Encapsulation of Orange Peel Oil in Biopolymeric Nanocomposites to Control Its Release under Different Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
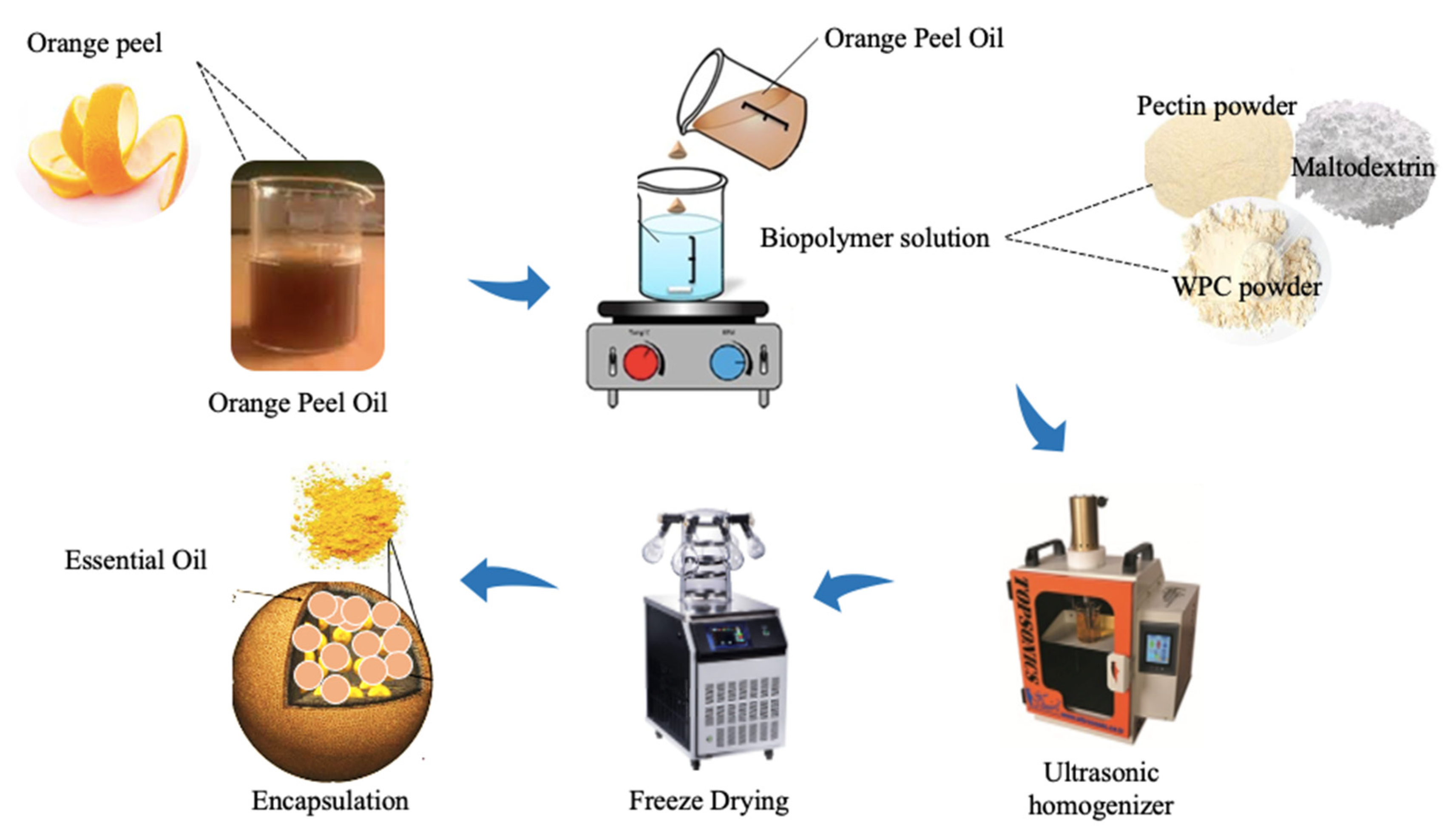
2.2. Encapsulation Process of Orange Peel Oil in Whey Protein–Pectin Complexes
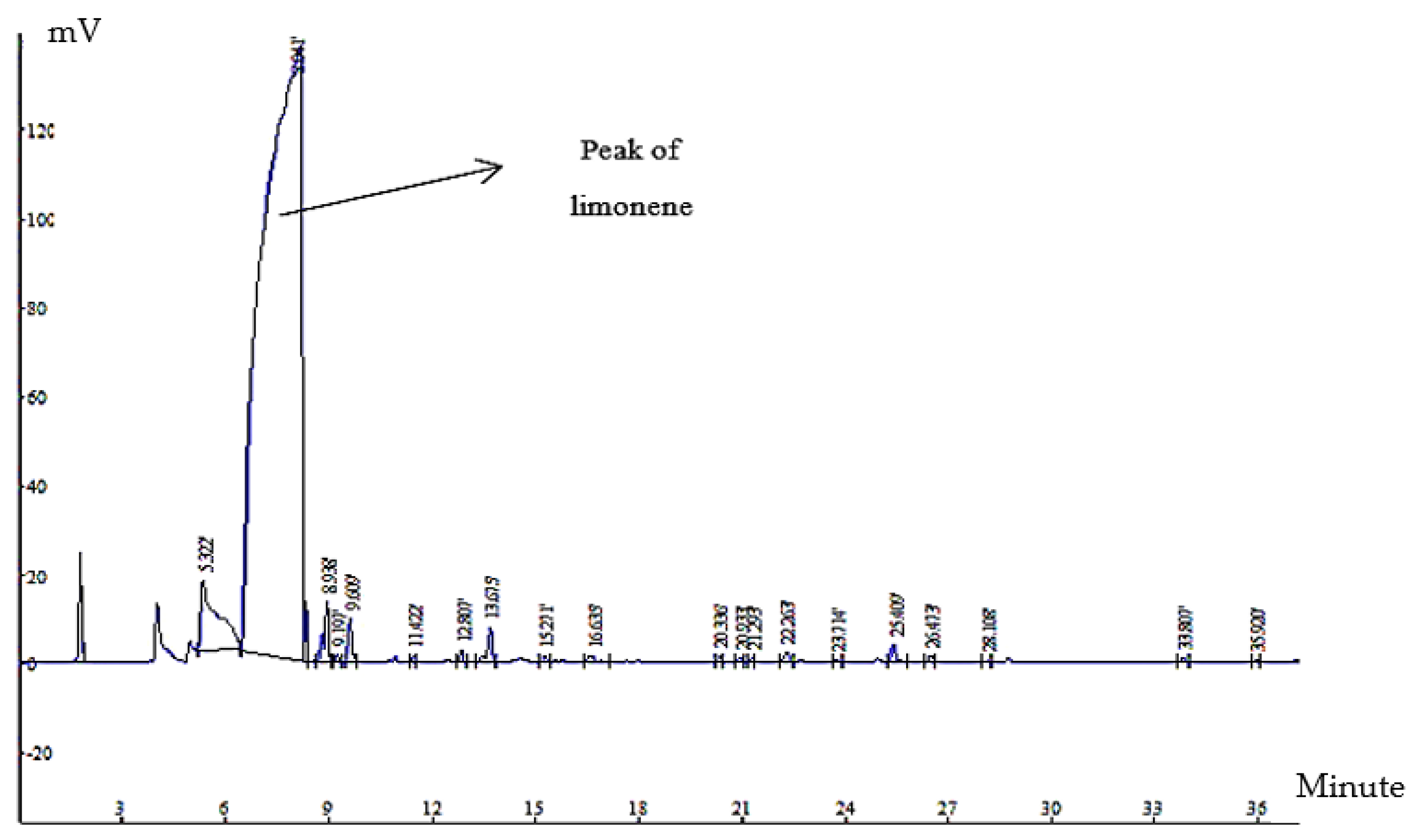
2.3. Encapsulation Efficiency of Orange Peel Oil
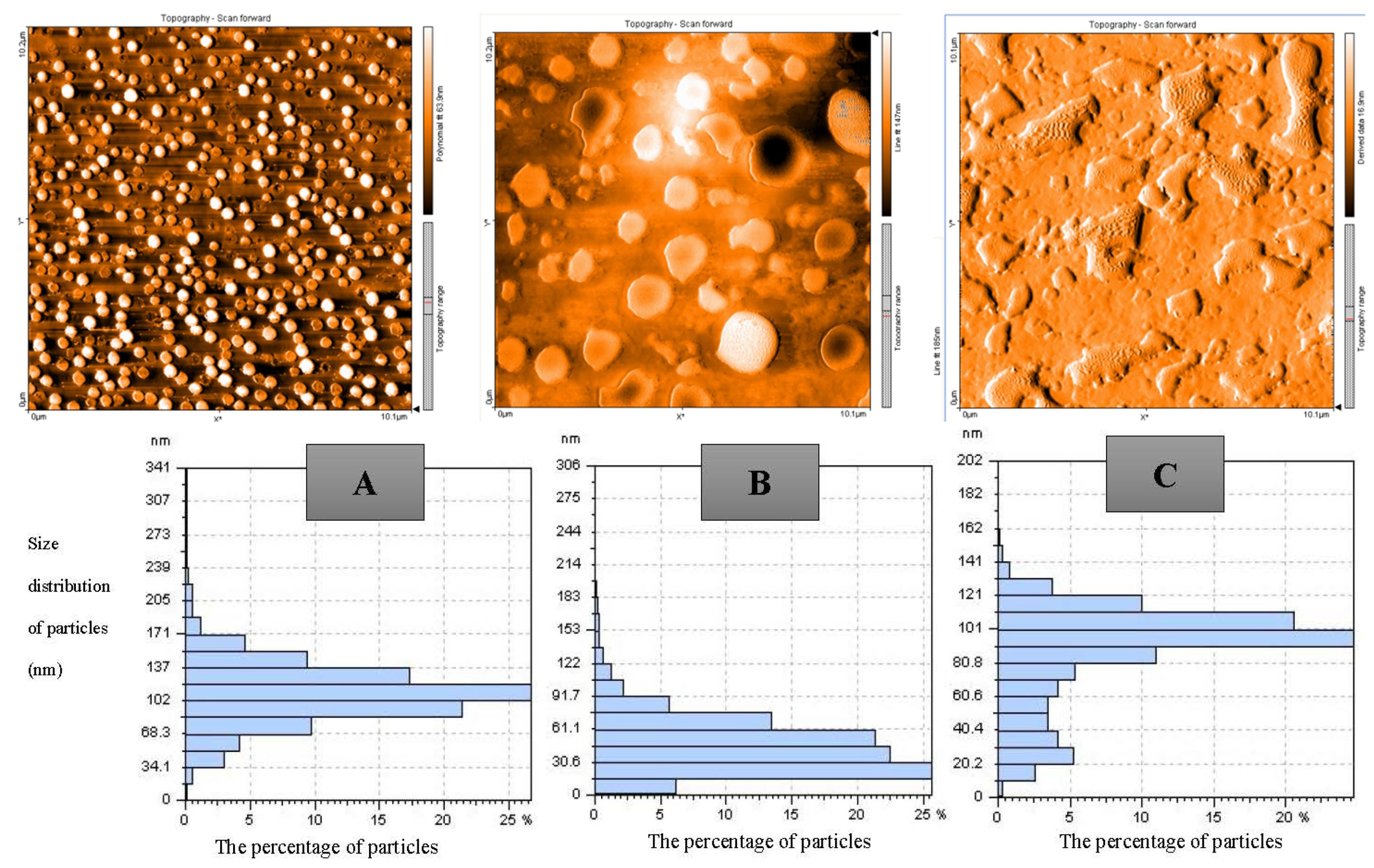
2.4. Atomic Force Microscopy Analysis
2.5. Release Profile Analysis of Orange Peel Oil
2.6. Determination of the Release Profile in Simulated Salivary Conditions
2.7. Statistical Analysis
3. Results and Discussion
3.1. Results of Encapsulation Efficiency for Powders
3.2. Evaluating the Morphology and Size of Nanocomposites
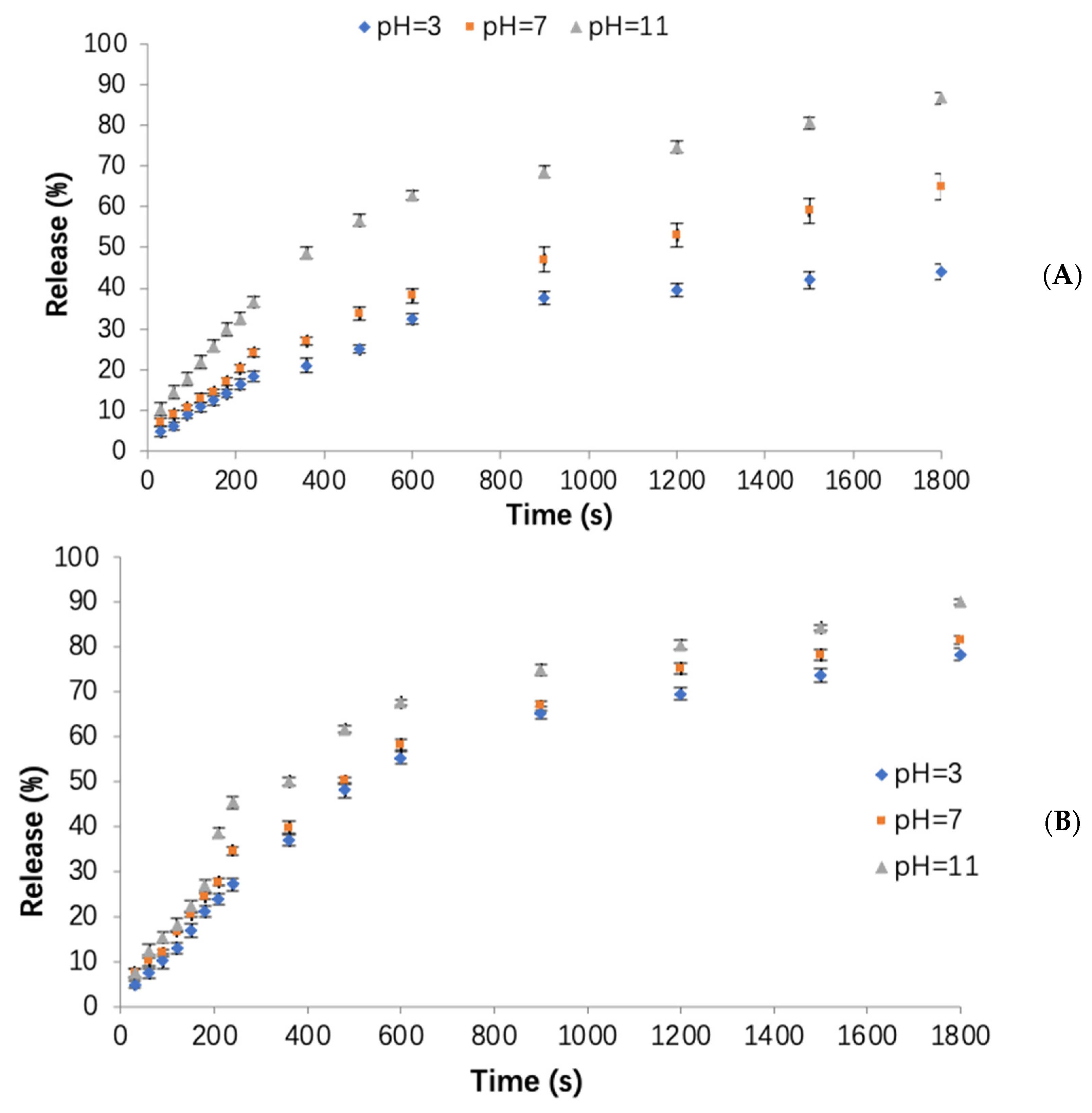
3.3. Influence of Different pH Values on the Release of Orange Peel Oil
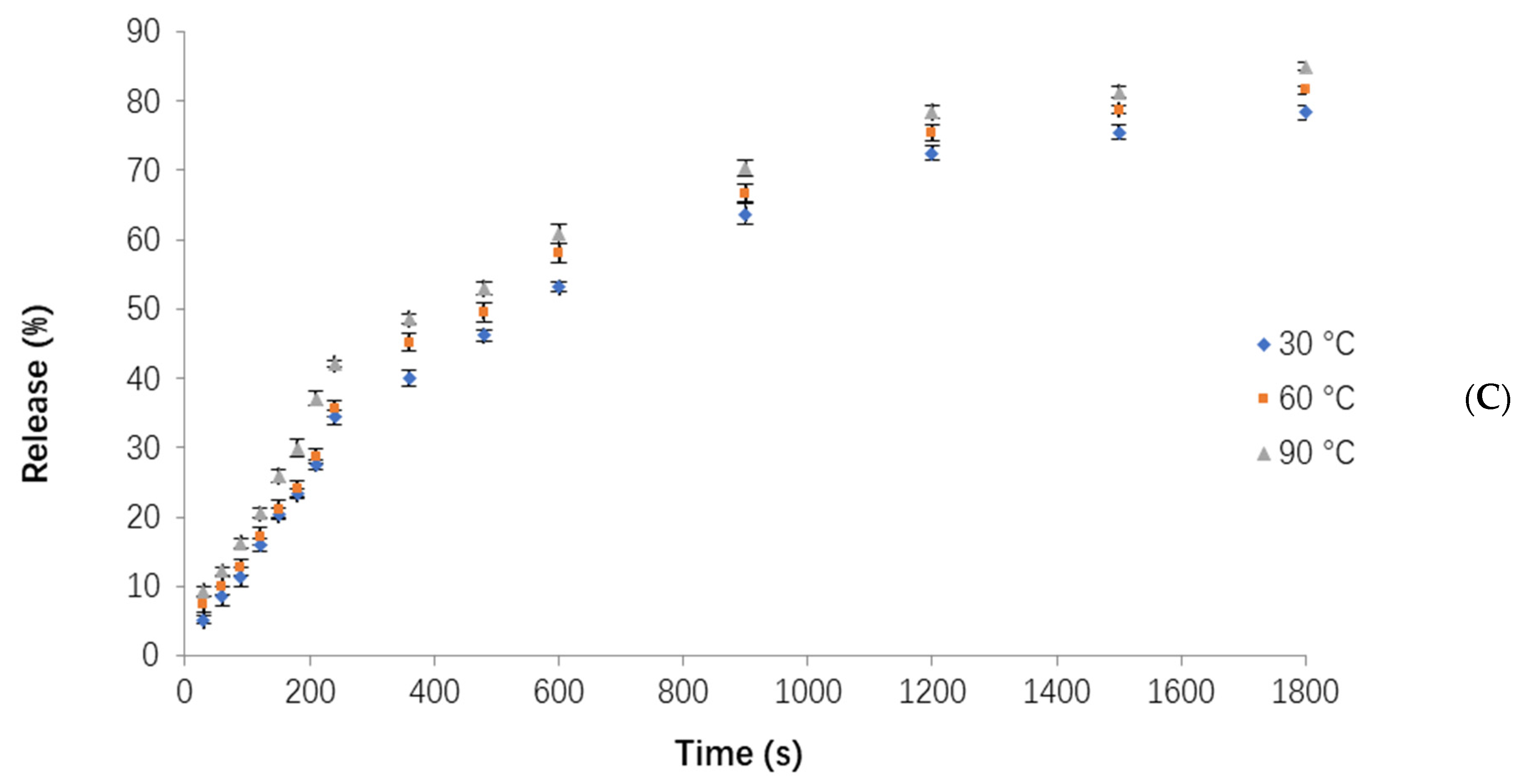
3.4. Influence of Different Temperatures on the Release of Orange Peel Oil
3.5. Modeling of the Orange Peel Oil Release Profile in Different Conditions
3.6. The Release of Orange Peel Oil in Simulated Salivary Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghasemi, S.; Jafari, S.; Assadpour, E.; Khomeiri, M. Production of pectin-whey protein nano-complexes as carriers of orange peel oil. Carbohydr. Polym. 2017, 177, 369–377. [Google Scholar] [CrossRef]
