Analysis of Volatile Organic Compounds in Milk during Heat Treatment Based on E-Nose, E-Tongue and HS-SPME-GC-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Heat Treatment
2.3. E-Nose Analysis
2.4. E-Tongue Analysis
2.5. Collection and Extraction of VOCs from Milk
2.6. GC-MS Analysis Conditions
2.7. Statistical Analysis
3. Results
3.1. E-Nose Analysis
3.2. E-Tongue Analysis
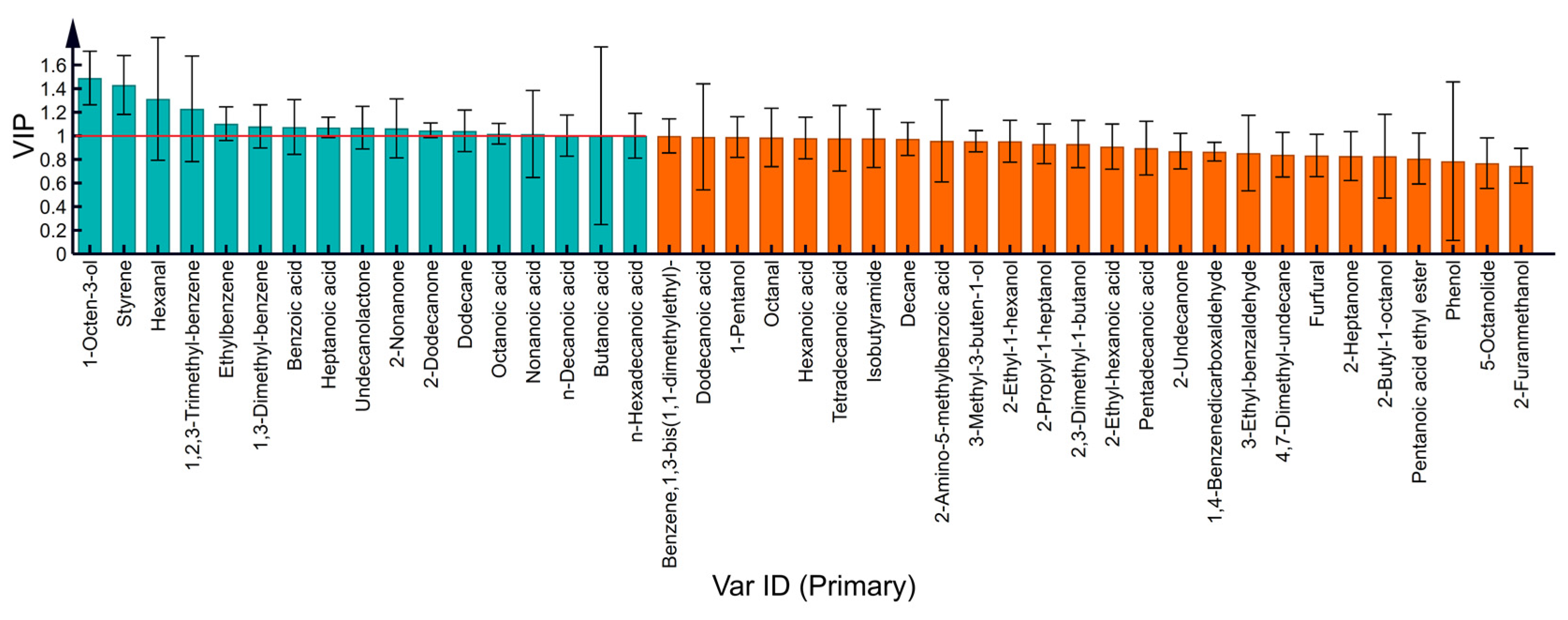
3.3. HS-SPME-GC-MS Analysis
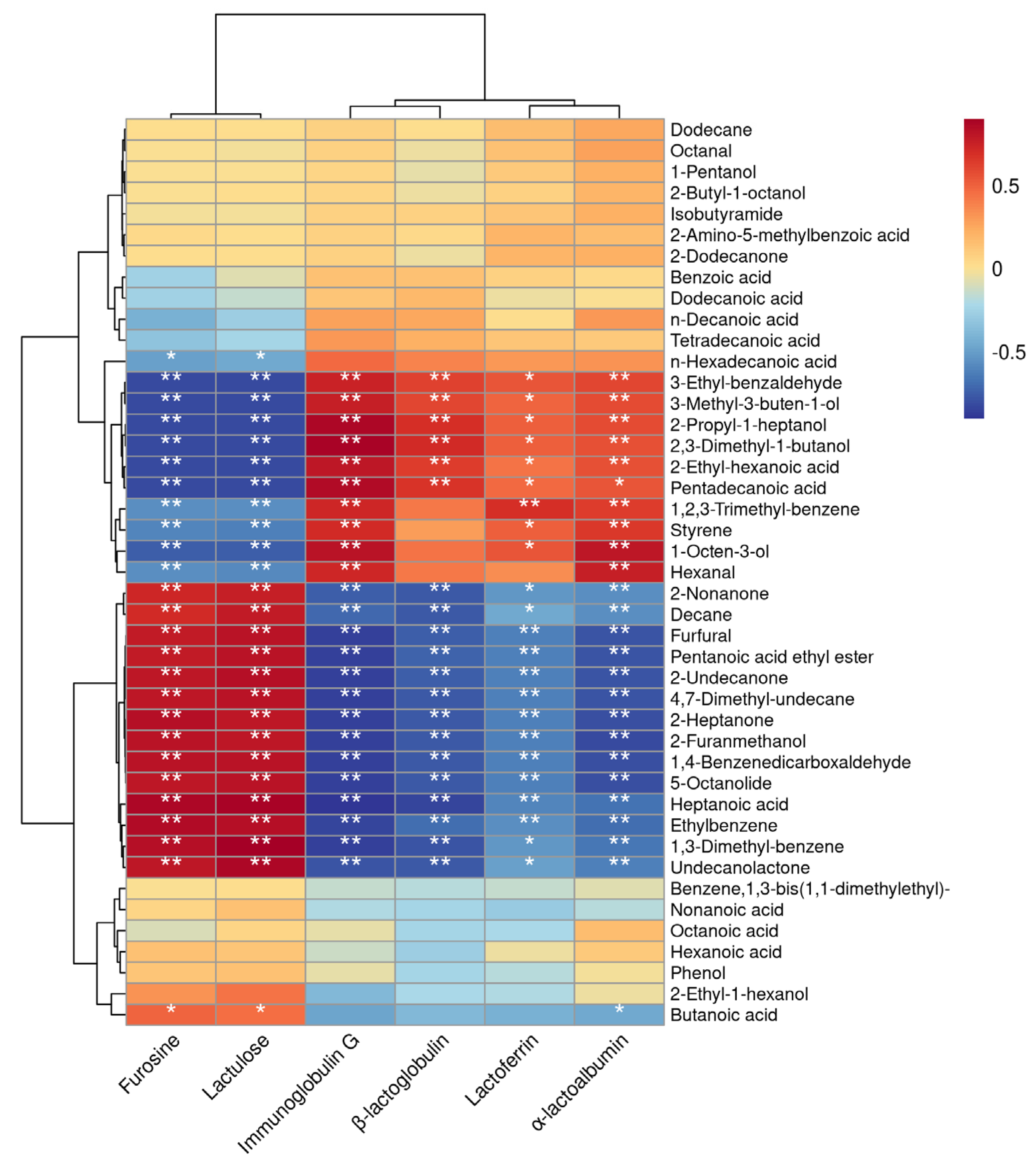
3.4. Correlation Analysis between Thermal Parameters and VOCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alegbeleye, O.O.; Guimarães, J.T.; Cruz, A.G.; Sant’Ana, A.S. Hazards of a ‘healthy’ trend? An appraisal of the risks of raw milk consumption and the potential of novel treatment technologies to serve as alternatives to pasteurization. Trends Food Sci. Technol. 2018, 82, 148–166. [Google Scholar] [CrossRef]
- Jo, Y.; Benoist, D.M.; Barbano, D.M.; Drake, M.A. Flavor and flavor chemistry differences among milks processed by high-temperature, short-time pasteurization or ultra-pasteurization. J. Dairy Sci. 2018, 101, 3812–3828. [Google Scholar] [CrossRef] [PubMed]
- Andreoletti, O.; Baggesen, D.L.; Bolton, D.; Butaye, P.; Cook, P.; Davies, R.; Escamez, P.S.F.; Griffin, J.; Hald, T.; Havellar, A.; et al. Scientific Opinion on the public health risks related to the consumption of raw drinking milk. Efsa J. 2015, 13, 3940. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Bremer, P.; Oey, I.; Everett, D.W. Bacterial inactivation in whole milk using pulsed electric field processing. Int. Dairy J. 2014, 35, 49–56. [Google Scholar] [CrossRef]
- Bogahawaththa, D.; Buckow, R.; Chandrapala, J.; Vasiljevic, T. Comparison between thermal pasteurization and high pressure processing of bovine skim milk in relation to denaturation and immunogenicity of native milk proteins. Innov. Food Sci. Emerg. 2018, 47, 301–308. [Google Scholar] [CrossRef]
- Bezie, A. The Effect of Different Heat Treatment on the Nutritional Value of Milk and Milk Products and Shelf-Life of Milk Products. A Review. J. Dairy Vet. Sci. 2019, 11, 555822. [Google Scholar] [CrossRef]
- Amador-Espejo, G.G.; Gallardo-Chacón, J.J.; Juan, B.; Trujillo, A.J. Effect of ultra-high-pressure homogenization at moderate inlet temperatures on volatile profile of milk. J. Food Process. Eng. 2017, 40, e12548. [Google Scholar] [CrossRef]
- Al-Attabi, Z.; D’Arcy, B.R.; Deeth, H.C. Volatile sulfur compounds in pasteurised and UHT milk during storage. Dairy Sci. Technol. 2014, 94, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Contarini, G.; Pavolo, M. Volatile fraction of milk: Comparison between purge and trap and solid phase microextraction techniques. J. Agric. Food Chem. 2002, 50, 7350–7355. [Google Scholar] [CrossRef]
