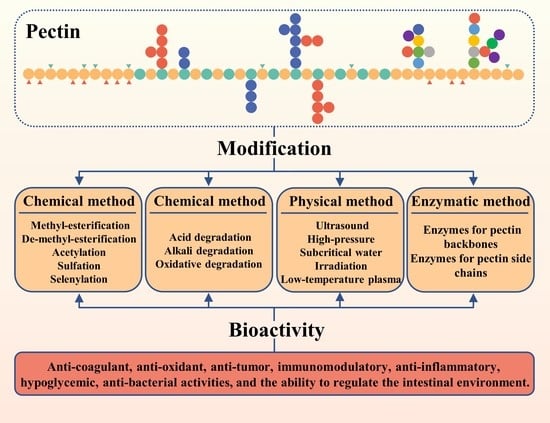
The Preparation and Potential Bioactivities of Modified Pectins: A Review
Abstract
:1. Introduction
2. Chemical Modification of Pectins
2.1. Introducing New Functional Groups
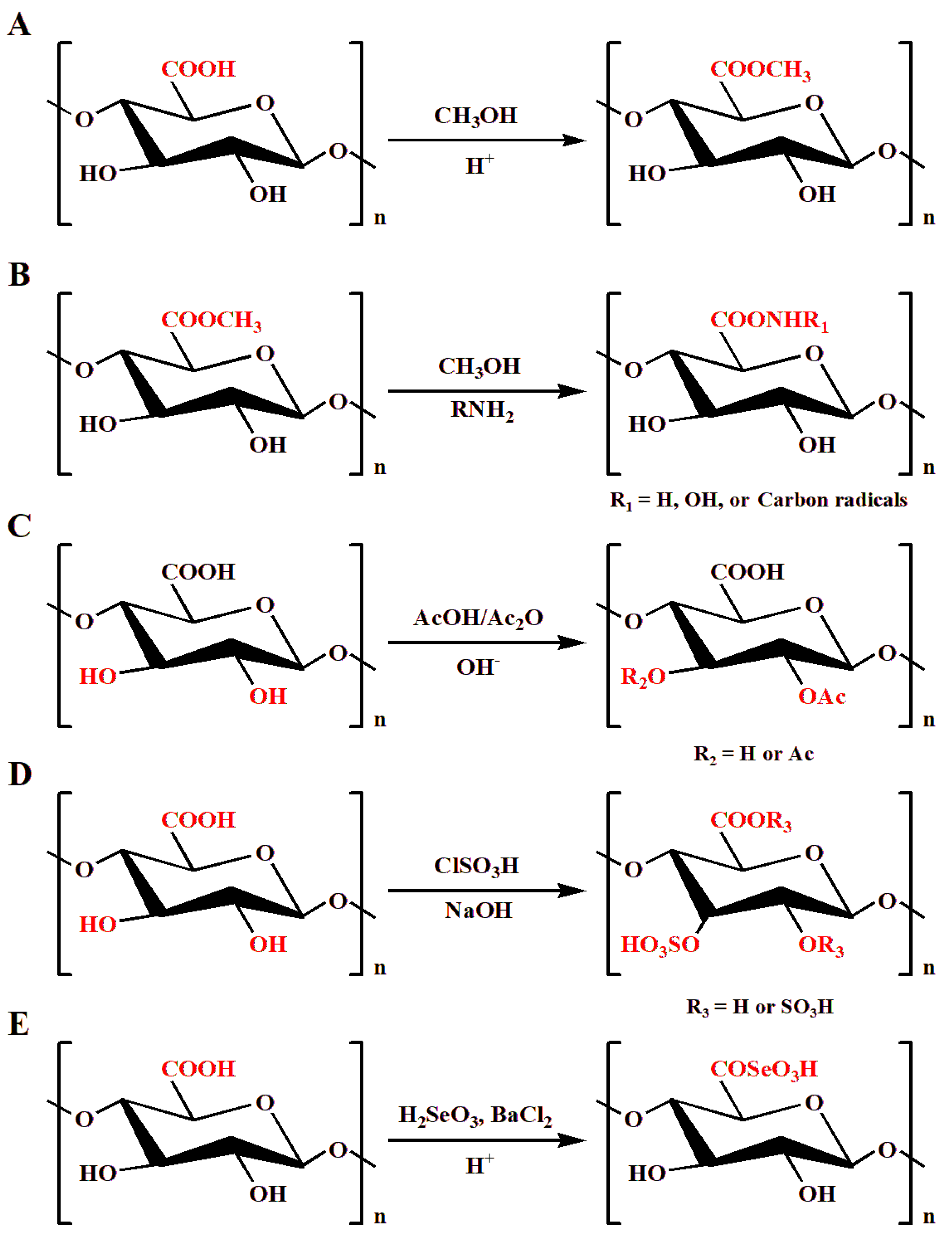
2.1.1. Methyl Esterification
2.1.2. Acetylation
2.1.3. Sulfation
2.1.4. Selenylation
2.2. Degradation of Pectins
2.2.1. Acid Degradation
2.2.2. Alkali Degradation
2.2.3. Oxidative Degradation
3. Physical Modification of Pectins
3.1. Ultrasound Modification
3.2. High-Pressure Modification
3.3. Subcritical Water Modification
3.4. Irradiation Modification
3.5. Low-Temperature Plasma Modification
3.6. Other Physical Modifications
4. Enzymatic Modification of Pectins
4.1. Modification of Pectin Backbones
4.1.1. Pectin Esterase
4.1.2. Pectin Hydrolase
4.1.3. Pectate Lyase
4.1.4. RG-I Acetylesterase, Hydrolase, and Lyase
4.2. Modification of Pectin Neutral Side Chains
5. Potential Bioactivities of Modified Pectins
5.1. Anti-Coagulant Activity
5.2. Anti-Oxidant Activity
5.3. Ability to Regulate the Intestinal Environment
5.3.1. Regulating Ability Based on Methyl Ester Groups/DM
5.3.2. Regulating Ability Based on Mw
5.3.3. Regulating Ability Based on Domains
5.4. Anti-Tumor Activity
5.5. Immunomodulatory Activity
5.6. Anti-Inflammatory Activity
5.7. Hypoglycemic Activity
5.8. Anti-Bacterial Activity
6. Conclusions and Future Prospects Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Liu, G.; Liang, L.; Yu, G.; Li, Q. Pumpkin Polysaccharide Modifies the Gut Microbiota during Alleviation of Type 2 Diabetes in Rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Hou, Z.; Hu, X.; Luan, L.; Yu, C.; Wang, X.; Chen, S.; Ye, X. Prebiotic potential of RG-I pectic polysaccharides from Citrus subcompressa by novel extraction methods. Food Hydrocoll. 2021, 124, 107213. [Google Scholar] [CrossRef]
- Larsen, N.; De Souza, C.B.; Krych, L.; Cahú, T.B.; Wiese, M.; Kot, W.; Hansen, K.M.; Blennow, A.; Venema, K.; Jespersen, L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Xiong, B.; Zhang, W.; Wu, Z.; Liu, R.; Yang, C.; Hui, A.; Huang, X.; Xian, Z. Preparation, characterization, antioxidant and anti-inflammatory activities of acid-soluble pectin from okra (Abelmoschus esculentus L.). Int. J. Biol. Macromol. 2021, 181, 824–834. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Wei, Y.; Yu, G.; Li, F.; Li, Q. Structural Characterization and Mechanisms of Macrophage Immunomodulatory Activity of a Pectic Polysaccharide from Cucurbita Moschata Duch. Carbohydr. Polym. 2021, 269, 118288. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A Novel Low-Molecular-Mass Pumpkin Polysaccharide: Structural Characterization, Antioxidant Activity, and Hypoglycemic Potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Li, F.; Zhao, J.; Wei, Y.; Jiao, X.; Li, Q. Holistic Review of Polysaccharides Isolated from Pumpkin: Preparation Methods, Structures and Bioactivities. Int. J. Biol. Macromol. 2021, 193, 541–552. [Google Scholar] [CrossRef]
- Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Wang, H.; Yu, W.; Li, Q. Structural Diversity and Physicochemical Properties of Polysaccharides Isolated from Pumpkin (Cucurbita Moschata) by Different Methods. Food Res. Int. 2023, 163, 112157. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Zhao, J.; Zhang, L.; Li, Q. In Vivo Pharmacokinetic Study of a Cucurbita Moschata Polysaccharide after Oral Administration. Int. J. Biol. Macromol. 2022, 203, 19–28. [Google Scholar] [CrossRef]
- Chaouch, M.A.; Hammi, K.M.; Dhahri, M.; Mansour, M.B.; Maaroufi, M.R.; Le Cerf, D.; Majdoub, H. Access to New Anticoagulant by Sulfation of Pectin-like Polysaccharides Isolated from Opuntia Ficus Indica Cladodes. Int. J. Biol. Macromol. 2018, 120, 1794–1800. [Google Scholar] [CrossRef]
- Huang, L.; Huang, M.; Shen, M.; Wen, P.; Wu, T.; Hong, Y.; Yu, Q.; Chen, Y.; Xie, J. Sulfated Modification Enhanced the Antioxidant Activity of Mesona Chinensis Benth Polysaccharide and Its Protective Effect on Cellular Oxidative Stress. Int. J. Biol. Macromol. 2019, 136, 1000–1006. [Google Scholar] [CrossRef]
- Huang, R.; Shen, M.; Yu, Y.; Liu, X.; Xie, J. Physicochemical Characterization and Immunomodulatory Activity of Sulfated Chinese Yam Polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, H.; Wang, L.; Liu, F.; Pan, S. Preparation and Prebiotic Potential of Pectin Oligosaccharides Obtained from Citrus Peel Pectin. Food Chem. 2018, 244, 232–237. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Trends in “Green” and Novel Methods of Pectin Modification—A Review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar] [CrossRef]
- Chen, T.T.; Zhang, Z.H.; Wang, Z.W.; Chen, Z.L.; Ma, H.; Yan, J.K. Effects of Ultrasound Modification at Different Frequency Modes on Physicochemical, Structural, Functional, and Biological Properties of Citrus Pectin. Food Hydrocoll. 2021, 113, 106484. [Google Scholar] [CrossRef]
- Gamonpilas, C.; Buathongjan, C.; Sangwan, W.; Rattanaprasert, M.; Weizman, K.C.; Klomtun, M.; Phonsatta, N.; Methacanon, P. Production of Low Molecular Weight Pectins via Electron Beam Irradiation and Their Potential Prebiotic Functionality. Food Hydrocoll. 2021, 113, 106551. [Google Scholar] [CrossRef]
- Mudugamuwa Arachchige, M.P.; Mu, T.; Ma, M. Effect of High Hydrostatic Pressure-Assisted Pectinase Modification on the Pb2+ Adsorption Capacity of Pectin Isolated from Sweet Potato Residue. Chemosphere 2021, 262, 128102. [Google Scholar] [CrossRef]
- Ma, X.; Wang, D.; Chen, W.; Ismail, B.B.; Wang, W.; Lv, R.; Ding, T.; Ye, X.; Liu, D. Effects of Ultrasound Pretreatment on the Enzymolysis of Pectin: Kinetic Study, Structural Characteristics and Anti-Cancer Activity of the Hydrolysates. Food Hydrocoll. 2018, 79, 90–99. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.M.; Li, T.; Liang, R.H.; Luo, S.J. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef]
- Liang, W.; Liao, J.; Qi, J.R.; Jiang, W.; Yang, X. Physicochemical Characteristics and Functional Properties of High Methoxyl Pectin with Different Degree of Esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.K.; Choi, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. The Characterization, Selenylation and Anti-Inflammatory Activity of Pectic Polysaccharides Extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2018, 111, 311–318. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Chemically Modified Polysaccharides: Synthesis, Characterization, Structure Activity Relationships of Action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Xie, L.; Shen, M.; Hong, Y.; Ye, H.; Huang, L.; Xie, J. Chemical Modifications of Polysaccharides and Their Anti-Tumor Activities. Carbohydr. Polym. 2020, 229, 115436. [Google Scholar] [CrossRef]
- Matricardi, P.; Dentini, M.; Crescenzi, V.; Ross-Murphy, S.B. Gelation of Chemically Cross-Linked Polygalacturonic Acid Derivatives. Carbohydr. Polym. 1995, 27, 215–220. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Jarvis, M.C. Acetylation and Methylation of Homogalacturonans 1: Optimisation of the Reaction and Characterisation of the Products. Carbohydr. Polym. 1999, 39, 201–207. [Google Scholar] [CrossRef]
- Rosenbohm, C.; Lundt, I.; Christensen, T.M.I.E.; Young, N.W.G. Chemically Methylated and Reduced Pectins: Preparation, Characterisation by 1H NMR Spectroscopy, Enzymatic Degradation, and Gelling Properties. Carbohydr. Res. 2003, 338, 637–649. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Thibault, J.F. Degradation of Pectins in Alkaline Conditions: Kinetics of Demethylation. Carbohydr. Res. 1996, 286, 139–150. [Google Scholar] [CrossRef]
- Ralet, M.C.; Bonnin, E.; Thibault, J.F. Chromatographic Study of Highly Methoxylated Lime Pectins Deesterified by Different Pectin Methyl-Esterases. J. Chromatogr. B Biomed. Sci. Appl. 2001, 753, 157–166. [Google Scholar] [CrossRef]
- Bae, I.Y.; Joe, Y.N.; Rha, H.J.; Lee, S.; Yoo, S.H.; Lee, H.G. Effect of Sulfation on the Physicochemical and Biological Properties of Citrus Pectins. Food Hydrocoll. 2009, 23, 1980–1983. [Google Scholar] [CrossRef]
- Vityazev, F.V.; Golovchenko, V.V.; Patova, O.A.; Drozd, N.N.; Makarov, V.A.; Shashkov, A.S.; Ovodov, Y.S. Synthesis of Sulfated Pectins and Their Anticoagulant Activity. Biochemistry 2010, 75, 759–768. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, X.; Yin, X.; Chen, S. Sulfation of Citrus Pectin by Pyridine-Sulfurtrioxide Complex and Its Anticoagulant Activity. LWT-Food Sci. Technol. 2015, 60, 1162–1167. [Google Scholar] [CrossRef]