- Ghasemi, S.; Jafari, S.; Assadpour, E.; Khomeiri, M. Nanoencapsulation of d-limonene within nanocarriers produced by pectin-whey protein complexes. Food Hydrocoll. 2018, 77, 152–162. [Google Scholar] [CrossRef]
- Estrella-Osuna, D.E.; Tapia-Hernández, J.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Ornelas-Paz, J.D.; Del-Toro-Sánchez, C.L.; Ocaño-Higuera, V.M.; Rodríguez-Félix, F.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A. Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release. Nanomaterials 2022, 12, 2303. [Google Scholar] [CrossRef]
- Jones, O.; Decker, E.; McClements, D. Thermal analysis of β-lactoglobulin complexes with pectins or carrageenan for production of stable biopolymer particles. Food Hydrocoll. 2010, 24, 239–248. [Google Scholar] [CrossRef]
- Jafari, S.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Jafari, S.; Fathi, M.; Mandala, I. Emerging product formation. In Food Waste Recovery; Elsevier: New York, NY, USA, 2015; pp. 293–317. [Google Scholar]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-polysaccharide nanoparticles as matrices for antioxidant compounds: A strategy for prevention of chronic degenerative diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M.; Maghsoudlou, Y. Evaluation of folic acid release from spray dried powder particles of pectin-whey protein nano-capsules. Int. J. Biol. Macromol. 