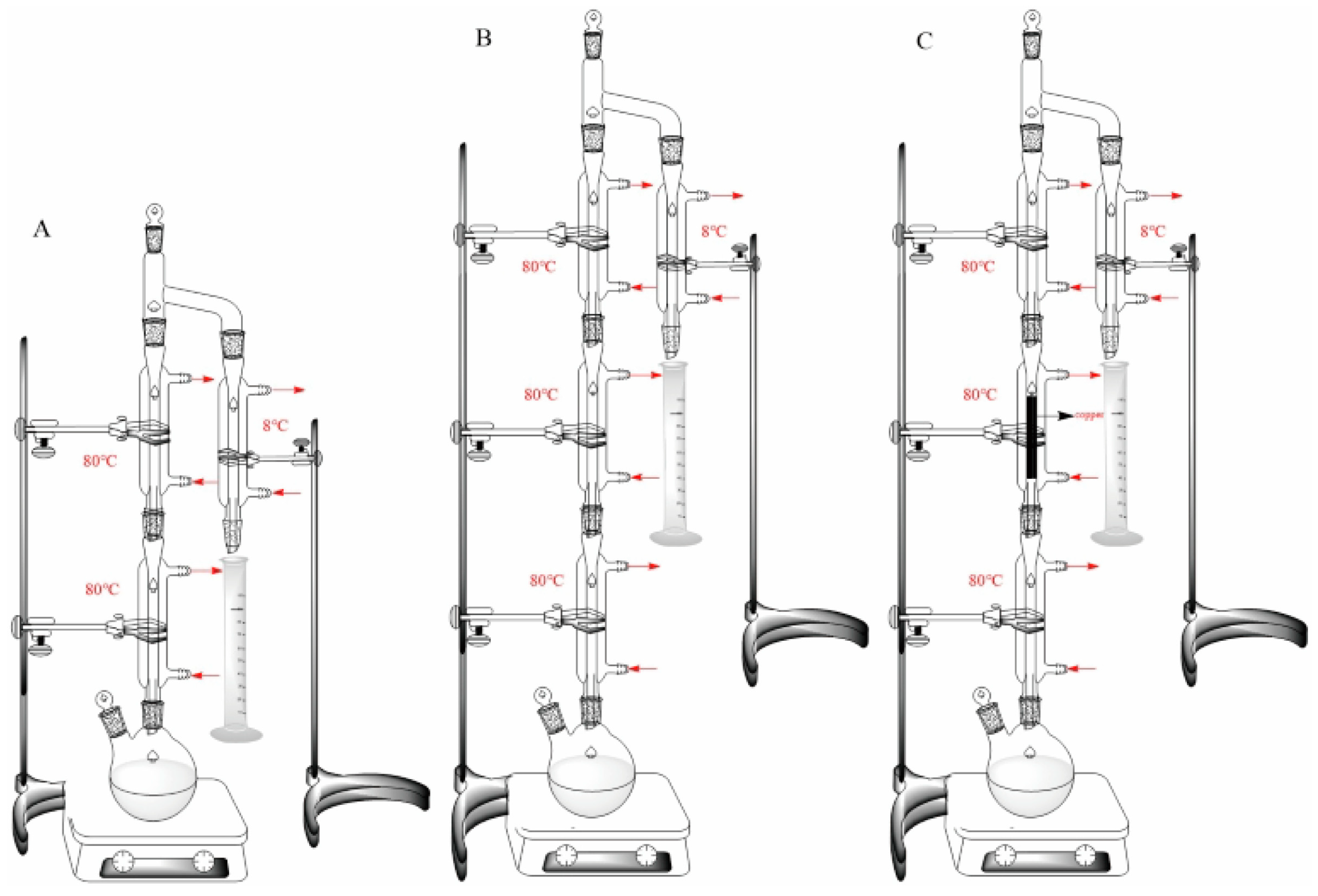
2.4.2. The Effect of the Optimized Distillation Device and Copper Wires on the Formation of EC
2.5. The Simulation of Solid Distillation for Baijiu
2.6. Analytical Method
3. Results and Discussion
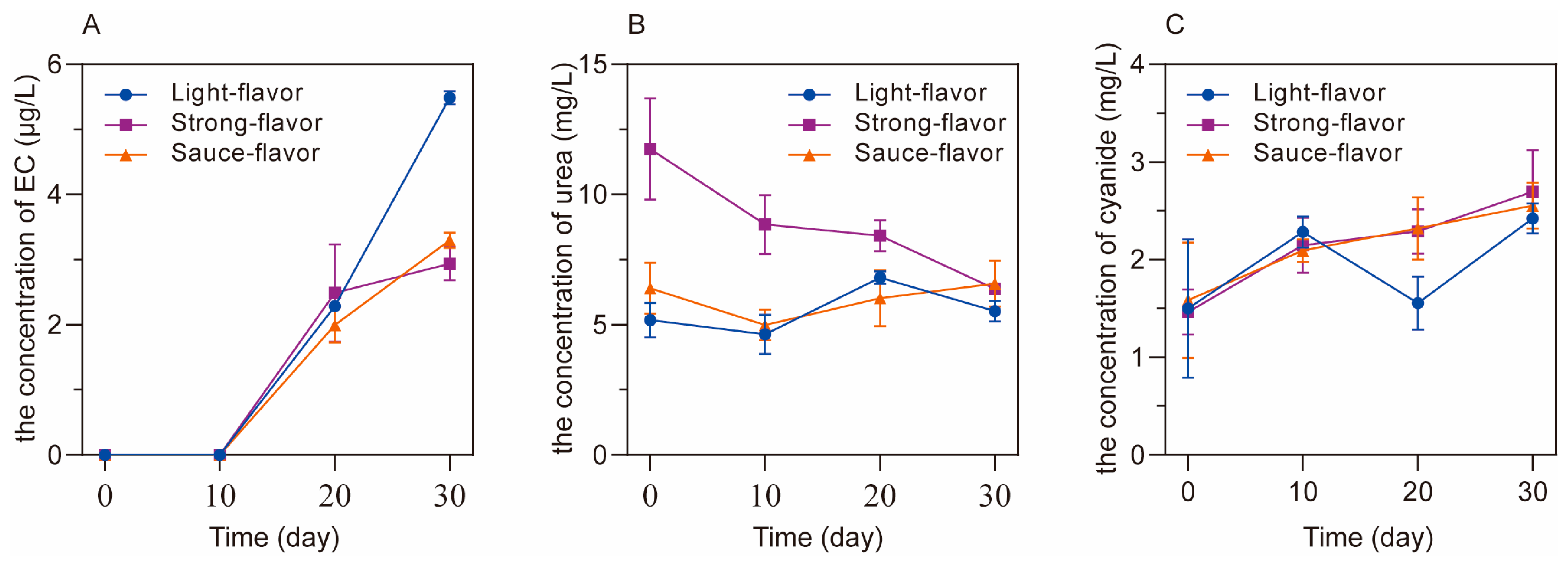
3.1. The Concentrations of EC, Urea and Cyanide during the Simulated Fermentation of Different Flavors of Baijiu
3.2. The Effects of Temperature, pH, Ethanol and Metal Ions on the Formation of EC during the Aqueous Reaction between Precursors and Ethanol
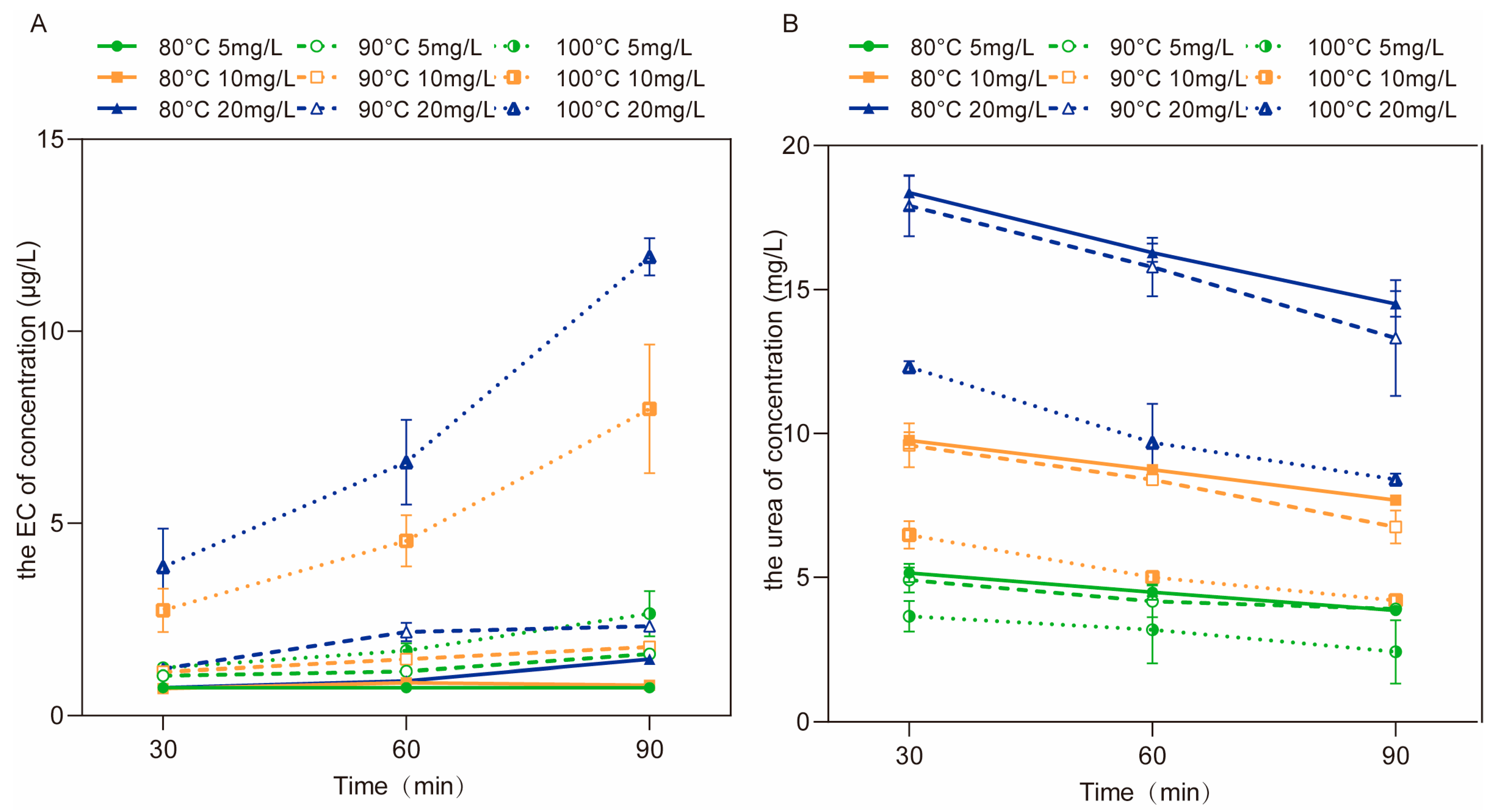
3.2.1. The Concentrations of EC and Urea after Aqueous Reactions at Different Temperatures
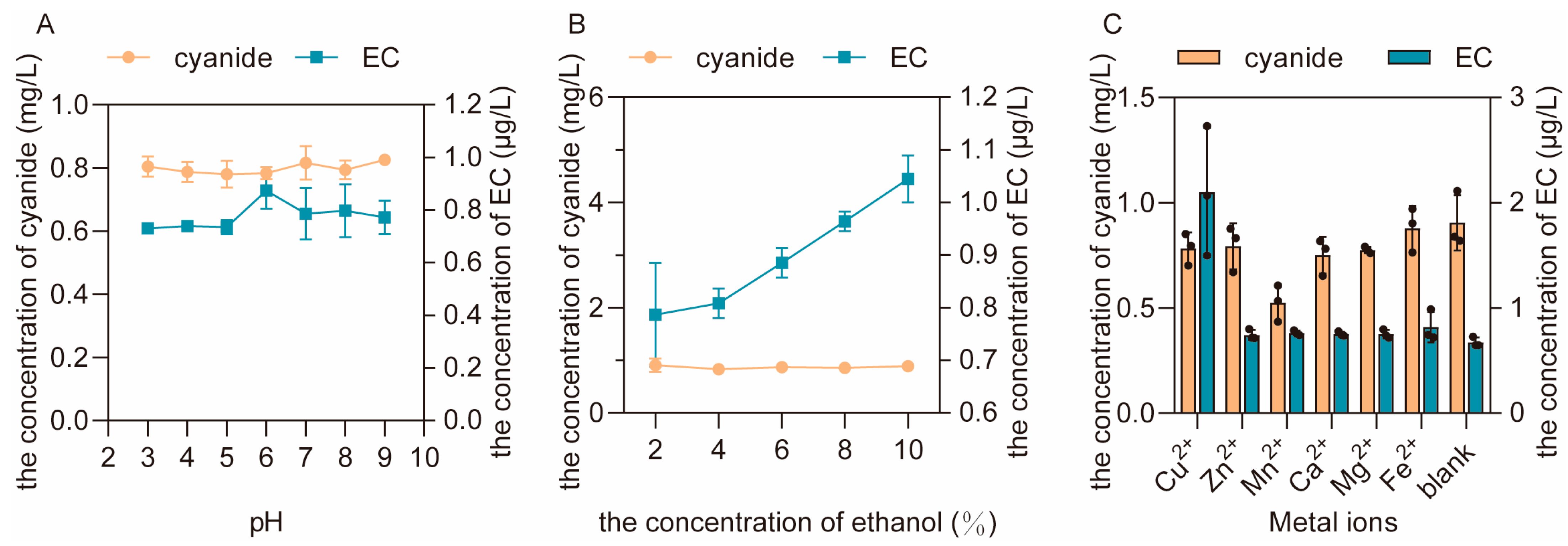
3.2.2. The Effects of pH Value, Alcohol Concentration and Metal Ions on the Formation of EC and the Residue of Urea
3.2.3. The Concentrations of EC and Cyanide after Aqueous Reactions at Different Temperatures
3.2.4. The Effects of pH Value, Alcohol Concentration and Metal Ions on the Formation of EC and the Residue of Cyanide
3.3. The Effect of Different Precursors and Devices on the Formation of EC during Gaseous Reactions
3.3.1. The Concentration of EC at Different Initial Concentrations of Urea and Cyanide during Distillation
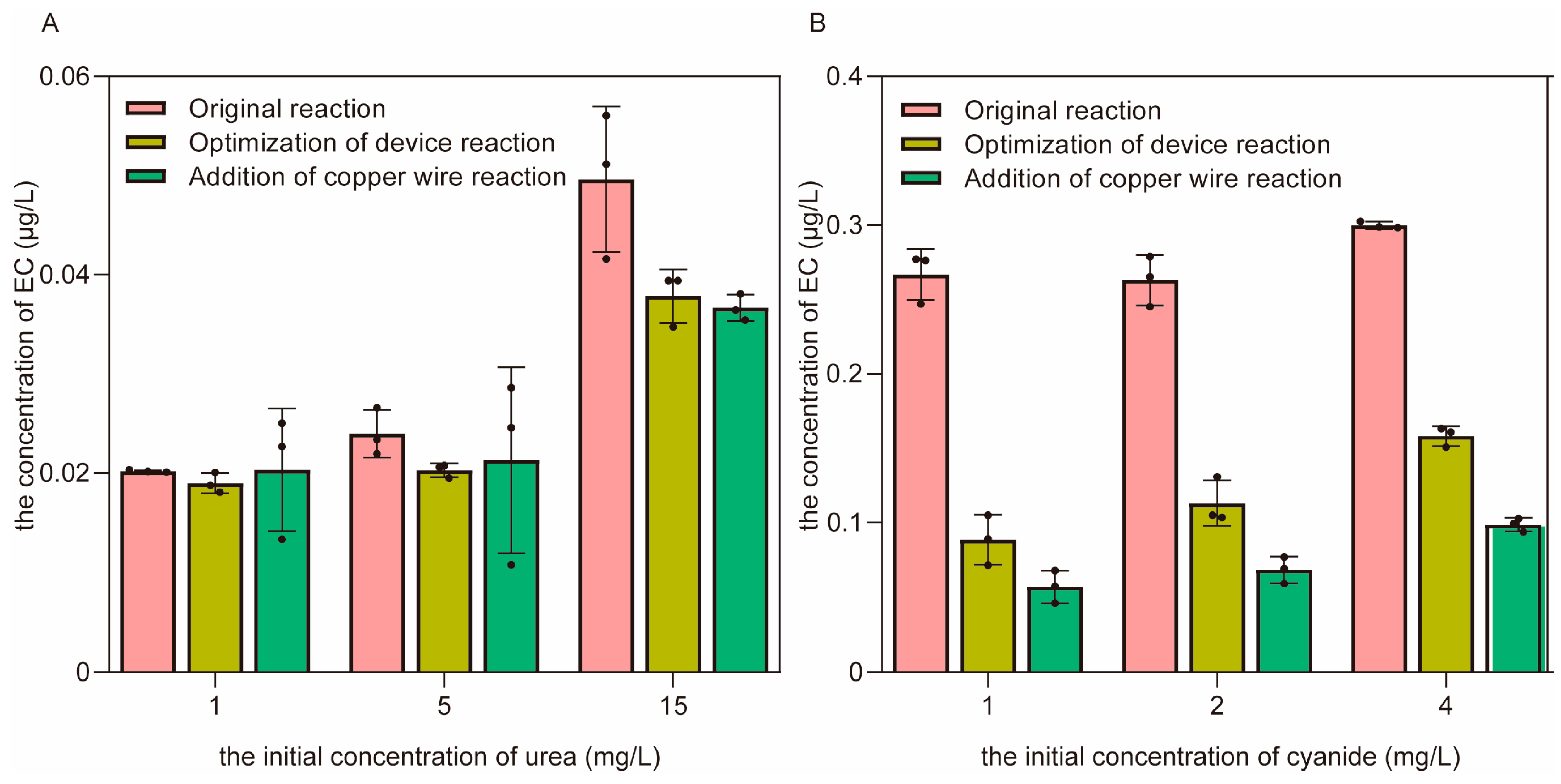
3.3.2. The Control of EC Formation through the Optimization of the Distillation Device and the Addition of Copper Wires in the Simulated Distillation System