- Román, Y.; de Oliveira Barddal, H.P.; Iacomini, M.; Sassaki, G.L.; Cipriani, T.R. Anticoagulant and Antithrombotic Effects of Chemically Sulfated Fucogalactan and Citrus Pectin. Carbohydr. Polym. 2017, 174, 731–739. [Google Scholar] [CrossRef]
- Chen, J.; Mei, M.S.; Xu, Y.; Xiong, S.; Zhao, Y.; Liu, R.; Shi, S.; Wang, H.; Wang, S. The Impact of the Methyl Esters of Homogalacturonan on Cellular Uptake Dependent Hypoglycemic Activity in IR-HepG2 Cells. Carbohydr. Polym. 2022, 293, 119741. [Google Scholar] [CrossRef]
- Pillai, P.K.S.; Stone, A.K.; Guo, Q.; Guo, Q.; Wang, Q.; Nickerson, M.T. Effect of Alkaline De-Esterified Pectin on the Complex Coacervation with Pea Protein Isolate under Different Mixing Conditions. Food Chem. 2019, 284, 227–235. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, H.; Wei, M.; Zhu, C. Effects of Enzymatic Treatment on the Physicochemical Properties and Antioxidant Activity of Hawthorn Pectin. Mater. Today Commun. 2022, 30, 103225. [Google Scholar] [CrossRef]
- Zhou, Y.; Mei, Y.; Luo, T.; Chen, W.; Zhong, Q.; Chen, H.; Chen, W. Study on the Relationship between Emulsion Properties and Interfacial Rheology of Sugar Beet Pectin Modified by Different Enzymes. Molecules 2021, 26, 2829. [Google Scholar] [CrossRef]
- Ahmad, M.M. Recent Trends in Chemical Modification and Antioxidant Activities of Plants-Based Polysaccharides: A Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Maas, N.C.; Gracher, A.H.P.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M.; Cipriani, T.R. Sulfation Pattern of Citrus Pectin and Its Carboxy-Reduced Derivatives: Influence on Anticoagulant and Antithrombotic Effects. Carbohydr. Polym. 2012, 89, 1081–1087. [Google Scholar] [CrossRef] [Green Version]
- De Sousa, A.L.N.; Ribeiro, A.C.B.; Santos, D.G.; Ricardo, N.M.P.S.; Ribeiro, M.E.N.P.; Cavalcanti, E.S.B.; Cunha, A.P.; Ricardo, N.M.P.S. ModificaÇÃO QuÍmica Da Pectina Do MelÃo Caipira (Cucumis Melo VAR. Acidulus). Quim. Nova 2017, 40, 554–560. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated Modification of Polysaccharides: Synthesis, Characterization and Bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, J.; Qiu, S.; Li, Y.; Wang, D.; Liu, C.; Li, X.; Hou, R.; Yue, C.; Liu, J.; et al. Optimization of Selenylation Modification for Garlic Polysaccharide Based on Immune-Enhancing Activity. Carbohydr. Polym. 2016, 136, 560–569. [Google Scholar] [CrossRef]
- Wei, D.; Chen, T.; Yan, M.; Zhao, W.; Li, F.; Cheng, W.; Yuan, L. Synthesis, Characterization, Antioxidant Activity and Neuroprotective Effects of Selenium Polysaccharide from Radix Hedysari. Carbohydr. Polym. 2015, 125, 161–168. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Rokita, B.; Lotfy, S.; Ulanski, P.; Rosiak, J.M. Degradation of Chitosan and Starch by 360-KHz Ultrasound. Carbohydr. Polym. 2005, 60, 175–184. [Google Scholar] [CrossRef]
- Coenen, G.J.; Bakx, E.J.; Verhoef, R.P.; Schols, H.A.; Voragen, A.G.J. Identification of the Connecting Linkage between Homo- or Xylogalacturonan and Rhamnogalacturonan Type I. Carbohydr. Polym. 2007, 70, 224–235. [Google Scholar] [CrossRef]
- Morris, V.J.; Belshaw, N.J.; Waldron, K.W.; Maxwell, E.G. The Bioactivity of Modified Pectin Fragments. Bioact. Carbohydr. Diet. Fibre 2013, 1, 21–37. [Google Scholar] [CrossRef]
- Garna, H.; Mabon, N.; Nott, K.; Wathelet, B.; Paquot, M. Kinetic of the Hydrolysis of Pectin Galacturonic Acid Chains and Quantification by Ionic Chromatography. Food Chem. 2006, 96, 477–484. [Google Scholar] [CrossRef]
- Li, S.; Yang, G.; Yan, J.; Wu, D.; Hou, Y.; Diao, Q.; Zhou, Y. Polysaccharide Structure and Immunological Relationships of RG-I Pectin from the Bee Pollen of Nelumbo Nucifera. Int. J. Biol. Macromol. 2018, 111, 660–666. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Sun, L.; Ji, L.; Zhu, J.; Fan, Y.; Tai, G.; Zhou, Y. Further Analysis of the Structure and Immunological Activity of an RG-I Type Pectin from Panax Ginseng. Carbohydr. Polym. 2012, 89, 519–525. [Google Scholar] [CrossRef]
- Bemiller, J.N.; Kumari, G.V. beta-elimination in uronic acids: Evidence for an ElcB mechanism. Carbohydr. Res. 1972, 25, 419–428. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Yu, S.; Guo, X.; Ai, C.; Tang, X.; Chen, H.; Lin, J.; Zhang, X.; Meng, H. Effects of PH and Temperature on the Structure, Rheological and Gel-Forming Properties of Sugar Beet Pectins. Food Hydrocoll. 2021, 116, 106646. [Google Scholar] [CrossRef]
- Kirtchev, N.; Panchev, I.; Kratchanov, C. Kinetics of Acid-catalysed De-esterification of Pectin in a Heterogeneous Medium. Int. J. Food Sci. Technol. 1989, 24, 479–486. [Google Scholar] [CrossRef]
- Sajjaanantakul, T.; Van BUREN, J.P.; Downing, D.L. Effect of Methyl Ester Content on Heat Degradation of Chelator-Soluble Carrot Pectin. J. Food Sci. 1989, 54, 1272–1277. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Liu, S.; Wei, C.; Yan, L.; Ding, T.; Linhardt, R.J.; Liu, D.; Ye, X.; Chen, S. Pectic Oligosaccharides Hydrolyzed from Citrus Canning Processing Water by Fenton Reaction and Their Antiproliferation Potentials. Int. J. Biol. Macromol. 2019, 124, 1025–1032. [Google Scholar] [CrossRef]
- Zhi, Z.; Chen, J.; Li, S.; Wang, W.; Huang, R.; Liu, D.; DIng, T.; Linhardt, R.J.; Chen, S.; Ye, X. Fast Preparation of RG-I Enriched Ultra-Low Molecular Weight Pectin by an Ultrasound Accelerated Fenton Process. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yeung, Y.K.; Kang, Y.R.; So, B.R.; Jung, S.K.; Chang, Y.H. Structural, Antioxidant, Prebiotic and Anti-Inflammatory Properties of Pectic Oligosaccharides Hydrolyzed from Okra Pectin by Fenton Reaction. Food Hydrocoll. 2021, 118, 106779. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Zheng, Y.; Zhang, H.; Chen, J.; Yan, L.; Ding, T.; Linhardt, R.J.; Orfila, C.; Liu, D.; et al. Fast Preparation of Rhamnogalacturonan I Enriched Low Molecular Weight Pectic Polysaccharide by Ultrasonically Accelerated Metal-Free Fenton Reaction. Food Hydrocoll. 2019, 95, 551–561. [Google Scholar] [CrossRef]
- Hu, W.; Chen, S.; Wu, D.; Zheng, J.; Ye, X. Ultrasonic-Assisted Citrus Pectin Modification in the Bicarbonate-Activated Hydrogen Peroxide System: Chemical and Microstructural Analysis. Ultrason. Sonochem. 2019, 58, 104576. [Google Scholar] [CrossRef]
- Cao, J.; Yang, J.; Yue, K.; Wang, Z. Preparation of Modified Citrus Pectin (MCP) Using an Advanced Oxidation Process with Hydroxyl Radicals Generated by UV-H2O2. Food Hydrocoll. 2020, 102, 105587. [Google Scholar] [CrossRef]
- Bekli, S.; Aktas, B.; Gencer, D.; Aslim, B. Biochemical and Molecular Characterizations of a Novel PH- and Temperature-Stable Pectate Lyase from Bacillus Amyloliquefaciens S6 for Industrial Application. Mol. Biotechnol. 2019, 61, 681–693. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Montilla, A.; Moreno, F.J.; Villamiel, M. Modification of Citrus and Apple Pectin by Power Ultrasound: Effects of Acid and Enzymatic Treatment. Ultrason. Sonochem. 2017, 38, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Ogutu, F.O.; Mu, T.H. Ultrasonic Degradation of Sweet Potato Pectin and Its Antioxidant Activity. Ultrason. Sonochem. 2017, 38, 726–734. [Google Scholar] [CrossRef]
- Chen, X.; Qi, Y.; Zhu, C.; Wang, Q. Effect of Ultrasound on the Properties and Antioxidant Activity of Hawthorn Pectin. Int. J. Biol. Macromol. 2019, 131, 273–281. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Lan, X.; Gong, S.; Wu, J.; Wang, Z. Effects of High Hydrostatic Pressure and High Pressure Homogenization Processing on Characteristics of Potato Peel Waste Pectin. Carbohydr. Polym. 2018, 196, 474–482. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.H.; Liu, W.; Liu, C.M.; Li, T.; Tu, Z.C.; Wan, J. Degradation of High-Methoxyl Pectin by Dynamic High Pressure Microfluidization and Its Mechanism. Food Hydrocoll. 2012, 28, 121–129. [Google Scholar] [CrossRef]
- Zhong, L.; Li, X.; Duan, M.; Song, Y.; He, N.; Che, L. Impacts of High Hydrostatic Pressure Processing on the Structure and Properties of Pectin. LWT 2021, 148, 111793. [Google Scholar] [CrossRef]
- Yan, J.K.; Wu, L.X.; Cai, W.D.; Xiao, G.S.; Duan, Y.; Zhang, H. Subcritical Water Extraction-Based Methods Affect the Physicochemical and Functional Properties of Soluble Dietary Fibers from Wheat Bran. Food Chem. 2019, 298, 124987. [Google Scholar] [CrossRef]
- Ramirez, C.S.V.; Temelli, F.; Saldaña, M.D.A. Production of Pea Hull Soluble Fiber-Derived Oligosaccharides Using Subcritical Water with Carboxylic Acids. J. Supercrit. Fluids 2021, 178, 105349. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. The Potential of Subcritical Water as a “Green” Method for the Extraction and Modification of Pectin: A Critical Review. Food Res. Int. 2022, 161, 111849. [Google Scholar] [CrossRef]