- Troise, A.D.; Fiore, A.; Colantuono, A.; Kokkinidou, S.; Peterson, D.G.; Fogliano, V. Effect of olive mill wastewater phenol compounds on reactive carbonyl species and Maillard reaction end-products in ultra-high-temperature-treated milk. J. Agric. Food Chem. 2014, 62, 10092–10100. [Google Scholar] [CrossRef]
- Bittante, G.; Cologna, N.; Cecchinato, A.; De Marchi, M.; Penasa, M.; Tiezzi, F.; Endrizzi, I.; Gasperi, F. Monitoring of sensory attributes used in the quality payment system of Trentingrana cheese. J. Dairy Sci. 2011, 94, 5699–5709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez-Landaverde, P.A.; Torres, J.A.; Qian, M.C. Quantification of trace volatile sulfur compounds in milk by solid-phase microextraction and gas chromatography-pulsed flame photometric detection. J. Dairy Sci. 2006, 89, 2919–2927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zabbia, A.; Buys, E.M.; De Kock, H.L. Undesirable Sulphur and Carbonyl Flavor Compounds in UHT Milk: A Review. Crit. Rev. Food Sci. 2012, 52, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Valero, E.; Villamiel, M.; Miralles, B.; Sanz, J.; Martinez-Castro, I. Changes in flavour and volatile components during storage of whole and skimmed UHT milk. Food Chem. 2001, 72, 51–58. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.A.; Qian, M.C. Quantitative determination of thermally derived off-flavor compounds in milk using solid-phase microextraction and gas chromatography. J. Dairy Sci 2005, 88, 3764–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkins, M.L.; Elliott, A.J.; D’Arcy, B.R.; Deeth, H.C. Stale flavour volatiles in Australian commercial UHT milk during storage. Aust. J. Dairy Technol. 2005, 60, 231–237. [Google Scholar]
- He, W.; Ren, F.; Wang, Y.; Gao, X.; Song, J. Application of GC-IMS in Detection of Food Flavor Substances. IOP Conf. Ser. Earth Environ. Sci. 2020, 545, 012030. [Google Scholar] [CrossRef]
- Dong, D.; Zheng, W.; Jiao, L.; Lang, Y.; Zhao, X. Chinese vinegar classification via volatiles using long-optical-path infrared spectroscopy and chemometrics. Food Chem. 2016, 194, 95–100. [Google Scholar] [CrossRef]
- Nie, J.; Li, Y.; Xing, J.; Chao, J.; Qin, X.; Li, Z. Comparison of two types of vinegar with different aging times by NMR-based metabolomic approach. J. Food Biochem. 2019, 43, e12835. [Google Scholar] [CrossRef]
- Magan, N.; Pavlou, A.; Chrysanthakis, I. Milk-sense: A volatile sensing system recognises spoilage bacteria and yeasts in milk. Sens. Actuators B Chem. 2001, 72, 28–34. [Google Scholar] [CrossRef]
- Marsili, R.T. SPME-MS-MVA as an Electronic Nose for the Study of Off-Flavors in Milk. J. Agric. Food Chem. 1999, 47, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Sun, L.; Du, S.; Ge, G.T.; Wang, Z.J.; Li, Y.Y.; Bao, J.; Zhao, M.; Si, Q.; Hao, J.F.; et al. Effects of Harvesting Period and Storage Duration on Volatile Organic Compounds and Nutritive Qualities of Alfalfa. Agriculture 2022, 12, 1115. [Google Scholar] [CrossRef]