3.4. The Most Effective Strategy to Reduce the Concentration of EC in Different Flavors of Baijiu
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Wang, D.; Fan, W.L.; Mu, X.Q.; Chen, J. Traditional Chinese biotechnology. Adv. Biochem. Eng. Biot. 2010, 122, 189–233. [Google Scholar]
- Tu, W.; Cao, X.; Cheng, J.; Li, L.; Zhang, T.; Wu, Q.; Xiang, P.; Shen, C.; Li, Q. Chinese baijiu: The perfect works of microorganisms. Front. Microbiol. 2022, 13, 919044. [Google Scholar] [CrossRef]
- Guan, T.; Tian, X.; Wu, J.; Luo, J.; Peng, Z.; Yang, H.; Zhao, X.; Zhang, J. Investigation and risk assessment of ethyl carbamate in Chinese baijiu. LWT-Food Sci. Technol. 2021, 152, 112340. [Google Scholar] [CrossRef]
- Riachi, L.G.; Santos, A.; Moreira, R.F.; De Maria, C.A. A review of ethyl carbamate and polycyclic aromatic hydrocarbon contamination risk in cachaca and other Brazilian sugarcane spirits. Food Chem. 2014, 149, 159–169. [Google Scholar] [CrossRef]
- Li, G.; Zhong, Q.; Wang, D.; Zhang, X.; Gao, H.; Shen, S. Determination and formation of ethyl carbamate in Chinese spirits. Food Control 2015, 56, 169–176. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Huang, X.; Xiao, Z.; Yang, Y.; Yu, Q.; Chen, S.; He, L.; Liu, A.; Liu, S.; et al. A review on mechanistic overview on the formation of toxic substances during the traditional fermented food processing. Food Rev. Int. 2021, 1–18. [Google Scholar] [CrossRef]