- Klinchongkon, K.; Khuwijitjaru, P.; Adachi, S. Degradation Kinetics of Passion Fruit Pectin in Subcritical Water. Biosci. Biotechnol. Biochem. 2017, 81, 712–717. [Google Scholar] [CrossRef] [Green Version]
- Klinchongkon, K.; Khuwijitjaru, P.; Adachi, S. Properties of Subcritical Water-Hydrolyzed Passion Fruit (Passiflora edulis) Pectin. Food Hydrocoll. 2018, 74, 72–77. [Google Scholar] [CrossRef]
- Odueke, O.B.; Farag, K.W.; Baines, R.N.; Chadd, S.A. Irradiation Applications in Dairy Products: A Review. Food Bioprocess Technol. 2016, 9, 751–767. [Google Scholar] [CrossRef]
- Pillai, S.D.; Shayanfar, S. Electron Beam Technology and Other Irradiation Technology Applications in the Food Industry. Top. Curr. Chem. 2017, 375, 249–268. [Google Scholar] [CrossRef]
- Ayyad, K.; Hassanien, F.; Ragab, M. The effect of y irradiation on the structure of pectin. Mol. Nutr. Food Res. 1990, 34, 465–468. [Google Scholar] [CrossRef]
- Sjöberg, A.M. The Effects of γ Irradiation on the Structure of Apple Pectin. Food Hydrocoll. 1987, 1, 271–276. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A Novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, R.k. Cold Plasma Treatment of Dairy Proteins in Relation to Functionality Enhancement. Trends Food Sci. Technol. 2020, 102, 30–36. [Google Scholar] [CrossRef]
- Zielinska, S.; Cybulska, J.; Pieczywek, P.; Zdunek, A.; Kurzyna-Szklarek, M.; Staniszewska, I.; Liu, Z.L.; Pan, Z.; Xiao, H.W.; Zielinska, M. Structural Morphology and Rheological Properties of Pectin Fractions Extracted from Okra Pods Subjected to Cold Plasma Treatment. Food Bioprocess Technol. 2022, 15, 1168–1181. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Impact of Atmospheric Pressure Cold Plasma on the Rheological and Gelling Properties of High Methoxyl Apple Pectin. Food Hydrocoll. 2022, 129, 107639. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. Atmospheric Pressure Pin-to-Plate Cold Plasma Modification of High Methoxyl Apple Pectin: Impact on Functional Properties. J. Agric. Food Res. 2022, 9, 100356. [Google Scholar] [CrossRef]
- Hu, W.; Li, P.; Guo, D.; Zhang, B.; Tao, D.; Li, J.; Zhong, W.; Zang, H.; Xu, Y.; Ma, F. Effect of Solution Pulsed Plasma Process on the Degradation and Physicochemical Properties of Pectin. Food Hydrocoll. 2023, 136, 108236. [Google Scholar] [CrossRef]
- Hua, X.; Yang, H.; Din, P.; Chi, K.; Yang, R. Rheological Properties of Deesterified Pectin with Different Methoxylation Degree. Food Biosci. 2018, 23, 91–99. [Google Scholar] [CrossRef]
- Santos, E.E.; Amaro, R.C.; Bustamante, C.C.C.; Guerra, M.H.A.; Soares, L.C.; Froes, R.E.S. Extraction of Pectin from Agroindustrial Residue with an Ecofriendly Solvent: Use of FTIR and Chemometrics to Differentiate Pectins According to Degree of Methyl Esterification. Food Hydrocoll. 2020, 107, 105921. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR Spectroscopy, a Reliable Method for Routine Analysis of the Degree of Methylesterification of Pectin in Different Fruit- and Vegetable-Based Matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef]
- Grasdalen, H.; Einar Bakøy, O.; Larsen, B. Determination of the Degree of Esterification and the Distribution of Methylated and Free Carboxyl Groups in Pectins by 1H-n.m.r. Spectroscopy. Carbohydr. Res. 1988, 184, 183–191. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; He, X.; Wei, X. Preparation, Structural Characterization and Bioactivities of Se-Containing Polysaccharide: A Review. Int. J. Biol. Macromol. 2018, 120, 82–92. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.K.; Chang, Y.H. Effects of Selenylation Modification on Structural and Antioxidant Properties of Pectic Polysaccharides Extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2017, 104, 1124–1132. [Google Scholar] [CrossRef]
- Tao, W.; An, X.; Guo, Z.; Yang, N.; Wu, M.; Oliveira, H.; Zhang, R.; He, J. Structural Characterization, Acute Toxicity Assessment and Protective Effects of Selenylated Apple Pectin on Dextran Sulfate Sodium-Induced Ulcerative Colitis. Food Funct. 2022, 13, 7320–7332. [Google Scholar] [CrossRef]
- Keijbets, M.J.H.; Pilnik, W. β-Elimination of Pectin in the Presence of Anions and Cations. Carbohydr. Res. 1974, 33, 359–362. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, C.; Qiu, W.Y.; Chen, T.T.; Yang, Y.; Wang, W.H.; Zhang, H.N. Ultrasonic Treatment at Different PH Values Affects the Macromolecular, Structural, and Rheological Characteristics of Citrus Pectin. Food Chem. 2021, 341, 128216. [Google Scholar] [CrossRef]
- Larsen, L.R.; van der Weem, J.; Caspers-Weiffenbach, R.; Schieber, A.; Weber, F. Effects of Ultrasound on the Enzymatic Degradation of Pectin. Ultrason. Sonochem. 2021, 72, 105465. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Wang, D.; Ding, T.; Ye, X.; Liu, D. Degradation Kinetics and Structural Characteristics of Pectin under Simultaneous Sonochemical-Enzymatic Functions. Carbohydr. Polym. 2016, 154, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Iordache, M.; Jelen, P. High pressure microfluidization treatment of heat denatured whey proteins for improved functionality. Innov. Food Sci. Emerg. 2003, 4, 367–376. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhong, J.Z.; Liu, W.; Tu, Z.C.; Wan, J.; Cai, X.F.; Song, X.Y. Relationship between Functional Properties and Aggregation Changes of Whey Protein Induced by High Pressure Microfluidization. J. Food Sci. 2011, 76, E341–E347. [Google Scholar] [CrossRef]
- Shpigelman, A.; Kyomugasho, C.; Christiaens, S.; Van Loey, A.M.; Hendrickx, M.E. The Effect of High Pressure Homogenization on Pectin: Importance of Pectin Source and PH. Food Hydrocoll. 2015, 43, 189–198. [Google Scholar] [CrossRef]
- Silvestri, S.; Gabrielson, G. Degradation of Tragacanth by High Shear and Turbulent Forces during Microfluidization. Int. J. Pharm. 1991, 73, 163–169. [Google Scholar] [CrossRef]
- Sun, Y.; Yao, J.; Zhang, L.; Chen, F.; Hu, X.; Zhang, Y. New Evidence on Pectin-Related Instantaneous Pressure Softening Mechanism of Asparagus Lettuce under High Pressure Processing. Food Sci. Technol. Int. 2019, 25, 337–346. [Google Scholar] [CrossRef]
- Arachchige, M.P.M.; Mu, T.; Ma, M. Structural, Physicochemical and Emulsifying Properties of Sweet Potato Pectin Treated by High Hydrostatic Pressure and/or Pectinase: A Comparative Study. J. Sci. Food Agric. 2020, 100, 4911–4920. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, F.; Lan, X.; Gong, S.; Wang, Z. Characteristics of Pectin from Black Cherry Tomato Waste Modified by Dynamic High-Pressure Microfluidization. J. Food Eng. 2018, 216, 90–97. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.H.; Liu, W.; Li, T.; Liu, C.M.; Wu, S.S.; Wang, Z.J. Pectic-Oligosaccharides Prepared by Dynamic High-Pressure Microfluidization and Their in Vitro Fermentation Properties. Carbohydr. Polym. 2013, 91, 175–182. [Google Scholar] [CrossRef]
- Liu, C.M.; Liang, L.; Shuai, X.X.; Liang, R.H.; Chen, J. Dynamic High-Pressure Microfluidization-Treated Pectin under Different Ethanol Concentrations. Polymers 2018, 10, 1410. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.; Chen, Q.; Huang, M.; Liu, F.; Pan, S. Physiochemical, Rheological and Emulsifying Properties of Low Methoxyl Pectin Prepared by High Hydrostatic Pressure-Assisted Enzymatic, Conventional Enzymatic, and Alkaline de-Esterification: A Comparison Study. Food Hydrocoll. 2019, 93, 146–155. [Google Scholar] [CrossRef]
- Ma, J.; Tong, P.; Chen, Y.; Wang, Y.; Ren, H.; Gao, Z.; Yue, T.; Long, F. The Inhibition of Pectin Oligosaccharides on Degranulation of RBL-2H3 Cells from Apple Pectin with High Hydrostatic Pressure Assisted Enzyme Treatment. Food Chem. 2022, 371, 131097. [Google Scholar] [CrossRef]
- Pińkowska, H.; Krzywonos, M.; Wolak, P.; Złocińska, A. Production of Uronic Acids by Hydrothermolysis of Pectin as a Model Substance for Plant Biomass Waste. Green Process. Synth. 2019, 8, 683–690. [Google Scholar] [CrossRef]
- Ramirez, C.S.V.; Temelli, F.; Saldaña, M.D.A. Carboxylic Acid-Catalyzed Hydrolysis of Rhamnogalacturonan in Subcritical Water Media. J. Supercrit. Fluids 2021, 175, 105268. [Google Scholar] [CrossRef]
- Ho, J.K.; Jo, C.; Joong, H.K.; Jun, H.S.; Bong, J.A.; Myung, W.B. Antioxidant and Cancer Cell Proliferation Inhibition Effect of Citrus Pectin-Oligosaccharide Prepared by Irradiation. J. Med. Food 2006, 9, 313–320. [Google Scholar] [CrossRef]
- Momeni, M.; Tabibiazar, M.; Khorram, S.; Zakerhamidi, M.; Mohammadifar, M.; Valizadeh, H.; Ghorbani, M. Pectin Modification Assisted by Nitrogen Glow Discharge Plasma. Int. J. Biol. Macromol. 2018, 120, 2572–2578. [Google Scholar] [CrossRef]