2017, 95, 238–247. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Fathi, M.; Mozafari, M.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Technol. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Fathi, M.; Varshosaz, J.; Mohebbi, M.; Shahidi, F. Hesperetin-loaded solid lipid nanoparticles and nanostructure lipid carriers for food fortification: Preparation, characterization, and modeling. Food Bioprocess Technol. 2013, 6, 1464–1475. [Google Scholar] [CrossRef]
- Jiménez-Alvarado, R.; Beristain, C.; Medina-Torres, L.; Román-Guerrero, A.; Vernon-Carter, E. Ferrous bisglycinate content and release in W1/O/W2 multiple emulsions stabilized by protein–polysaccharide complexes. Food Hydrocoll. 2009, 23, 2425–2433. [Google Scholar] [CrossRef]
- Hosseini, S.; Emam-Djomeh, Z.; Sabatino, P.; Van der Meeren, P. Nanocomplexes arising from protein-polysaccharide electrostatic interaction as a promising carrier for nutraceutical compounds. Food Hydrocoll. 2015, 50, 16–26. [Google Scholar] [CrossRef]
- Zimet, P.; Livney, Y. Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2009, 23, 1120–1126. [Google Scholar] [CrossRef]
- Bagheri, L.; Madadlou, A.; Yarmand, M.; Mousavi, M. Nanoencapsulation of date palm pit extract in whey protein particles generated via desolvation method. Food Res. Int. 2013, 51, 866–871. [Google Scholar] [CrossRef]
- Jafari, S.; He, Y.; Bhandari, B. Encapsulation of nanoparticles of d-limonene by spray drying: Role of emulsifiers and emulsifying techniques. Dry. Technol. 2007, 25, 1069–1079. [Google Scholar] [CrossRef]
- Peinado, I.; Lesmes, U.; Andrés, A.; McClements, J. Fabrication and morphological characterization of biopolymer particles formed by electrostatic complexation of heat treated lactoferrin and anionic polysaccharides. Langmuir 2010, 26, 9827–9834. [Google Scholar] [CrossRef]
- Matias, R.; Ribeiro, P.; Sarraguça, M.; Lopes, J. A UV spectrophotometric method for the determination of folic acid in pharmaceutical tablets and dissolution tests. Anal. Methods 2014, 6, 3065–3071. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm 2010, 67, 217–223. [Google Scholar]
- Ades, H.; Kesselman, E.; Ungar, Y.; Shimoni, E. Complexation with starch for encapsulation and controlled release of menthone and menthol. LWT-Food Sci. Technol. 2012, 45, 277–288. [Google Scholar] [CrossRef]