- Shen, D.Y.; Song, H.L.; Zou, T.T.; Wan, S.Y.; Li, M.K. Characterization of odor-active compounds in moso bamboo (Phyllostachys pubescens Mazel) leaf via gas chromatography-ion mobility spectrometry, one- and two-dimensional gas chromatography-olfactory-mass spectrometry, and electronic nose. Food Res. Int. 2022, 152, 110916. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Ning, F.J.; Zhao, L.; Ming, H.H.; Zhang, J.P.; Yu, W.J.; Yi, S.X.; Luo, L.P. Quality assessment of royal jelly based on physicochemical properties and flavor profiles using HS-SPME-GC/MS combined with electronic nose and electronic tongue analyses. Food Chem. 2023, 403, 134392. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-Y.; Cao, W.-X.; Bai, J.-R.; Zhou, X.-J.; Meng, J.-F. Analysis of flavor characteristics of Nitraria tangutorum Bobr fruit at different maturity. J. Food Saf. Qual. 2022, 13, 6677–6681. [Google Scholar] [CrossRef]
- Bottiroli, R.; Troise, A.D.; Aprea, E.; Fogliano, V.; Gasperi, F.; Vitaglione, P. Understanding the effect of storage temperature on the quality of semi-skimmed UHT hydrolyzed-lactose milk: An insight on release of free amino acids, formation of volatiles organic compounds and browning. Food Res. Int. 2021, 141, 110120. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Ma, Y.; Fu, X.; Cao, Q.; Zhang, Y.; You, C. Effects of heat treatment, homogenization pressure, and overprocessing on the content of furfural compounds in liquid milk. J. Sci. Food Agric. 2020, 100, 5276–5282. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, D.; Peterson, D.G. Identification of bitter peptides in whey protein hydrolysate. J. Agric. Food Chem. 2014, 62, 5719–5725. [Google Scholar] [CrossRef]
- Leksrisompong, P.P.; Miracle, R.E.; Drake, M. Characterization of flavor of whey protein hydrolysates. J. Agric. Food Chem. 2010, 58, 6318–6327. [Google Scholar] [CrossRef]
- Dutta Banik, D.; Medler, K.F. Bitter, sweet, and umami signaling in taste cells: It’s not as simple as we thought. Curr. Opin. Physiol. 2021, 20, 159–164. [Google Scholar] [CrossRef]
- Jo, Y.; Carter, B.G.; Barbano, D.M.; Drake, M.A. Identification of the source of volatile sulfur compounds produced in milk during thermal processing. J. Dairy Sci. 2019, 102, 8658–8669. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.P.; Barbano, D.M.; Drake, M.A. The influence of ultra-pasteurization by indirect heating versus direct steam injection on skim and 2% fat milks. J. Dairy Sci. 2017, 100, 1688–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, S.; Jansson, T.; Eggers, N.; Clausen, M.R.; Larsen, L.B.; Jensen, H.B.; Ray, C.; Sundgren, A.; Andersen, H.J.; Bertram, H.C. Storage-induced changes in the sensory characteristics and volatiles of conventional and lactose-hydrolyzed UHT processed milk. Eur. Food Res. Technol. 2015, 240, 1247–1257. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, S.; Lu, J.; Pang, X.; Xu, X.; Lv, J.; Zhang, S. Effect of different heat treatments on the Maillard reaction products, volatile compounds and glycation level of milk. Int. Dairy J. 2021, 123, 105182. [Google Scholar] [CrossRef]