- Calce, E.; Bugatti, V.; Vittoria, V.; De Luca, S. Solvent-Free Synthesis of Modified Pectin Compounds Promoted by Microwave Irradiation. Molecules 2012, 17, 12234–12242. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.H. Pulsed Electric Field-Assisted Modification of Pectin from Sugar Beet Pulp. Carbohydr. Polym. 2013, 92, 1700–1704. [Google Scholar] [CrossRef]
- Chen, J.; Wu, S.S.; Liang, R.H.; Liu, W.; Liu, C.M.; Shuai, X.X.; Wang, Z.J. The Effect of High Speed Shearing on Disaggregation and Degradation of Pectin from Creeping Fig Seeds. Food Chem. 2014, 165, 1–8. [Google Scholar] [CrossRef]
- Kotnala, B.; Shashirekha, M.N.; Vasu, P. Purification and Characterization of a Salt-Dependent Pectin Methylesterase from Carica Papaya Fruit Mesocarp-Exocarp Tissue. J. Food Sci. 2018, 83, 2062–2070. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, J.; Zhang, Z.; Ma, L.; Xu, T.; Yu, H.; Zhang, Q.; Chen, Y. Overexpression of Escherichia Coli Acetyl Esterase Using a Strategy of Multi-Copy Promoters. Waste Biomass Valorization 2018, 9, 561–570. [Google Scholar] [CrossRef]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; Mortensen, A.; et al. Safety Evaluation of the Food Enzyme Containing Endo-Polygalacturonase, Pectinesterase, Pectin Lyase and Non-Reducing End α-l-Arabinofuranosidase Activities from the Aspergillus Niger Strain PEC. EFSA J. 2022, 20, 1–17. [Google Scholar] [CrossRef]
- Amin, F.; Bhatti, H.N.; Bilal, M.; Asgher, M. Multiple Parameter Optimizations for Enhanced Biosynthesis of Exo-Polygalacturonase Enzyme and Its Application in Fruit Juice Clarification. Int. J. Food Eng. 2017, 13, 465–468. [Google Scholar] [CrossRef]
- Fahmy, A.S.; El-Beih, F.M.; Mohamed, S.A.; Abdel-Gany, S.S.; Abd-Elbaky, E.A. Characterization of an Exopolygalacturonase from Aspergillus Niger. Appl. Biochem. Biotechnol. 2008, 149, 205–217. [Google Scholar] [CrossRef]
- Seegmiller, C.G.; Jansen, E.F. Polymethylgalacturonase, an Enzyme Causing the Glycosidic Hydrolysis of Esterified Pectic Substances. J. Biol. Chem. 1952, 195, 327–336. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Zhang, D.; Du, G.; Chen, J. Improved Production of Polygalacturonate Lyase by Combining a PH and Online Methanol Control Strategy in a Two-Stage Induction Phase with a Shift in the Transition Phase. J. Ind. Microbiol. Biotechnol. 2010, 37, 323–333. [Google Scholar] [CrossRef]
- Sesmero, R.; Mitchell, J.R.; Mercado, J.A.; Quesada, M.A. Rheological Characterisation of Juices Obtained from Transgenic Pectate Lyase-Silenced Strawberry Fruits. Food Chem. 2009, 116, 426–432. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, Y.; Li, Q.; Zhu, B. Pectinolytic Lyases: A Comprehensive Review of Sources, Category, Property, Structure, and Catalytic Mechanism of Pectate Lyases and Pectin Lyases. Bioresour. Bioprocess. 2021, 8, 79. [Google Scholar] [CrossRef]
- Nighojkar, A.; Patidar, M.K.; Nighojkar, S. Pectinases: Production and Applications for Fruit Juice Beverages. In Processing and Sustainability of Beverages; Woodhead Publishing, Abington Hall: Cambridge, UK, 2019; pp. 235–273. [Google Scholar]
- Silva, I.R.; Jers, C.; Meyer, A.S.; Mikkelsen, J.D. Rhamnogalacturonan I Modifying Enzymes: An Update. N. Biotechnol. 2016, 33, 41–54. [Google Scholar] [CrossRef]
- Itoh, T.; Ochiai, A.; Mikami, B.; Hashimoto, W.; Murata, K. A Novel Glycoside Hydrolase Family 105: The Structure of Family 105 Unsaturated Rhamnogalacturonyl Hydrolase Complexed with a Disaccharide in Comparison with Family 88 Enzyme Complexed with the Disaccharide. J. Mol. Biol. 2006, 360, 573–585. [Google Scholar] [CrossRef]
- Schols, H.A.; Geraeds, C.C.J.M.; Searle-van Leeuwen, M.F.; Kormelink, F.J.M.; Voragen, A.G.J. Rhamnogalacturonase: A Novel Enzyme That Degrades the Hairy Regions of Pectins. Carbohydr. Res. 1990, 206, 105–115. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yamada, H.; Kunishige, Y.; Takenaka, S.; Nakazawa, M.; Ueda, M.; Sakamoto, T. Identification of a Novel Penicillium Chrysogenum Rhamnogalacturonan Rhamnohydrolase and the First Report of a Rhamnogalacturonan Rhamnohydrolase Gene. Enzym. Microb. Technol. 2017, 98, 76–85. [Google Scholar] [CrossRef]
- Ochiai, A.; Itoh, T.; Mikami, B.; Hashimoto, W.; Murata, K. Structural Determinants Responsible for Substrate Recognition and Mode of Action in Family 11 Polysaccharide Lyases. J. Biol. Chem. 2009, 284, 10181–10189. [Google Scholar] [CrossRef] [Green Version]
- Beldman, G.; Searle-van Leeuwen, M.J.F.; De Ruiter, G.A.; Siliha, H.A.; Voragen, A.G.J. Degradation of Arabinans by Arabinanases from Aspergillus Aculeatus and Aspergillus Niger. Carbohydr. Polym. 1993, 20, 159–168. [Google Scholar] [CrossRef]
- Holck, J.; Hjernø, K.; Lorentzen, A.; Vigsnæs, L.K.; Hemmingsen, L.; Licht, T.R.; Mikkelsen, J.D.; Meyer, A.S. Tailored Enzymatic Production of Oligosaccharides from Sugar Beet Pectin and Evidence of Differential Effects of a Single DP Chain Length Difference on Human Faecal Microbiota Composition after in Vitro Fermentation. Process Biochem. 2011, 46, 1039–1049. [Google Scholar] [CrossRef]
- Pillai, P.K.S.; Morales-Contreras, B.E.; Wicker, L.; Nickerson, M.T. Effect of Enzyme De-Esterified Pectin on the Electrostatic Complexation with Pea Protein Isolate under Different Mixing Conditions. Food Chem. 2020, 305, 125433. [Google Scholar] [CrossRef]
- Humerez-Flores, J.N.; Verkempinck, S.H.E.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Targeted Modifications of Citrus Pectin to Improve Interfacial Properties and the Impact on Emulsion Stability. Food Hydrocoll. 2022, 132, 107841. [Google Scholar] [CrossRef]
- Humerez-Flores, J.N.; Kyomugasho, C.; Gutiérrez-Ortiz, A.A.; De Bie, M.; Panozzo, A.; Van Loey, A.M.; Moldenaers, P.; Hendrickx, M.E. Production and Molecular Characterization of Tailored Citrus Pectin-Derived Compounds. Food Chem. 2022, 367, 130635. [Google Scholar] [CrossRef]
- Oosterveld, A.; Beldman, G.; Voragen, A.G.J. Enzymatic Modification of Pectic Polysaccharides Obtained from Sugar Beet Pulp. Carbohydr. Polym. 2002, 48, 73–81. [Google Scholar] [CrossRef]
- Combo, A.M.M.; Aguedo, M.; Quiévy, N.; Danthine, S.; Goffin, D.; Jacquet, N.; Blecker, C.; Devaux, J.; Paquot, M. Characterization of Sugar Beet Pectic-Derived Oligosaccharides Obtained by Enzymatic Hydrolysis. Int. J. Biol. Macromol. 2013, 52, 148–156. [Google Scholar] [CrossRef]
- Abbott, D.W.; Boraston, A.B. A Family 2 Pectate Lyase Displays a Rare Fold and Transition Metal-Assisted β-Elimination. J. Biol. Chem. 2007, 282, 35328–35336. [Google Scholar] [CrossRef] [Green Version]
- Bonnin, E.; Ralet, M.C.; Thibault, J.F.; Schols, H.A. Enzymes for the valorisation of fruit- and vegetable-based co-products. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Waldron, K.W., Ed.; Woodhead Publishing, Abington Hall: Cambridge, UK, 2009; pp. 257–285. [Google Scholar]
- Lima, J.O.; Pereira, J.F.; de Araújo, E.F.; Queiroz, M.V. de Pectin Lyase Overproduction by Penicillium Griseoroseum Mutants Resistant to Catabolite Repression. Braz. J. Microbiol. 2017, 48, 602–606. [Google Scholar] [CrossRef]
- Sukhumsiirchart, W.; Kawanishi, S.; Deesukon, W.; Chansiri, K.; Kawasaki, H.; Sakamoto, T. Purification, Characterization, and Overexpression of Thermophilic Pectate Lyase of Bacillus Sp. Rn1 Isolated from a Hot Spring in Thailand. Biosci. Biotechnol. Biochem. 2009, 73, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Guo, Z.; Cao, S.; Zhu, B. Elucidating the Degradation Pattern of a New Cold-Tolerant Pectate Lyase Used for Efficient Preparation of Pectin Oligosaccharides. Bioresour. Bioprocess. 2021, 8, 121. [Google Scholar] [CrossRef]
- Zheng, L.; Guo, Z.; Xu, Y.; Zhu, B.; Yao, Z. Biochemical Characterization and Immobilization of a Novel Pectate Lyase ErPL2 for Efficient Preparation of Pectin Oligosaccharides. Int. J. Biol. Macromol. 2022, 204, 532–539. [Google Scholar] [CrossRef]
- Mutter, M.; Beldman, G.; Schols, H.A.; Gerard, A.; Voragen, J. Rhamnogalacturonan α-L-Rhamnopyranohydrolase (A Novel Enzyme Specific for the Terminal Nonreducing Rhamnosyl Unit in Rhamnogalacturonan Regions of Pectin). Plant Physiol. 1994, 106, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Pitson, S.M.; Mutter, M.; Van Den Broek, L.A.M.; Voragen, A.G.J.; Beldman, G. Stereochemical Course of Hydrolysis Catalysed by α-L-Rhamnosyl and α-D-Galacturonosyl Hydrolases from Aspergillus Aculeatus. Biochem. Biophys. Res. Commun. 1998, 242, 552–559. [Google Scholar] [CrossRef]