- Gowd, V.; Su, H.; Karlovsky, P.; Chen, W. Ethyl carbamate: An emerging food and environmental toxicant. Food Chem. 2018, 248, 312–321. [Google Scholar] [CrossRef]
- Weber, J.V.; Sharypov, V.I. Ethyl carbamate in foods and beverages: A review. Environ. Chem. Lett. 2008, 7, 233–247. [Google Scholar] [CrossRef]
- Bruno, S.; Vaitsman, D.; Kunigami, C.; Brasil, M. Influence of the distillation processes from Rio de Janeiro in the ethyl carbamate formation in Brazilian sugar cane spirits. Food Chem. 2007, 104, 1345–1352. [Google Scholar] [CrossRef]
- Jiao, Z.; Dong, Y.; Chen, Q. Ethyl carbamate in fermented beverages: Presence, analytical chemistry, formation mechanism, and mitigation proposals. Compr. Rev. Food Sci. Food 2014, 13, 611–626. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Zhang, M.P. Ethyl carbamate in Chinese liquor (baijiu): Presence, analysis, formation, and control. Appl. Microbiol. Biot. 2021, 105, 4383–4395. [Google Scholar] [CrossRef]
- Wang, S.; Tian, X.; Tian, L.; Guo, Q.; Liu, Y.; Zhao, F.; Zhang, J.; Li, D.; Luo, J.; He, Z.; et al. Degradation of ethyl carbamate in strong-flavor baijiu by the microbial combination culture. Food Control 2023, 145, 109447. [Google Scholar] [CrossRef]