- Colahan-Sederstrom, P.M.; Peterson, D.G. Inhibition of key aroma compound generated during ultrahigh-temperature processing of bovine milk via epicatechin addition. J. Agric. Food Chem. 2005, 53, 398–402. [Google Scholar] [CrossRef]
- Mu, S.; Stieger, M.; Boesveldt, S. Olfactory discrimination of fat content in milks is facilitated by differences in volatile compound composition rather than odor intensity. Food Chem. 2022, 393, 133357. [Google Scholar] [CrossRef]
- Van Hekken, D.L.; Iandola, S.; Tomasula, P.M. Short communication: Volatiles in microfluidized raw and heat-treated milk. J. Dairy Sci. 2019, 102, 8819–8824. [Google Scholar] [CrossRef]
- Alothman, M.; Lusk, K.A.; Silcock, P.J.; Bremer, P.J. Relationship between total microbial numbers, volatile organic compound composition, and the sensory characteristics of whole fresh chilled pasteurized milk. Food Packag. Shelf Life 2018, 15, 69–75. [Google Scholar] [CrossRef]
- Pegolo, S.; Bergamaschi, M.; Gasperi, F.; Biasioli, F.; Cecchinato, A.; Bittante, G. Integrated PTR-ToF-MS, GWAS and biological pathway analyses reveal the contribution of cow’s genome to cheese volatilome. Sci. Rep. 2018, 8, 17002. [Google Scholar] [CrossRef]
- Zhou, T.; Huo, R.; Kwok, L.Y.; Li, C.; Ma, Y.; Mi, Z.; Chen, Y. Effects of applying Lactobacillus helveticus H9 as adjunct starter culture in yogurt fermentation and storage. J. Dairy Sci. 2019, 102, 223–235. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Peng, C.; Wang, J.; Guo, S.; Sun, Z.; Zhang, H. Mesopic fermentation contributes more to the formation of important flavor compounds and increased growth of Lactobacillus casei Zhang than does high temperature during milk fermentation and storage. J. Dairy Sci. 2022, 105, 4857–4867. [Google Scholar] [CrossRef] [PubMed]
- NY/T 939—2016; Identification of Reconstituted Milk in Pasteurized and UHT Milk. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2016.
- T/TDSTIA 006—2019; Determination of Lactoferrin in Milk and Dairy Products-Liquid Chromatography Method. Tianjin Dairy Science and Technology Innovation Association: Tianjin, China, 2019.
- T/TDSTIA 002—2021; Determination of α-lactoalbumin in Milk and Dairy Products-Liquid Chromatography Method. Tianjin Dairy Science and Technology Innovation Association: Tianjin, China, 2021.
- T/TDSTIA 007—2019; Determination of β-lactoglobulin in Milk and Dairy Products-Liquid Chromatography Method. Tianjin Dairy Science and Technology Innovation Association: Tianjin, China, 2019.
- T/TDSTIA003-2021; Determination of Immunoglobulin G in Milk and Dairy Products-High Performance Liquid Chromatography Method. Tianjin Dairy Science and Technology Innovation Association: Tianjin, China, 2021.