- Amaechi, B.; Higham, S. In vitro remineralisation of eroded enamel lesions by saliva. J. Dent. 2001, 29, 371–376. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: Influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Kanemori, T.; Kanemaru, M.; Takai, N.; Mizuno, Y.; Yoshida, H. Performance evaluation of salivary amylase activity monitor. Biosens. Bioelectron. 2004, 20, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Lutz, R.; Aserin, A.; Wicker, L.; Garti, N. Release of electrolytes from W/O/W double emulsions stabilized by a soluble complex of modified pectin and whey protein isolate. Colloids Surf. B Biointerfaces 2009, 74, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Jafari, S.; Assadpour, E.; Esfanjani, A. Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2016, 82, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, J. Encapsulation and Controlled Release Technologies in Food Systems; John Wiley & Sons: New York, NY, USA, 2016. [Google Scholar]
- Liu, F.; Avena-Bustillos, R.; Chiou, B.-S.; Li, Y.; Ma, Y.; Williams, T.; Wood, D.; McHugh, T.; Zhong, F. Controlled-release of tea polyphenol from gelatin films incorporated with different ratios of free/nanoencapsulated tea polyphenols into fatty food simulants. Food Hydrocoll. 2017, 62, 212–221. [Google Scholar] [CrossRef]
- Esfanjani, A.; Jafari, S.; Assadpoor, E.; Mohammadi, A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J. Food Eng. 2015, 165, 149–155. [Google Scholar] [CrossRef]
- Mircioiu, I.; Anuta, V.; Ibrahim, N.; Mircioiu, C. Dissolution of tamoxifen in biorelevant media. A two phase release model. Farmacia 2012, 6, 315–324. [Google Scholar]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef]


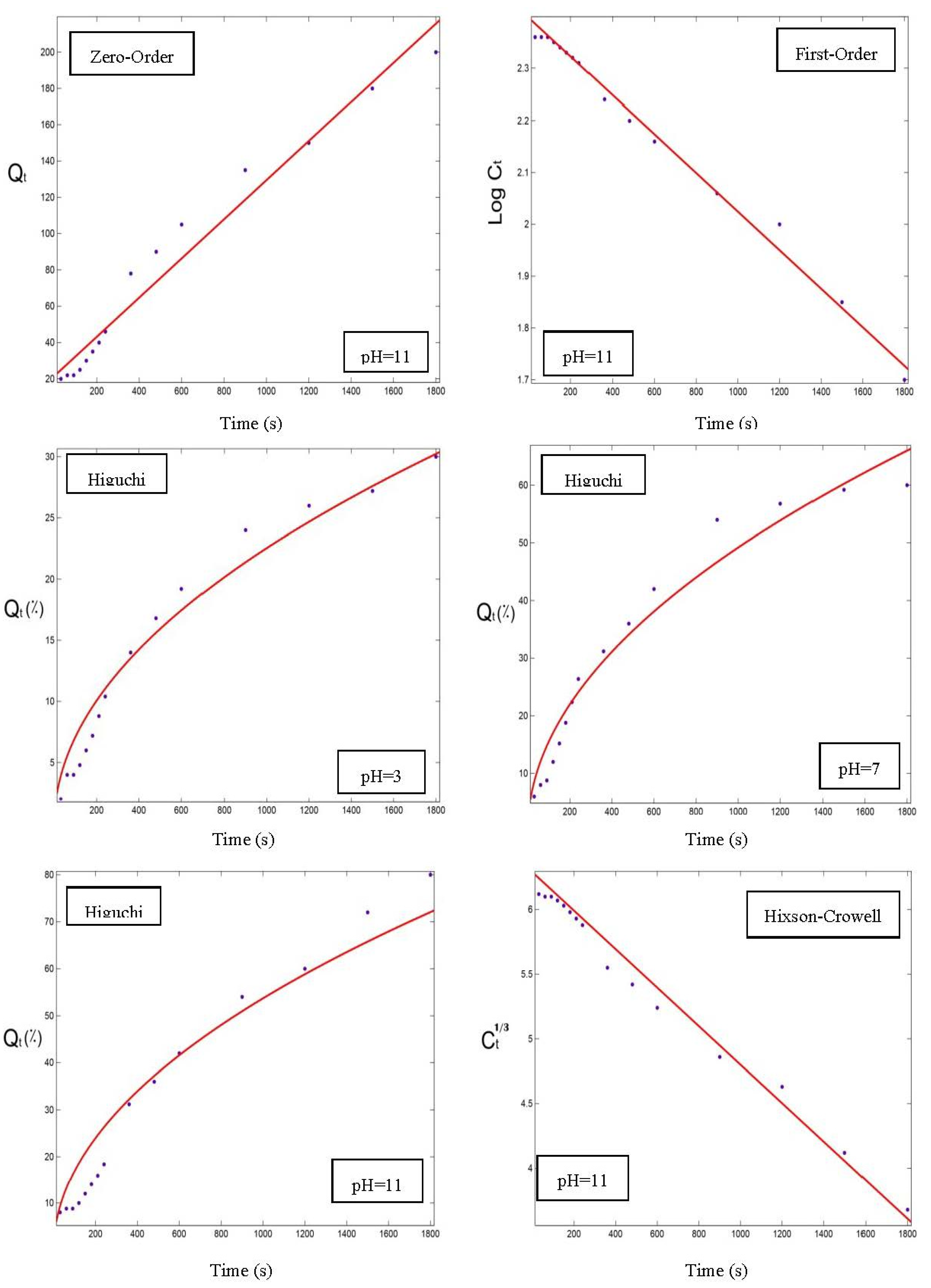
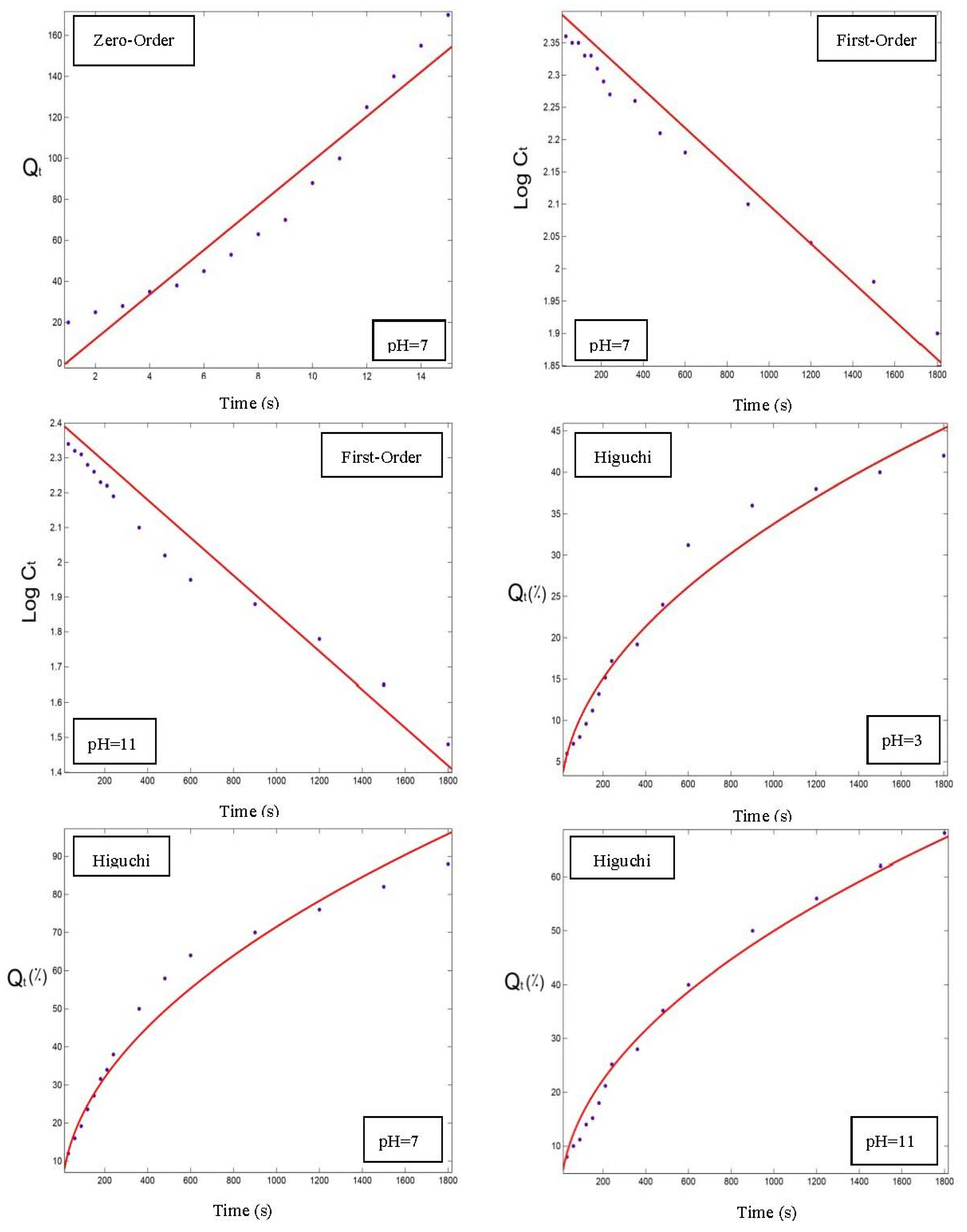

| Zero-Order | First-Order | |||||
|---|---|---|---|---|---|---|
| Treatments | R2 | K | RMSE | R2 | K | RMSE |
| T = 30 °C, pH = 3 | 0.90 ± 0.01 | 0.04 ± 0.01 | 7.64 ± 0.05 | 0.83 ± 0.01 | 0.001 ± 0.000 | 0.02 ± 0.00 |
| T = 30 °C, pH = 7 | 0.87 ± 0.02 | 0.08 ± 0.01 | 18.57 ± 0.08 | 0.84 ± 0.02 | 0.001 ± 0.000 | 0.05 ± 0.00 |
| T = 30 °C, pH = 11 | 0.97 ± 0.01 | 0.11 ± 0.02 | 11.82 ± 0.05 | 0.99 ± 0.01 | 0.002 ± 0.000 | 0.02 ± 0.00 |
| T = 60 °C, pH = 3 | 0.89 ± 0.02 | 0.05 ± 0.01 | 11.07 ± 0.09 | 0.75 ± 0.03 | 0.001 ± 0.000 | 0.04 ± 0.00 |
| T = 60 °C, pH = 7 | 0.95 ± 0.01 | 0.09 ± 0.01 | 11.89 ± 0.05 | 0.94 ± 0.01 | 0.002 ± 0.000 | 0.03 ± 0.00 |
| T = 60 °C, pH = 11 | 0.87 ± 0.02 | 0.10 ± 0.02 | 23.76 ± 0.10 | 0.93 ± 0.01 | 0.003 ± 0.000 | 0.07 ± 0.01 |
| T = 90 °C, pH = 3 | 0.81 ± 0.03 | 0.06 ± 0.01 | 17.15 ± 0.11 | 0.33 ± 0.04 | 0.001 ± 0.000 | 0.09 ± 0.01 |