- Ochiai, A.; Itoh, T.; Kawamata, A.; Hashimoto, W.; Murata, K. Plant Cell Wall Degradation by Saprophytic Bacillus Subtilis Strains: Gene Clusters Responsible for Rhamnogalacturonan Depolymerization. Appl. Environ. Microbiol. 2007, 73, 3803–3813. [Google Scholar] [CrossRef] [Green Version]
- Kunishige, Y.; Iwai, M.; Nakazawa, M.; Ueda, M.; Tada, T.; Nishimura, S.; Sakamoto, T. Crystal Structure of Exo-Rhamnogalacturonan Lyase from Penicillium Chrysogenum as a Member of Polysaccharide Lyase Family 26. FEBS Lett. 2018, 592, 1378–1388. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, M.A.; Eberhard, S.; Albersheim, P.; Darvill, A.G. Requirement of Borate Cross-Linking of Cell Wall Rhamnogalacturonan II for Arabidopsis Growth. Science 2001, 294, 846–849. [Google Scholar] [CrossRef]
- Øbro, J.; Harholt, J.; Scheller, H.V.; Orfila, C. Rhamnogalacturonan I in Solanum Tuberosum Tubers Contains Complex Arabinogalactan Structures. Phytochemistry 2004, 65, 1429–1438. [Google Scholar] [CrossRef]
- Sousa, A.G.; Nielsen, H.L.; Armagan, I.; Larsen, J.; Sørensen, S.O. The Impact of Rhamnogalacturonan-I Side Chain Monosaccharides on the Rheological Properties of Citrus Pectin. Food Hydrocoll. 2015, 47, 130–139. [Google Scholar] [CrossRef]
- Klaassen, M.T.; Trindade, L.M. RG-I Galactan Side-Chains Are Involved in the Regulation of the Water-Binding Capacity of Potato Cell Walls. Carbohydr. Polym. 2020, 227, 115353. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Zhang, H.; Wu, D.; Ye, X.; Linardt, R.J.; Chen, S. Gelling Mechanism of RG-I Enriched Citrus Pectin: Role of Arabinose Side-Chains in Cation- and Acid-Induced Gelation. Food Hydrocoll. 2020, 101, 105536. [Google Scholar] [CrossRef]
- Noguchi, M.; Hasegawa, Y.; Suzuki, S.; Nakazawa, M.; Ueda, M.; Sakamoto, T. Determination of Chemical Structure of Pea Pectin by Using Pectinolytic Enzymes. Carbohydr. Polym. 2020, 231, 115738. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Park, J.J.; Park, G.D.; Lee, W.Y. Enzymatic Hydrolysis Modifies Emulsifying Properties of Okra Pectin. Foods 2022, 11, 1497. [Google Scholar] [CrossRef]
- Da Silva Cerqueira Leite, K.M.; Tadiotti, A.C.; Baldochi, D.; Oliveira, O.M.M.F. Partial Purification, Heat Stability and Kinetic Characterization of the Pectinmethylesterase from Brazilian Guava, Paluma Cultivars. Food Chem. 2006, 94, 565–572. [Google Scholar] [CrossRef]
- Yeoman, C.J.; Han, Y.; Dodd, D.; Schroeder, C.M.; Mackie, R.I.; Cann, I.K.O. Thermostable enzymes as biocatalysts in the biofuel industry. Adv. Appl. Microbiol. 2010, 70, 1–55. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.C.; Satyanarayana, T. Thermostable and Alkalistable Exopolygalacturonase of Bacillus Pumilus Dcsr1: Characteristics and Applicability. Int. J. Biol. Macromol. 2020, 164, 3340–3348. [Google Scholar] [CrossRef]
- Rahman, M.S.; Choi, Y.S.; Kim, Y.K.; Park, C.; Yoo, J.C. Production of Novel Polygalacturonase from Bacillus Paralicheniformis CBS32 and Application to Depolymerization of Ramie Fiber. Polymers. 2019, 11, 1525. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Huo, W.; Dai, X.J.; Dang, Y. Preparation of Low-Molecular-Weight Citrus Pectin by Recombinant Bacillus Subtilis Pectate Lyase and Promotion of Growth of Bifidobacterium Longum. Catal. Commun. 2018, 107, 39–42. [Google Scholar] [CrossRef]
- Yang, G.; Chen, W.; Tan, H.; Li, K.; Li, J.; Yin, H. Biochemical Characterization and Evolutionary Analysis of a Novel Pectate Lyase from Aspergillus Parasiticus. Int. J. Biol. Macromol. 2020, 152, 180–188. [Google Scholar] [CrossRef]
- Cipriani, T.R.; Gracher, A.H.P.; De Souza, L.M.; Fonseca, R.J.C.; Belmiro, C.L.R.; Gorin, P.A.J.; Sassaki, G.L.; Iacomini, M. Influence of Molecular Weight of Chemically Sulfated Citrus Pectin Fractions on Their Antithrombotic and Bleeding Effects. Thromb. Haemost. 2009, 101, 860–866. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xu, L.; Jia, Y.; Xue, Z.; Zhang, M.; Phisalaphong, M.; Chen, H. Ultrasound-Assisted Modified Pectin from Unripe Fruit Pomace of Raspberry (Rubus Chingii Hu): Structural Characterization and Antioxidant Activities. LWT 2020, 134, 110007. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, X.; Hu, W.; Zhu, K.; Yu, C.; He, Q.; Linhardt, R.J.; Ye, X.; Chen, S. Anti-Inflammation Effects of Highly Purified Low-Mw RG-I Pectins on LPS-Activated Macrophages. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100283. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, C.; Zhao, S.; Tian, G.; Wang, F.; Li, C.; Wang, F.; Zheng, J. Alkali + Cellulase-Extracted Citrus Pectins Exhibit Compact Conformation and Good Fermentation Properties. Food Hydrocoll. 2020, 108, 106079. [Google Scholar] [CrossRef]
- Dongowski, G.; Lorenz, A.; Proll, J. The Degree of Methylation Influences the Degradation of Pectin in the Intestinal Tract of Rats and in Vitro. J. Nutr. 2002, 132, 1935–1944. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, E.G.; Colquhoun, I.J.; Chau, H.K.; Hotchkiss, A.T.; Waldron, K.W.; Morris, V.J.; Belshaw, N.J. Modified Sugar Beet Pectin Induces Apoptosis of Colon Cancer Cells via an Interaction with the Neutral Sugar Side-Chains. Carbohydr. Polym. 2016, 136, 923–929. [Google Scholar] [CrossRef]
- Li, P.; Xia, J.; Nie, Z.; Shan, Y. Pectic Oligosaccharides Hydrolyzed from Orange Peel by Fungal Multi-Enzyme Complexes and Their Prebiotic and Antibacterial Potentials. LWT Food Sci. Technol. 2016, 69, 203–210. [Google Scholar] [CrossRef]
- Fan, L.; Jiang, L.; Xu, Y.; Zhou, Y.; Shen, Y.; Xie, W.; Long, Z.; Zhou, J. Synthesis and Anticoagulant Activity of Sodium Alginate Sulfates. Carbohydr. Polym. 2011, 83, 1797–1803. [Google Scholar] [CrossRef]
- Hao, H.; Cui, C.; Xing, Y.; Jia, X.; Ma, B.; Kang, W.; Li, T.; Gao, M.; Xu, C. Sulfation of the Extracellular Polysaccharide from the Edible Fungus Stropharia Rugosoannulata with Its Antioxidant Activity. J. Futur. Foods 2023, 3, 37–42. [Google Scholar] [CrossRef]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Scholte, J.; Borewicz, K.; van den Bogert, B.; Smidt, H.; Scheurink, A.J.W.; Gruppen, H.; Schols, H.A. Effects of Pectin Supplementation on the Fermentation Patterns of Different Structural Carbohydrates in Rats. Mol. Nutr. Food Res. 2016, 60, 2256–2266. [Google Scholar] [CrossRef]
- Fåk, F.; Jakobsdottir, G.; Kulcinskaja, E.; Marungruang, N.; Matziouridou, C.; Nilsson, U.; Stålbrand, H.; Nyman, M. The Physico-Chemical Properties of Dietary Fibre Determine Metabolic Responses, Short-Chain Fatty Acid Profiles and Gut Microbiota Composition in Rats Fed Low- and High-Fat Diets. PLoS ONE 2015, 10, e0127252. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Lazarte, A.; Kachrimanidou, V.; Villamiel, M.; Rastall, R.A.; Moreno, F.J. In Vitro Fermentation Properties of Pectins and Enzymatic-Modified Pectins Obtained from Different Renewable Bioresources. Carbohydr. Polym. 2018, 199, 482–491. [Google Scholar] [CrossRef]
- Di, R.; Vakkalanka, M.S.; Onumpai, C.; Chau, H.K.; White, A.; Rastall, R.A.; Yam, K.; Hotchkiss, A.T. Pectic Oligosaccharide Structure-Function Relationships: Prebiotics, Inhibitors of Escherichia Coli O157:H7 Adhesion and Reduction of Shiga Toxin Cytotoxicity in HT29 Cells. Food Chem. 2017, 227, 245–254. [Google Scholar] [CrossRef]
- Khodaei, N.; Karboune, S. Enzymatic Generation of Galactose-Rich Oligosaccharides/Oligomers from Potato Rhamnogalacturonan I Pectic Polysaccharides. Food Chem. 2016, 197, 406–414. [Google Scholar] [CrossRef]
- Moro Cantu-Jungles, T.; do Nascimento, G.E.; Zhang, X.; Iacomini, M.; Cordeiro, L.M.C.; Hamaker, B.R. Soluble Xyloglucan Generates Bigger Bacterial Community Shifts than Pectic Polymers during in Vitro Fecal Fermentation. Carbohydr. Polym. 2019, 206, 389–395. [Google Scholar] [CrossRef]
- Ishisono, K.; Mano, T.; Yabe, T.; Kitaguchi, K. Dietary Fiber Pectin Ameliorates Experimental Colitis in a Neutral Sugar Side Chain-Dependent Manner. Front. Immunol. 2019, 10, 2979. [Google Scholar] [CrossRef] [Green Version]
- Onumpai, C.; Kolida, S.; Bonnin, E.; Rastall, R.A. Microbial Utilization and Selectivity of Pectin Fractions with Various Structures. Appl. Environ. Microbiol. 2011, 77, 5747–5754. [Google Scholar] [CrossRef] [Green Version]
- Kedir, W.M.; Deresa, E.M.; Diriba, T.F. Pharmaceutical and Drug Delivery Applications of Pectin and Its Modified Nanocomposites. Heliyon 2022, 8, e10654. [Google Scholar] [CrossRef]