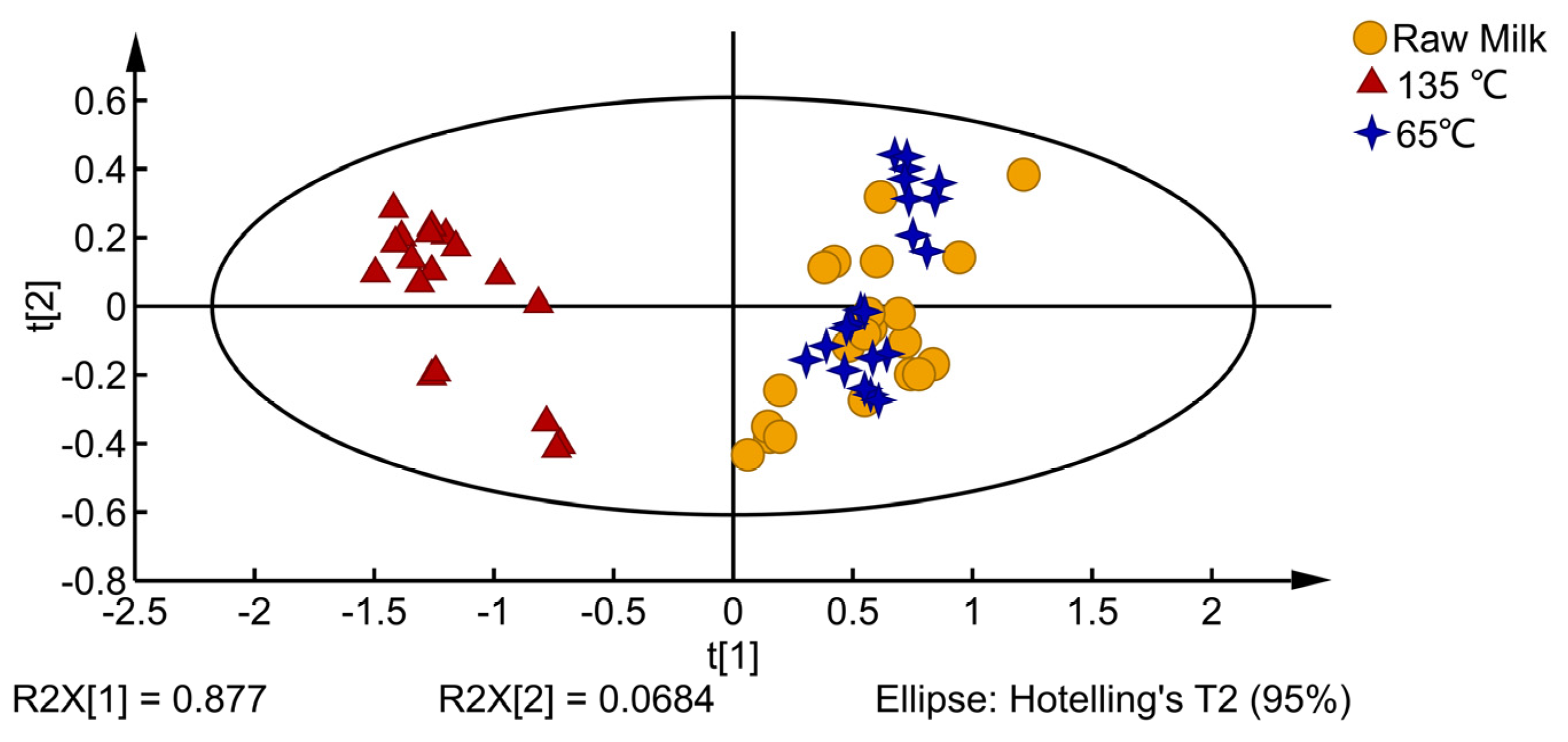
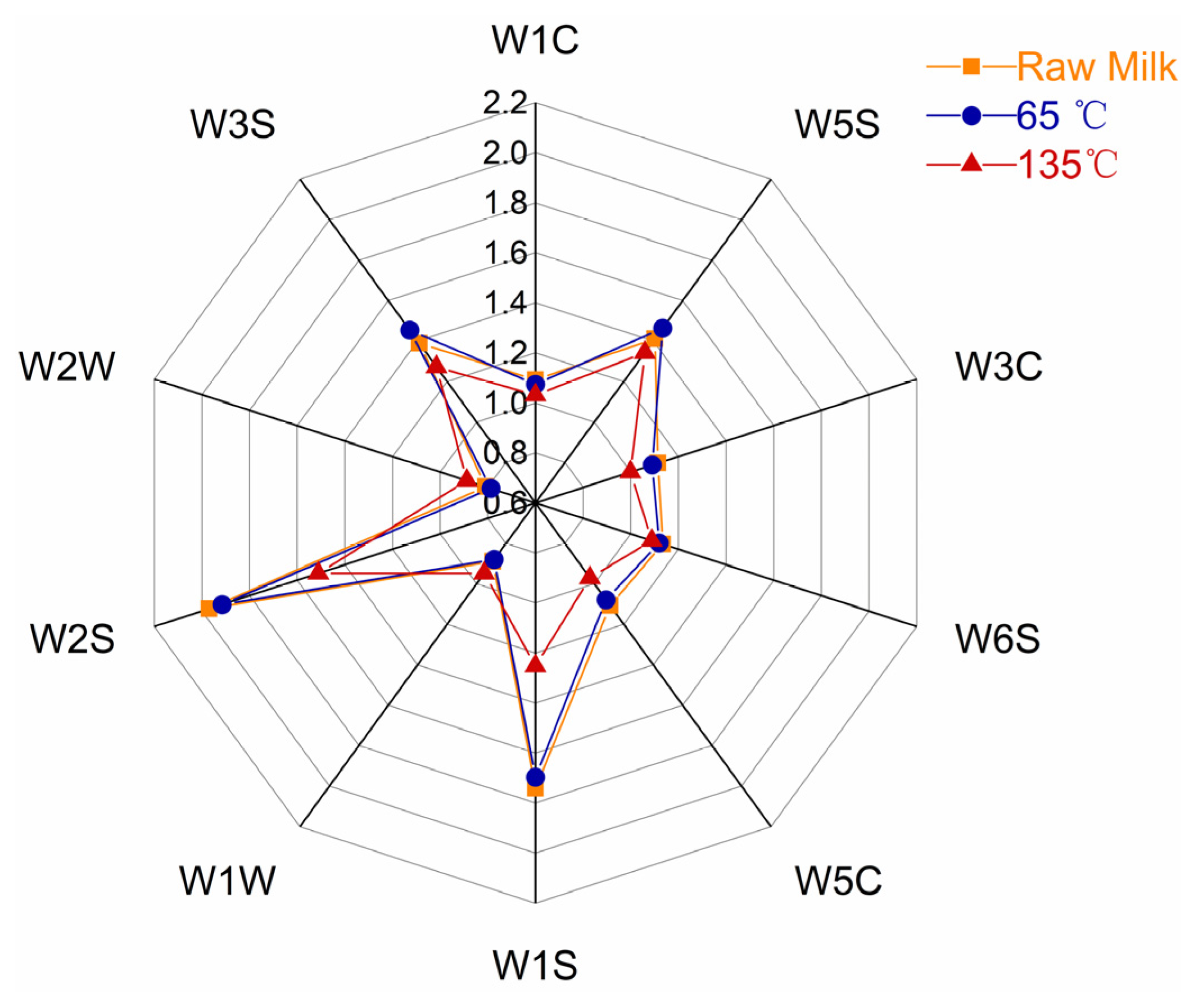


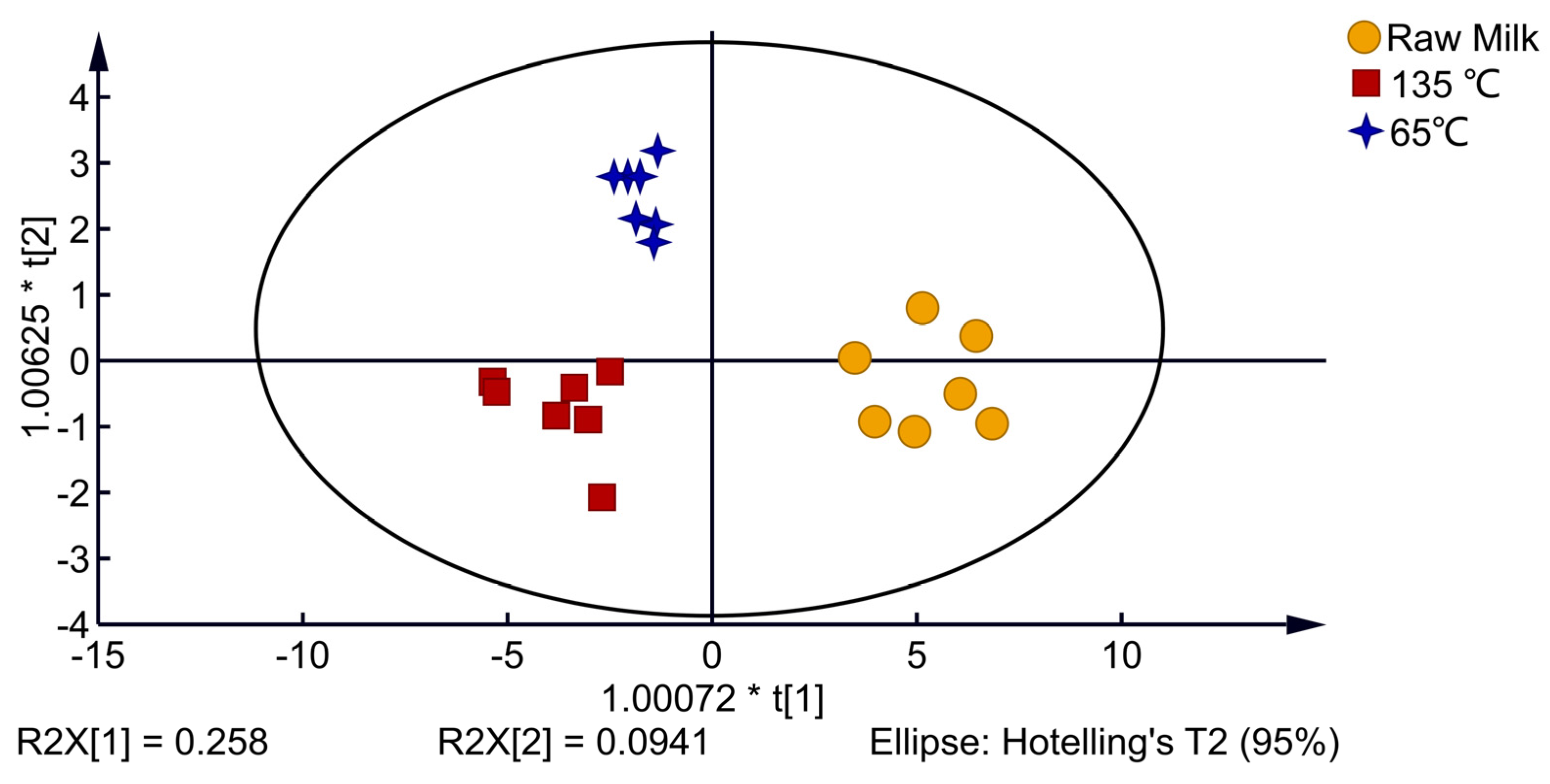
| No. | Designation | Performance Description | Main Sensitive Substance | Detection Threshold (mL/m3) |
|---|---|---|---|---|
| 1 | W5S | nitrogen oxides | NO2 | 1 |
| 2 | W1S | methyl | CH4 | 100 |
| 3 | W2S | alcohols, ketones and aldehydes | CO | 100 |
| 4 | W3C | ammonia | C6H6 | 10 |
| 5 | W1C | benzene | C7H8 | 10 |
| 6 | W5C | short-chain aromatic compounds and olefin | C3H8 | 1 |
| 7 | W1W | sulfur compounds | H2S | 1 |
| 8 | W2W | organic sulfides | H2S | 1 |
| 9 | W6S | hydrogen | H2 | 100 |
| 10 | W3S | long-chain alkanes | CH4 | 100 |
| NO | Designation | Performance |
|---|---|---|
| 1 | CT0 | Saltiness |
| 2 | CA0 | Sourness |
| 3 | AAE | Umami, Aftertaste-U |
| 4 | AE1 | Astringency, Aftertaste-A |
| 5 | C00 | Bitterness, Aftertaste-B |
| 6 | GL1 | Sweetness |
| Sourness | Saltiness | Bitterness | Astringency | Aftertaste-B | Aftertaste-A | Umami | Sweetness | Aftertaste-U | |
|---|---|---|---|---|---|---|---|---|---|
| Tasteless | −13 | −6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Raw milk | −45.71 ± 0.14 c | 0.18 ± 0.44 b | 7.81 ± 0.19 b | 1.94 ± 0.18 b | 1.00 ± 0.07 a | 0.37 ± 0.03 a | 6.79 ± 0.11 b | 11.08 ± 0.23 a | 4.91 ± 0.11 b |
| 65 °C | −41.41 ± 0.15 b | 3.29 ± 0.44 a | 7.88 ± 0.25 b | 3.19 ± 0.14 a | 0.18 ± 0.19 b | 0.33 ± 0.02 a | 7.21 ± 0.08 ab | 8.92 ± 0.17 b | 6.59 ± 0.22 a |