| T = 90 °C, pH = 7 | 0.82 ± 0.01 | 0.08 ± 0.01 | 22.63 ± 0.09 | 0.65 ± 0.02 | 0.002 ± 0.000 | 0.10 ± 0.01 |
| T = 90 °C, pH = 11 | 0.88 ± 0.01 | 0.10 ± 0.02 | 21.93 ± 0.20 | 0.92 ± 0.01 | 0.003 ± 0.000 | 0.08 ± 0.00 |
| Higuchi | Hixson–Crowell | |||||
| Treatments | R2 | K | RMSE | R2 | K | RMSE |
| T = 30 °C, pH = 3 | 0.96 ± 0.01 | 0.71 ± 0.10 | 1.92 ± 0.03 | 0.80 ± 0.01 | 0.000 ± 0.000 | 0.10 ± 0.00 |
| T = 30 °C, pH = 7 | 0.96 ± 0.01 | 1.56 ± 0.21 | 6.30 ± 0.07 | 0.78 ± 0.01 | 0.001 ± 0.000 | 0.26 ± 0.01 |
| T = 30 °C, pH = 11 | 0.94 ± 0.01 | 1.70 ± 0.28 | 6.30 ± 0.11 | 0.98 ± 0.01 | 0.001 ± 0.000 | 0.11 ± 0.00 |
| T = 60 °C, pH = 3 | 0.97 ± 0.01 | 1.07 ± 0.16 | 2.27 ± 0.04 | 0.70 ± 0.02 | 0.001 ± 0.000 | 0.18 ± 0.01 |
| T = 60 °C, pH = 7 | 0.99 ± 0.01 | 1.58 ± 0.26 | 2.35 ± 0.04 | 0.89 ± 0.01 | 0.001 ± 0.000 | 0.20 ± 0.01 |
| T = 60 °C, pH = 11 | 0.96 ± 0.02 | 2.26 ± 0.18 | 4.79 ± 0.06 | 0.82 ± 0.01 | 0.002 ± 0.000 | 0.40 ± 0.01 |
| T = 90 °C, pH = 3 | 0.83 ± 0.02 | 1.65 ± 0.32 | 6.27 ± 0.08 | 0.14 ± 0.02 | 0.001 ± 0.000 | 0.41 ± 0.01 |
| T = 90 °C, pH = 7 | 0.88 ± 0.01 | 2.06 ± 0.04 | 6.99 ± 0.07 | 0.47 ± 0.02 | 0.002 ± 0.000 | 0.49 ± 0.02 |
| T = 90 °C, pH = 11 | 0.94 ± 0.01 | 2.37 ± 0.06 | 5.97 ± 0.08 | 0.78 ± 0.03 | 0.002 ± 0.000 | 0.45 ± 0.02 |