- Cui, K.; Wu, Q.; Xu, Y. Biodegradation of ethyl carbamate and urea with Lysinibacillus sphaericus mt33 in Chinese liquor fermentation. J. Agric. Food Chem. 2018, 66, 1583–1590. [Google Scholar] [CrossRef]
- Shen, T.; Wu, Q.; Xu, Y. Biodegradation of cyanide with Saccharomyces cerevisiae in baijiu fermentation. Food Control 2021, 127, 108107. [Google Scholar] [CrossRef]
- Wu, Q.; Lin, J.; Cui, K.; Du, R.; Zhu, Y.; Xu, Y. Effect of microbial interaction on urea metabolism in Chinese liquor fermentation. J. Agric. Food Chem. 2017, 65, 11133–11139. [Google Scholar] [CrossRef]
- Wu, D.; Xie, W.; Li, X.; Cai, G.; Lu, J.; Xie, G. Metabolic engineering of Saccharomyces cerevisiae using the CRISPR/Cas9 system to minimize ethyl carbamate accumulation during Chinese rice wine fermentation. Appl. Microbiol. Biot. 2020, 104, 4435–4444. [Google Scholar] [CrossRef]
- Guo, X.W.; Li, Y.Z.; Guo, J.; Wang, Q.; Huang, S.Y.; Chen, Y.F.; Du, L.P.; Xiao, D.G. Reduced production of ethyl carbamate for wine fermentation by deleting CAR1 in Saccharomyces cerevisiae. J. Ind. Microbiol. Biot. 2016, 43, 671–679. [Google Scholar] [CrossRef]
- Alcarde, A.R.; Souza, L.M.; Bortoletto, A.M. Ethyl carbamate kinetics in double distillation of sugar cane spirit. Part 2: Influence of type of pot still. J. Inst. Brew. 2012, 118, 352–355. [Google Scholar] [CrossRef]