| 135 °C | 5.77 ± 4.36 a | 1.43 ± 1.70 ab | 15.10 ± 2.10 a | 2.37 ± 0.71 ab | 0.07 ± 0.08 b | 0.18 ± 0.04 b | 7.73 ± 0.95 a | 8.35 ± 0.35 c | 1.18 ± 0.26 c |
| NO | Compound | Retention Time | CAS# | Formula | RI(Ref) | RI | Raw Milk (μg/L) | 65 °C (μg/L) | 135 °C (μg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | 1 | Hexanal | 8.386 | 66-25-1 | C6H12O | 1095 | 1008 | 13.57 ± 2.57 | 22.28 ± 5.34 | ND |
| 2 | Octanal | 12.677 | 124-13-0 | C8H16O | 1289 | 1272 | ND | 2.53 ± 0.42 | ND | |
| 3 | Furfural | 15.205 | 98-01-1 | C5H4O2 | 1439 | 1452 | ND | ND | 2.55 ± 0.59 | |
| 4 | 3-Ethyl-benzaldehyde | 18.318 | 34246-54-3 | C9H10O | 1700 | 0.89 ± 0.25 | ND | ND | ||
| 5 | 1,4-Benzenedicarboxaldehyde | 22.701 | 623-27-8 | C8H6O2 | 2119 | ND | ND | 0.47 ± 0.09 | ||
| Alcohols | 1 | 2-Butyl-1-octano | 4.719 | 3913-02-8 | C12H26O | 1853 | ND | 3.90 ± 0.89 | ND | |
| 2 | 2,3-Dimethyl-1-butanol | 4.722 | 19550-30-2 | C6H14O | 0.63 ± 0.12 | ND | ND | |||
| 3 | 2-Propyl-1-heptanol | 6.221 | 10042-59-8 | C10H22O | 862 | 5.54 ± 0.90 | ND | ND | ||
| 4 | 3-Methyl-3-buten-1-ol | 12.103 | 763-32-6 | C5H10O | 1236 | 1234 | 2.21 ± 0.24 | ND | ND | |
| 5 | 1-Pentanol | 12.191 | 71-41-0 | C5H12O | 1258 | 1240 | ND | 1.96 ± 0.24 | ND | |
| 6 | 1-Octen-3-ol | 15.124 | 3391-86-4 | C8H16O | 1458 | 1446 | 2.39 ± 0.47 | 1.73 ± 0.24 | ND | |
| 7 | 2-Ethyl-1-hexanol | 15.665 | 104-76-7 | C8H18O | 1484 | 1487 | 2.62 ± 0.62 | 4.59 ± 0.36 | 4.25 ± 1.05 | |