- Leclere, L.; Van Cutsem, P.; Michiels, C. Anti-Cancer Activities of PH- or Heat-Modified Pectin. Front. Pharmacol. 2013, 4, 128. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhang, F.; Liu, X.; Ange, K.S.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a Lectin Binding Rhamnogalacturonan-I Containing Pectic Polysaccharide from Pumpkin. Carbohydr. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Sun, L.; Peng, X.; Zhang, T.; Xue, H.; Tai, G.; Zhou, Y. The Inhibitory Effects of a Rhamnogalacturonan I (RG-I) Domain from Ginseng Pectin on Galectin-3 and Its Structure-Activity Relationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef] [Green Version]
- Ognyanov, M.; Nikolova, M.; Yanakieva, I.; Kussovski, V.; Kratchanova, M. Influence of Composition on the Biological Activity of Pectic Polysaccharides from Leek. J. BioSci. Biotech 2013, 2, 13–20. [Google Scholar]
- Hu, S.; Kuwabara, R.; Beukema, M.; Ferrari, M.; de Haan, B.J.; Walvoort, M.T.C.; de Vos, P.; Smink, A.M. Low Methyl-Esterified Pectin Protects Pancreatic β-Cells against Diabetes-Induced Oxidative and Inflammatory Stress via Galectin-3. Carbohydr. Polym. 2020, 249, 116863. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Noruzi, E.B.; Aliabadi, H.A.M.; Sheikhaleslami, S.; Akbarzadeh, A.R.; Hashemi, S.M.; Gorab, M.G.; Maleki, A.; Cohan, R.A.; Mahdavi, M.; et al. Recent Advances on Biomedical Applications of Pectin-Containing Biomaterials. Int. J. Biol. Macromol. 2022, 217, 1–18. [Google Scholar] [CrossRef]
- Jantrawut, P.; Bunrueangtha, J.; Suerthong, J.; Kantrong, N. Fabrication and Characterization of Low Methoxyl Pectin/Gelatin/Carboxymethyl Cellulose Absorbent Hydrogel Film for Wound Dressing Applications. Materials 2019, 12, 1628. [Google Scholar] [CrossRef] [Green Version]

| Modification Type | Modification Method | Mechanism/Basic Point | Main Influence Factor | Ref. |
|---|---|---|---|---|
| Chemical modification | Methyl esterification | Introduce -CH3 into the -OH on O-6 of GalpA by methanol under the catalysis of acid | Acid type and concentration; reaction temperature and time | [20,28,29,30,31,32,33,34] |
| De-methyl esterification | Remove -CH3 from GalpA by alkali, acid, amidation regent, and enzyme. Alkali method: remove -CH3 under the catalysis of alkali to generate carboxylate and methanol; acid method: remove -CH3 under high temperatures and strong acid conditions; amidation treatment: ammonolyze the methyl ester groups by the ammonia in methanol in alkali conditions; enzyme method: hydrolyze the methyl ester groups by pectin methylesterase | The type and concentration of alkali, acid, amidation regent, and enzyme; reaction temperature, pH value, and time | [20,29,34,35,36,37] | |
| Acetylation | Introduce acetyl into the -OH on O-2 and O-3 of GalpA by acetylation reagents | The type of reaction solvent and catalyst; the type and concentration of acetylation reagent; reaction temperature and time | [23,24,26] | |
| Sulfation | Replace the -OH of GalpA with the sulfate groups by sulfation reagents | The type and concentration of sulfation reagents; reaction temperature and time | [20,31,32,33,38,39,40,41] | |
| Chemical modification | Selenylation | Replace the -OH of GalpA with selenium functional groups with inorganic selenium | The concentration of inorganic selenium; reaction temperature and time | [22,42,43] |
| Acid degradation | Depolymerize pectin by using the tolerance differences between the different glycosidic bonds to acids; tolerance order: the glycosidic bonds between GalpA > the glycosidic bonds between GalpA and Rhap > the glycosidic bonds between neutral sugars | Acid type and concentration; reaction temperature and time | [44,45,46,47,48,49] | |
| Alkali degradation | β-elimination reaction: the process of cleaving C-O bonds at the β-position which results from the removal of the hydrogen atoms on C-5 of GalpA and the formation of the double bond between C-4 and C-5 | Reaction temperature, pH value, and time; cations in the reaction system | [50,51,52,53] | |
| Oxidative degradation | Fenton reaction: the oxidizing groups (·OH and ·O2−) generated from the decomposition of H2O2 under catalysis will combine with the hydrogen atoms attached to the carbon atoms of pectins and then attack the glycosidic bonds | Fe2+ concentration (for metal Fenton reaction); physical process parameters (for non-metal Fenton reaction); H2O2 concentration; reaction temperature and time | [54,55,56,57,58,59] | |
| Physical modification | Ultrasound modification | The implosion of cavitation bubbles produced by ultrasonic power generates high shear forces, which can break the glycosidic bonds; the collapse of the cavitation bubbles promotes the dissociation of water molecules to produce -OH and -H radicals and the formation of H2O2 to attack the glycosidic bonds | US intensity/frequency; duty cycle; reaction temperature, pH value, and time | [15,16,44,58,60,61,62,63,64] |
| High-pressure modification | HHP transfers pressure by liquid medium (usually water) to depolymerize pectin; HPH utilizes the forces of high-velocity impact, high-frequency vibration, cavitation, high shear stress, instantaneous pressure drop, and high pressure generated by fluid flowing through a small gap (a few hundred micrometers) in a short period of time (less than 5 s) to depolymerize pectin | Process pressure; reaction temperature, pH value, and time; cycle number (for HPH) | [65,66,67] | |
| Subcritical water modification | Under high temperature and pressure conditions, water is rapidly hydrolyzed to H+ and OH−, which have high catalytic activity and reactivity to depolymerize pectin | Reaction temperature and pressure; reaction pH value (acid type) and time | [68,69,70,71,72] | |
| Irradiation modification | Gamma irradiation and electron beam are used to modify pectin | Irradiation dosage; reaction time | [17,73,74,75,76] | |
| Low-temperature plasma modification | ROS, NOS, UV irradiation, electrons, and unstable radicals generated by low-temperature plasma can depolymerize pectin | Reaction voltage and time | [15,77,78,79,80,81,82] |
| Name | Abbr. | E.C. No. | Site and Product | Ref. |
|---|---|---|---|---|
| Pectin methylesterase | PME | 3.1.1.11 | Hydrolyze methyl esters in GalpA, releasing methanol. | [112] |
| Pectin acetylesterase | PAE | 3.1.1.6 | Hydrolyze acetyl esters of GalpA in the HG domain, releasing ethanol. | [113] |
| Endo-polygalacturonase | Endo-PG | 3.2.1.15 | Randomly hydrolyze α-1,4-glycosidic bonds in polygalacturonic acid, releasing oligogalacturonic acid. | [114] |
| Exo-polygalacturonase-1 | Exo-PG-1 | 3.2.1.67 | Hydrolyze the first α-1,4-glycosidic bonds from the non-reducing end of polygalacturonic acid, releasing mono- and oligogalacturonic acid. | [115] |
| Exo-polygalacturonase-2 | Exo-PG-2 | 3.2.1.82 | Hydrolyze the second α-1,4-glycosidic bonds from the non-reducing end of polygalacturonic acid, releasing di- and oligogalacturonic acid. | [116] |
| Endo-polymethylgalacturonase | Endo-PMG | — | Randomly hydrolyze α-1,4-glycosidic bonds in methyl-esterified polygalacturonic acid, releasing methyl-esterified oligogalacturonic acid. | [117] |
| Exo-polymethylgalacturonase | Exo-PMG | — | Hydrolyze the first α-1,4-glycosidic bonds from the non-reducing end of the methyl-esterified polygalacturonic acid, releasing methyl-esterified mono- and oligogalacturonic acid. | [114] |
| Endo-polygalacturonate lyase | Endo-PGL | 4.2.2.2 | Randomly cleave the α-1,4-glycosidic bonds in polygalacturonic acid by a β-elimination reaction, releasing oligogalacturonic acid with unsaturated GalpA and GalpA at the terminal end, respectively. | [118] |
| Exo-polygalacturonate lyase | Exo-PGL | 4.2.2.9 | Cleave the second α-1,4-glycosidic bonds from the reducing end of polygalacturonic acid by a β-elimination reaction, releasing unsaturated disaccharide and shortened polygalacturonic acid. | [119] |
| Endo-polymethylgalacturonate lyase | Endo-PMGL | 4.2.2.10 | Randomly cleave the α-1,4-glycosidic bonds in methyl-esterified polygalacturonic acid by a β-elimination reaction, releasing oligogalacturonic acid with unsaturated methyl-esterified GalpA and GalpA at the terminal end, respectively. | [120] |
| Exo-polymethylgalacturonate lyase | Exo-PMGL | — | Cleave the first α-1,4-glycosidic bonds from the non-reducing end of methyl-esterified polygalacturonic acid by a β-elimination reaction, releasing unsaturated methyl-esterified mono- and oligogalacturonic acid. | [121] |
| Rhamnogalacturonan acetylesterase | RGAE | 3.1.1.86 | Hydrolyze acetyl esters of GalpA in the RG-I domain, releasing ethanol. | [122] |
| Endo-rhamnogalacturonan hydrolase | RGH | 3.2.1.171 | Randomly hydrolyze the α-D-GalA-(1→2)-α-L-Rha glycosidic bonds in the RG-I backbone, releasing oligo-RG-I with GalpA and Rhap at the reducing end and non-reducing end, respectively. | [122] |