- Wu, P.; Cai, C.; Shen, X.; Wang, L.; Zhang, J.; Tan, Y.; Jiang, W.; Pan, X. Formation of ethyl carbamate and changes during fermentation and storage of yellow rice wine. Food Chem. 2014, 152, 108–112. [Google Scholar] [CrossRef]
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic compounds in alcoholic beverages: An update. Arch. Toxicol. 2016, 90, 2349–2367. [Google Scholar] [CrossRef]
- Tian, S.; Zeng, W.; Guan, X.; Zhou, J.; Du, G. Effects of fortified starter culture containing Saccharomyces cerevisiae and Lactobacillus fermentum on microbial community structure and ethyl carbamate. Food Control 2022, 137, 108890. [Google Scholar] [CrossRef]
- Zhou, K.; Patrignani, F.; Sun, Y.M.; Lanciotti, R.; Xu, Z.L. Inhibition of ethyl carbamate accumulation in soy sauce by adding quercetin and ornithine during thermal process. Food Chem. 2021, 343, 128528. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, S.; Zhang, J.; Xu, Y.; Wang, D. Kinetic modeling of ethyl carbamate formation from urea in Huangjiu during storage. Food Control 2021, 129, 108249. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lyu, J.; Kim, M.K.; Lee, K.G. Effect of citrulline, urea, ethanol, and urease on the formation of ethyl carbamate in soybean paste model system. Food Chem. 2015, 189, 74–79. [Google Scholar] [CrossRef]
- Takanao, M.; Terumichi, A.; Keietsu, A.; Nami, F.; Takeshi, H.; Masaoki, S.; Kinji, U. Determination of ethyl carbamate in soy sauce and its possible precursor. J. Agric. Food Chem. 1993, 41, 352–356. [Google Scholar]