| 8 | 2-Furanmethanol | 17.694 | 98-00-0 | C5H6O2 | 1664 | 1649 | ND | ND | 16.58 ± 6.22 | |
| Ketones | 1 | 2-Heptanone | 10.81 | 110-43-0 | C7H14O | 1184 | 1152 | ND | ND | 13.75 ± 1.79 |
| 2 | 2-Nonanone | 14.277 | 821-55-6 | C9H18O | 1389 | 1383 | 1.75 ± 0.28 b | 2.45 ± 0.43 b | 5.72 ± 0.75 a | |
| 3 | 2-Undecanone | 17.025 | 112-12-9 | C11H22O | 1599 | 1594 | ND | ND | 2.43 ± 0.42 | |
| 4 | 2-Dodecanone | 17.062 | 6175-49-1 | C12H24O | 1597 | ND | 0.47 ± 0.03 | ND | ||
| Esters | 1 | Pentanoic acid ethyl ester | 20.915 | 539-82-2 | C7H14O2 | 1939 | ND | ND | 2.82 ± 0.70 | |
| 2 | 5-Octanolide | 21.141 | 698-76-0 | C8H14O2 | 1956 | 1961 | ND | ND | 0.95 ± 0.42 | |
| 3 | Undecanolactone | 23.369 | 710-04-3 | C11H20O2 | 2190 | ND | 0.52 ± 0.07 b | 0.94 ± 0.16 a | ||
| Acids | 1 | Butanoic acid | 17.309 | 107-92-6 | C4H8O2 | 1637 | 1616 | 4.49 ± 0.47 b | 4.63 ± 0.62 b | 10.57 ± 2.61 a |
| 2 | 2-Amino-5-methylbenzoic acid | 18.811 | 2941-78-8 | C8H9NO2 | 1744 | ND | 40.43 ± 9.89 | ND | ||
| 3 | Hexanoic acid | 19.79 | 142-62-1 | C6H12O2 | 1851 | 1832 | 5.49 ± 0.91 | 6.90 ± 1.09 | 6.65 ± 1.81 | |
| 4 | 2-Ethyl-hexanoic acid | 20.901 | 149-57-5 | C8H16O2 | 1962.5 | 1938 | 1.05 ± 0.22 | ND | ND | |
| 5 | Heptanoic acid | 20.908 | 111-14-8 | C7H14O2 | 1960 | 1938 | ND | 0.75 ± 0.21 | 1.91 ± 0.24 | |
| 6 | Styrene | 21.988 | 124-07-2 | C8H16O2 | 2050 | 2045 | 8.63 ± 1.28 | 7.56 ± 1.56 | 8.88 ± 1.36 | |