| Exo-unsaturated rhamnogalacturonyl hydrolase | URGH | 3.2.1.172 | Hydrolyze the α-D-GalA-(1→2)-α-L-Rha glycosidic bonds of the oligo-RG-I with unsaturated GalpA at the non-reducing end, releasing unsaturated glucuronic acid. | [123] |
| Exo-rhamnogalacturonan galacturonohydrolase | RGGH | 3.2.1.173 | Hydrolyze the first α-D-GalA-(1→2)-α-L-Rha glycosidic bonds from the non-reducing end in the RG-I backbone, releasing mono-GalA and oligo-RG-I. | [124] |
| Exo-rhamnogalacturonan rhamnohydrolase | RGRH | 3.2.1.174 | Hydrolyze the first α-L-Rha-(1→4)-α-D-GalA glycosidic bonds from the non-reducing end in the RG-I backbone, releasing mono-Rha and oligo-RG-I. | [125] |
| Endo-rhamnogalacturonan lyase | RG-I endo-lyase | 4.2.2.23 | Randomly cleave the α-L-Rha-(1→4)-α-D-GalA glycosidic bonds in the RG-I backbone, releasing oligo-RG-I with Rhap (pyranose) and unsaturated GalpA (pyranose) at the reducing and non-reducing end. | [122] |
| Exo-rhamnogalacturonan lyase | RG-I exo-lyase | 4.2.2.24 | Cleave the α-L-Rha-(1→4)-α-D-GalA glycosidic bonds of the RG-I backbone with Rhap (pyranose) and unsaturated GalpA (pyranose) at the reducing and non-reducing end, releasing disaccharide and shortened RG-I with unsaturated GalpA (pyranose) at the non-reducing end. | [126] |
| β-D-galactosidase | — | 3.2.1.23 | Hydrolyze the first β-1,4-glycosidic bonds from the non-reducing end of galactan. | [122] |
| α-L-arabinofuranosidase | — | 3.2.1.55 | Hydrolyze the first α-1,5-glycosidic bonds from the non-reducing end of arabinan and AG-I. | [127] |
| Endo-galactanase | — | 3.2.1.89 | Randomly hydrolyze the β-1,4-glycosidic bonds in galactan and the β-1,3/4/6-glycosidic bonds in AG-I and AG-II. | [122] |
| Endo-arabinanase | — | 3.2.1.99 | Randomly hydrolyze the α-1,5-glycosidic bonds in arabinan and AG-I. | [127] |
| Pectin Source | Modification Condition | Pectin Changes Caused by Modification | Ref. | ||
|---|---|---|---|---|---|
| Chemical Structure and Property | Bioactivity | ||||
| Citrus | Sulfation: 1500 mg pectin + 15 mL FA + 10 mL CSA; 80–90 °C, 4 h | Sulfur content = 0→2.68%; DS = 0→0.15 | Anti-coagulant activity | APTT (pectin concentration = 25–100 mg/mL): 5 times that of native pectin; longer PT | [30] |
| Citrus | Pectin + DMF + SO3-Pyr (the ratio of ηSO3-Pyr to -OH = 18; the ratio of total reaction volume to weight of pectin = 100); 25 °C, 6 h | DS = 0→1.41; Mw: 4.14 × 105 g/mol→1.24 × 105 g/mol | Anti-coagulant activity | For each concentration increase (µg/mL) of sulfated pectin, PT and APTT increased, on average, 2.0 s and 5.7 s, respectively | [33] |
| Opuntia ficus indica cladodes | Sulfation: 400 mg pectin + 16 mL FA + 3 mL SO3-DMF; 50 °C, 3 h | Sulfate content = 0→2.13%, 7.04%; DS = 0→0.12, 0.46; Mw: 7890 × 10−3 g/mol→3870, 2100 × 10−3 g/mol; Neutral sugar content: 54.20%→52.72%, 21.44%; GalA content: 31.20%→18.30%, 14.59% | Anti-coagulant activity | Longer TT and APTT | [11] |
| Citrus | Sulfation: 200 mg pectin + 10 mL DMSO + 4 mL SO3-Pyr or 200 mg pectin TBA salt + 10 mL DMSO + 6 mL SO3-Pyr; 80 °C, 1–3 h | Sulfate content = 0→15–25%; Mw: 207 kDa→47–73 kDa; | Anti-coagulant activity | TT: 15.2 IU/mg; APTT: 51.96 IU/mg; inhibitory effect on thrombin (modified pectin concentration = 0.32 mg/mL): 60% | [32] |
| Citrus | Sulfation: 200 mg pectin + 40 mL FA + 40 mL Pyr + 16 mL CSA; 4 °C, 12 h | Almost 90% of -OH groups in pectins were sulfated | Anti-coagulant activity | Total inhibited the formation of venous thrombosis at a dose of 3.5 mg modified pectin/kg body weight of rat | [157] |
| Mesona chinensis Benth | Sulfation: 600 mg pectin + 100 mL FA + 6 mL Pyr + 3 mL CSA; 60 °C, 2 h | DS: 0→0.52; Mw: 157 kDa→177 kDa; Uronic acid content: 29.30%→33.27%; Monosaccharide composition (molar ratio): Gal: Glu: Xyl: GalA = 0.68: 1.49: 2.54: 6.33→Gal: Glu: Xyl: GalA = 0.48: 2.43: 3.87: 6.77 | Anti-oxidant activity | Scavenging abilities of DPPH radical and ·OH (pectin concentration = 1000 μg/mL): 75.11%→86.95%, 63.26%→68.93%; survival rate of RAW264.7 cells treated by H2O2 (pectin concentration = 1000 μg/mL): 78.58%→93.73%; SOD activity (pectin concentration = 1000 μg/mL): 1.12 Unit→1.29 unit; lower MDA content (pectin concentration = 1000 μg/mL): 96.88%→67.83% | [12] |
| Ulmus pumila L. | Selenylation: 1.0 g pectin + 50 mL 5% nitric acid (v/v) + sodium selenites (0.2, 0.4, 0.6, 0.8, and 1.0 g); room temperature, 24 h | Selenium content: 0→3.24, 5.14, 9.04, 10.67, 13.19 mg/g; Mw: 2.697 × 109 kDa→3.977, 4.338, 4.688, 5.220, 6.528 × 109 kDa | Anti-oxidant activity | Higher reducing power; higher scavenging abilities of nitrite and ·OH; higher SOD-like scavenging activity. | [88] |
| Hawthorn | Degradation by enzymes: 1% pectin solution (w/v) + enzymes, 40–50 °C, pH 3.5–7.5, 4–7 h | DM: 55.73%→38.56%, 41.05%, 26.62%; 40.46%; GalA content: 88.84%→82.82%, 92.01%, 69.47%; 90.04%; Mw: 288.41 kDa→6.51, 37.18, 3.59, 7.01 kDa | Anti-oxidant activity | IC 50 (·OH scavenging activity): 1.08 mg/mL→0.335, 0.779, 0.717, 0.481 mg/mL; IC 50 (ABTS·+ radical scavenging activity): 2.06 mg/mL→1.807, 1.924, 1.272, 1.874 mg/mL | [36] |
| Raspberry | Degradation by ultrasound: 2 mg/mL pectin + 50 mL distilled water; sonication, ice-bath, 400 W, 60 min | DM = 31.49%→28.07%; Neutral sugar = 21.42%→18.03%; Mw = 15.02 kDa→11.01 kDa; monosaccharide composition (%) = Gal: Rha: Ara: Glc: Xyl: Man: GalA = 8.45: 2.39: 9.15: 13.65: 2.17: 1.65: 62.48→8.14: 1.79: 7.08: 12.03: 1.62: 1.49: 67.76 | Anti-oxidant activity | DPPH radical scavenging activity: 0.75 mg AAE/mg→0.94 mg AAE/mg; ABTS·+ radical scavenging activity: 0.18 mg AAE/mg→0.32 mg AAE/mg; ferric reducing power: 0.39 mg AAE/mg→0.44 mg AAE/mg; The activity of human hepatic L02 cells with pre-treatment by pectins after H2O2 treatment (pectin concentration = 0.1 mg/mL): 83.39%→92.35% | [158] |
| Citrus | Degradation by irradiation: 2% pectin solution (w/v); irradiated (20 kGy, 14 °C,10 kGy/h) | Mw: 500 kDa→37 kDa | Anti-oxidant activity | β-carotene retention in β-carotene-linoleic acid bleaching assay (pectin concentration = 20 mg/mL): 21%→59%; DPPH radical scavenging activity (pectin concentration = 4 mg/mL): less than 20%→60% | [107] |
| Apple | Degradation by plasma: 10 g pectin + plasma treatment (230 V,1.2 kHz, 3, 6, 9, 12, 15 min); | Mw: 160,677.4 g/mol→about 40,000–12,000 g/mol; DE: 68.12%→54.21–65.78%; GalA content: 0.653%→0.658–0.816% | Anti-oxidant activity | Fe3+ reducing power (pectin concentration = 0.5% and 2%, plasma treatment = 15 min): 2.67 and 1.85 times that of natural pectin; higher DPPH radical scavenging activity | [81] |
| Citrus canning processing water | Degradation by ultrasound: pectin + 50 mM H2O2 & 10 mM ascorbic acid (0.5% m/v); ultrasound (3.8 W/mL, 22 kHz), 30 °C, 60 min | Mw of modified pectin: 2113, 3683, 7469 g/mol; Monosaccharide composition (molar ratio) of modified pectin: Ara: Gal: Rha: GalA: Glc: Xyl = 39.30: 7.85: 2.19: 45.15: 4.1: 1.59 (lowest Mw); 44.3: 7.79: 3.49: 42.31: 0.85: 0.78 (medium Mw); 50.47: 13.44: 11.23: 20.68: 2.8 (highest Mw) | Anti-oxidant activity | Lower inhibitory effect on the generation of ROS with the decrease in Mw | [159] |
| Citrus | Degradation by acid, alkali, enzymes, acid + enzymes, and alkali + enzymes | Mw of modified pectin: 1.39, 0.51, 0.95, 1.41, 1.12 × 105 g/mol; DM of modified pectin: 71.41%, 2.02%, 68.80%, 64.70%; 14.81% | Ability to regulate the intestinal environment | Modified pectin with Mw of 1.12 × 105 g/mol and DM of 14.81% is best for producing acetic acid, propionic acid, and butyric acid | [160] |
| — | Methyl esterification: 100 g pectin + 800 mL methanol/40 mL concentrated H2SO4, 6 d; then 1 L methanol/37.5 mL concentrated H2SO4, 4 °C, 6 d | DM of modified pectin: 34.4%, 70.8%, 92.6%; GalA content of modified pectin: 74.4%, 65.5%, 77.9% | Ability to regulate the intestinal environment | Acetate, propionate, butyrate, and total SCFAs content (pectin DM 34.4%→92.6%): 86.25%→71.62%; 15.11%→11.05%; 9.08%→5.77%; 110.62%→88.71%; Lactobacillus counts (21 d feeding, pectin DM 34.4%→92.6%): 7.60 log→6.26 log | [161] |
| Orange, lemon, lime, and sugar beet | De-methyl esterification and amidation | DM of modified pectin: 11.4–74.7%; Gal content of modified pectin: 9.1–30.7%; Ara content of modified pectin: 0.8–16.7%; Rha content of modified pectin: 1.7–3.7%; Glc content of modified pectin: 0.7–8.7%; GalA content of modified pectin: 70.7–83.0% | Ability to regulate the intestinal environment | Higher stimulation of F. prausnitzii with the increase in DM | [4] |