| Release (%) | ||
|---|---|---|
| Samples | 1st Minute | 2nd Minute |
| NC3 | 16.8 | 36.4 |
| NC6 | 26.1 | 49.3 |
| NC9 | 29.8 | 56.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, S.; Assadpour, E.; Kharazmi, M.S.; Jafarzadeh, S.; Zargar, M.; Jafari, S.M. Encapsulation of Orange Peel Oil in Biopolymeric Nanocomposites to Control Its Release under Different Conditions. Foods 2023, 12, 831. https://doi.org/10.3390/foods12040831
Ghasemi S, Assadpour E, Kharazmi MS, Jafarzadeh S, Zargar M, Jafari SM. Encapsulation of Orange Peel Oil in Biopolymeric Nanocomposites to Control Its Release under Different Conditions. Foods. 2023; 12(4):831. https://doi.org/10.3390/foods12040831
Chicago/Turabian StyleGhasemi, Sanaz, Elham Assadpour, Mohammad Saeed Kharazmi, Shima Jafarzadeh, Masoumeh Zargar, and Seid Mahdi Jafari. 2023. "Encapsulation of Orange Peel Oil in Biopolymeric Nanocomposites to Control Its Release under Different Conditions" Foods 12, no. 4: 831. https://doi.org/10.3390/foods12040831
APA StyleGhasemi, S., Assadpour, E., Kharazmi, M. S., Jafarzadeh, S., Zargar, M., & Jafari, S. M. (2023). Encapsulation of Orange Peel Oil in Biopolymeric Nanocomposites to Control Its Release under Different Conditions. Foods, 12(4), 831. https://doi.org/10.3390/foods12040831