- Aresta, M.; Boscolo, M.; Franco, D.W. Copper(II) catalysis in cyanide conversion into ethyl carbamate in spirits and relevant reactions. J. Agric. Food Chem. 2001, 49, 2819–2824. [Google Scholar] [CrossRef]
- Galinaro, C.; Franco, D. Ethyl carbamate formation in recently distilled sugar cane spirits; proposal for its control. Quím. Nova 2011, 34, 996–1000. [Google Scholar] [CrossRef]
- Galinaro, C.A.; Ohe, T.H.; da Silva, A.C.; da Silva, S.C.; Franco, D.W. Cyanate as an active precursor of ethyl carbamate formation in sugar cane spirit. J. Agric. Food Chem. 2015, 63, 7415–7420. [Google Scholar] [CrossRef]
- Zhang, W.; Si, G.; Ye, M.; Feng, S.; Cheng, F.; Li, J.; Mei, J.; Zong, S.; Wang, J.; Zhou, P. An efficient assay for simultaneous quantification of ethyl carbamate and phthalate esters in Chinese liquor by gas chromatography-mass spectrometry. Food Anal. Method 2017, 10, 3487–3495. [Google Scholar] [CrossRef]
- Qin, Y.; Duan, B.; Shin, J.A.; So, H.J.; Hong, E.S.; Jeong, H.G.; Lee, J.H.; Lee, K.T. Effect of fermentation on cyanide and ethyl carbamate contents in cassava flour and evaluation of their mass balance during lab-scale continuous distillation. Foods 2021, 10, 1089. [Google Scholar] [CrossRef]