| Apple | Degradation by high pressure: 1.84% pectin solution (w/v); 155 MPa, 63 °C, cycle number = 6 passes | Monosaccharide composition (wt%): GalA: Ara: Gal: Rha: Glc: Xyl = 71.68: 6.61: 7.45: 3.48: 0.56: 2.91→29.56: 18.35: 21.12: 9.12: 1.52: 8.42 | Ability to regulate the intestinal environment | Fermentation in vivo for 24 h: Bacteroides number: 8.54 log→7.85 log; Clostridia number: 7.91 log→7.21 log; total SCFAs content: 39.74 mM→59.85 mM; acetic acid content: 23.92 mM→31.64 mM; lactic acid content: 2.70 mM→12.57 mM; propionic acid content: 5.17 mM→7.89 mM | [101] |
| Pomelo | Degradation by irradiation: 40 mL 1% pectin solution (w/v), 3–250 kGy | DM: 68.2%→64.4% (40 kGy) and 72.4% (125 kGy); Mw: 193129 Da→9428 Da (40 kGy) and 1835 Da (125 kGy); monosaccharide composition (molar ratio): Fuc: Rha: Ara: Gal: Glc: Xyl: Man: GalA = 0.18: 3.52: 11.64: 9.36: 1.84: 1.18: 1.12: 71.15→0.21: 3.40: 10.46: 7.27: 1.95: 1.26: 1.49: 73.96 (40 kGy) and 0.22: 1.66: 10.56: 3.70: 2.13: 1.08: 1.28: 79.37 (125 kGy) | Ability to regulate the intestinal environment | Produce more butyric acid; increase the number of Eubacterium maltosivorans strain | [17] |
| Citrus | Degradation by irradiation: 2% pectin solution (w/v); irradiated (20 kGy, 14 °C,10 kGy/h) | Mw: 500 kDa→37 kDa | Anti-tumor activity | Higher inhibitory effects on skin (B16), colon (HT29), and human melanoma (SKMEL) cancer cells | [107] |
| Citrus | Degradation by ultrasound: pectin + 50 mM H2O2 & 10 mM ascorbic acid (0.5% m/v); ultrasound (11.4 W/mL, 22 kHz), 50 °C, 60 min | Mw: 791 kDa→7.65 kDa; monosaccharide composition (molar ratio): Ara: GalA: Gal: Rha: Fuc: Xyl = 44.55: 22.3: 18.4: 11.49: 2.3: 0.91→48.2: 14.36: 18.58: 13.46: 4.22: 1.18 | Anti-tumor activity | Cell viability of human breast cancer cell MCF-7 (pectin concentration = 500 µg/mL): 34.71%→56.39%; LDH content (pectin concentration = 500 µg/mL): 162.8% compared to the control group→138.3% compared to the control group | [57] |
| Citrus | Degradation by ultrasound: 25 mL pectin solution (5 mg/mL) + MH2O2: MNaHCO3 = 1: 2.5, 11.4 W/mL, 50 °C, 50 min | Mw: 1088 kDa→33 kDa; monosaccharide composition (molar ratio): Man: Rha: GlcA: GalA: Glc: Gal: Ara: Fuc = 1.12: 3.98: 0.49: 59.07: 4.84: 12.67: 15.94: 0.27→1.2: 4.81: 0.72: 54.39: 3.62: 16.42: 17.05: 1.81 | Anti-tumor activity | Growth inhibition rate of A549 lung cancer cell (pectin concentration = 1 mg/mL): about 15%→27.21% | [58] |
| Sugar beet | Degradation by enzymes: β-galactosidase + endo-galactanase (without galactan side chain); α-L-arabinofuranosidase + endo-arabinose + β-galactosidase + endo-galactanase (without all side chains) | β-1,4-galactan content: 100%→4–13% (without galactan side chain), 4–20% (without all side chains); terminal Ara content: 100%→36% (without galactan side chain), 36% (without all side chains) | Anti-tumor activity | Lower inhibitory effect on the proliferation of colon cancer cell HT29 as the removal of the galactan side chain | [162] |
| Chinese yam | Sulfation: 400 mg pectin + 100 mL FA + 10 mL CSA & Pyr (1: 3, v/v); 70 °C, 3 h | DS: 0→0.51; Mw: 33.33 kDa→37.04 kDa; Uronic acid: 21.15%→27.55%; monosaccharide composition (molar ratio): Rha:Gal:Glc:Xyl:GalA:GlcA = 1.77:11.36:13.44:1.53:15.47:1.67→0.13:13.51:12.98:1.25:16.22:0.91 | Immunomodulatory activity | Higher thymus index in immunosuppressed mice; higher proliferation effect on concanavalin-induced T-cells in high dosage; the absolute number of CD3+CD4+ (pectin concentration = 200 mg/kg body weight): 52.07→55.87; the ratio of CD4+/CD8+ (pectin concentration = 200 mg/kg body weight): 3.50→3.67; higher contents of TNF-α and IL-1β in high dosage; lower levels of serum antibody in high dosage | [13] |
| Citrus | Degradation by irradiation: 2% pectin solution (w/v); irradiated (20 kGy, 14 °C,10 kGy/h) | Mw: 500 kDa→37 kDa | Immunomodulatory activity | Spleen cell viability (pectin concentration = 1 mg/mL): 33.3%→69.3% | [107] |
| Bee pollen of Nelumbo nucifera | Degradation by acid: pectin + 0.1 M TFA, 80 °C, 8 h | Mw: 380 kDa→9 kDa; monosaccharide composition (molar ratio): Rha:GalA:Gal:Ara:GlcA:Glc = 11.5:12.0:41.2:29.7:2.0:3.6→15.3:18.1:50.7:0:13.3:2.7 | Immunomodulatory activity | Higher stimulative effect on macrophage phagocytosis; lower NO content | [48] |
| Panax ginseng | Degradation by acid: pectin + 0.1 M TFA, 80 °C, 6 h | Mw: 110 kDa→40 kDa; monosaccharide composition (molar ratio): Ara:Gal:Glc:Rha:GalA:GlcA = 40.9:44.4:2.9:4.1:5.3:2.0→0:76.1:0.6:10.1:8.6:4.6 | Immunomodulatory activity | Lower stimulative effect on lymphocyte proliferation; lower NO content | [49] |
| Apple | Selenylation: 1 g pectin + 100 mL 0.5% nitric acid solution (v/v) + 1 g sodium selenite; ultrasound treatment 240 W, 75 °C, 6 h | Mw: 517 kDa + 64.9 kDa + 18.8 kDa→603 kDa; monosaccharide composition (molar ratio): Rha:Ara:Gal:Glc:Xyl:GalA = 0.004:0.017:0.256:0.080:0.111:0.492→0.032:0.005:0.225:0.067:0.072:0.599 | Anti-inflammatory activity | The reduction in colon length (pectin concentration = 200 mg/mL): 13%→7%; lower IL-6 and TNF-α content; higher IL-10 content; higher activity of glutathione peroxidase; lower MPO content | [89] |
| Ulmus Pumila L. | Selenylation: 1 g pectin + 50 mL 0.5% nitric acid solution (v/v) + 0.2 g and 0.4 g sodium selenite, room temperature, 24 h | — | Anti-inflammatory activity | Higher inhibiting effects on the activation of p38 and the protein expression of iNOS; lower NO content | [22] |
| Okra pod | Degradation by Fenton reaction: 500 mL 1% pectin solution (v/v) deionized water + 3 mM, 5 mM, and 7 mM granular FeSO4 + 500 mL 2% H2O2 (v/v), room temperature, 2 h | Mw: 112.31 + 2.22 + 1.15 + 0.53 kDa→6.09 + 1.02 kDa (3 mM FeSO4), 4.89 + 2.48 + 1.22 kDa (5 mM FeSO4), 3.97 + 1.79 + 0.87 kDa (7 mM FeSO4); GalA content: 28.23 wt%→22.39, 13.61, 0.58 wt%; monosaccharide composition (molar ratio): Rha:Ara:Xyl:Man:Glu:Gal = 37.73:8.23:2.40:1.38:3.04:47.23→62.29:2.32:2.53:6.03:3.62:23.22 (3 mM FeSO4), 72.05:1.58:2.42:6.52:4.12:13.30 (5 mM FeSO4), 82.99:0.42:0.70:7.43:4.54:3.92 (7 mM FeSO4) | Anti-inflammatory activity | Higher inhibiting effects on the production of nitrite, the expression of iNOS, the phosphorylation of IκB kinase α/β and p65, and the degradation of IκBα | [56] |
| Ficus pumila L. | Methyl esterification: acidified methanol: 48.22 mL anhydrous MeOH + 1.78 mL acetyl chloride, 25 °C, 1 h; 200 mg pectin + 20 mL acidified methanol, 0.5–14 h; De-methyl esterification: 200 mg pectin + 0.02 M and 0.03 M NaOH, 4 °C, 30 min | DM of modified pectins: 3%, 16%, 25%, 34%, 45%, 54%, 63%, 76%, 84%, 94%; Mw of modified pectins: 42.7, 46.5, 50.5, 51.5, 52.6, 67.3, 48.4, 45.7, 43.8, 38.0 kDa | Hypoglycemic activity | Glucose consumption in IR-HepG2 cells: when DM is between 3% and 54%: DM3 < DM 16 < DM 25 < DM 34 < DM 45 < DM54; when DM is between 54% and 94%: DM54 > DM 63 > DM 76 > DM 84 > DM 94 | [21] |
| Citrus | Sulfation: 1.5 g pectin + 15 mL FA + 10 mL CSA, 80–90 °C, 4 h | Sulfur content = 0→2.68%; DS = 0→0.15 | Anti-bacterial activity | Inhibitory effect on Bacillus cereus and Vibrio fischeri (pectin concentration = 2.0 mg/mL): about 3%→20%, about 5%→58% | [30] |
| Orange peel | Degradation by enzymes: 4% pectin solution (w/v) + crude enzyme (pectinase (48.3 U/mL) + filter paper cellulase (FPase, 0.2 U/mL) + CMCase (2.8 U/mL) + xylanase (50.0 U/mL)), 45 °C, 6 h; then filter (membrane cut-off of 3 kDa and 1 kDa) | DPp of modified pectins: 6, 9, 19; monosaccharide composition (%): Rha:Ara:Gal:Glc:Xyl:Fru:GalA = 7.8:13.7:5.0:30.3:19.4:2.1:21.7→2.5:32.6:8.8:34.8:6.9:1.3:13.2 (modified pectin with DPp of 6), 3.2:17.6:14.8:36.9:1.3:2.0:24.2 (modified pectin with DPp of 9), 6.2:22.0:12.3:39.7:2.0:1.4:16.4 (modified pectin with DPp of 19) | Anti-bacterial activity | Minimum inhibitory concentrations of modified pectins against Staphylococcus aureus, Bacillus subtilis, and Escherichia coli. (mg/mL): 12.5, 12.5, 25 (modified pectin with DPp of 6); 12.5, 12.5, 25 (modified pectin with DPp of 9); 25, 25, 50 (modified pectin with DPp of 19) | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, X.; Li, F.; Zhao, J.; Wei, Y.; Zhang, L.; Yu, W.; Li, Q. The Preparation and Potential Bioactivities of Modified Pectins: A Review. Foods 2023, 12, 1016. https://doi.org/10.3390/foods12051016
Jiao X, Li F, Zhao J, Wei Y, Zhang L, Yu W, Li Q. The Preparation and Potential Bioactivities of Modified Pectins: A Review. Foods. 2023; 12(5):1016. https://doi.org/10.3390/foods12051016
Chicago/Turabian StyleJiao, Xu, Fei Li, Jing Zhao, Yunlu Wei, Luyao Zhang, Wenjun Yu, and Quanhong Li. 2023. "The Preparation and Potential Bioactivities of Modified Pectins: A Review" Foods 12, no. 5: 1016. https://doi.org/10.3390/foods12051016