- Silva, J.H.d.N.e.; Verruma-Bernardi, M.R.; Oliveira, A.L.d. Cachaça production in Brazil and its main contaminant (ethyl carbamate). Sci. Agric. 2020, 77, e20180135. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Fan, W.; Xu, Y.; Yang, J.; Xie, G.; Sun, L.; Xue, X. Influence of pilot scale pot still sencond distillation on ethyl carbamate and cyanide in baijiu and the quality of raw liquor. Food Ferment. Ind. 2022, 48, 53–57. [Google Scholar]
- Li, H.; Huang, W.; Shen, C.; Yi, B. Optimization of the distillation process of Chinese liquor by comprehensive experimental investigation. Food Bioprod. Process. 2012, 90, 392–398. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, Y.; Li, J.; Chen, J.; Zhao, X.; Du, G. Simulation and Control of the Formation of Ethyl Carbamate during the Fermentation and Distillation Processes of Chinese Baijiu. Foods 2023, 12, 821. https://doi.org/10.3390/foods12040821
Di Y, Li J, Chen J, Zhao X, Du G. Simulation and Control of the Formation of Ethyl Carbamate during the Fermentation and Distillation Processes of Chinese Baijiu. Foods. 2023; 12(4):821. https://doi.org/10.3390/foods12040821
Chicago/Turabian StyleDi, Yuhang, Jianghua Li, Jian Chen, Xinrui Zhao, and Guocheng Du. 2023. "Simulation and Control of the Formation of Ethyl Carbamate during the Fermentation and Distillation Processes of Chinese Baijiu" Foods 12, no. 4: 821. https://doi.org/10.3390/foods12040821