| 7 | Nonanoic acid | 23.011 | 112-05-0 | C9H18O2 | 2174 | 2151 | 1.02 ± 0.25 | 0.63 ± 0.17 | 1.24 ± 0.31 | |
| 8 | n-Decanoic acid | 23.984 | 334-48-5 | C10H20O2 | 2279 | 2257 | 10.74 ± 1.65 | 6.39 ± 1.13 | 7.03 ± 2.33 | |
| 9 | Benzoic acid | 25.419 | 65-85-0 | C7H6O2 | 2433 | 2421 | 5.03 ± 0.68 a | 2.25 ± 0.53 b | 4.20 ± 1.13 ab | |
| 10 | Dodecanoic acid | 25.817 | 143-07-7 | C12H24O2 | 2502 | 2469 | 7.83 ± 1.38 | 4.42 ± 0.82 | 7.66 ± 2.75 | |
| 11 | Tetradecanoic acid | 27.539 | 544-63-8 | C14H28O2 | 2692 | 2679 | 41.21 ± 6.08 a | 24.56 ± 5.45 b | 28.67 ± 8.32 ab | |
| 12 | Pentadecanoic acid | 28.528 | 1002-84-2 | C15H30O2 | 2803 | 2785 | 54.86 ± 11.44 | ND | ND | |
| 13 | n-Hexadecanoic acid | 29.75 | 57-10-3 | C16H32O2 | 2876 | 2894 | 48.43 ± 6.50 a | 28.23 ± 4.29 b | 26.20 ± 4.32 b | |
| Hydrocarbons | 1 | Decane | 6.225 | 124-18-5 | C10H22 | 863 | ND | 8.24 ± 1.31 | 8.93 ± 1.48 | |
| 2 | 4,7-Dimethyl-undecane | 7.369 | 17301-32-5 | C13H28 | 946 | ND | ND | 7.23 ± 1.76 | ||
| 3 | Ethylbenzene | 9.263 | 100-41-4 | C8H10 | 1122 | 1059 | ND | 7.27 ± 0.74 b | 14.87 ± 2.82 a | |
| 4 | 1,3-Dimethyl-benzene | 9.587 | 108-38-3 | C8H10 | 1140 | 1078 | ND | 7.94 ± 1.68 b | 17.05 ± 4.01 a | |
| 5 | Dodecane | 10.995 | 112-40-3 | C12H26 | 1163 | ND | 9.01 ± 1.01 | ND | ||
| 6 | Styrene | 12.001 | 100-42-5 | C8H8 | 1254 | 1227 | 1.59 ± 0.43 | 2.31 ± 0.47 | ND | |
| 7 | 1,2,3-Trimethyl-benzene | 12.39 | 526-73-8 | C9H12 | 1267 | 1253 | 1.70 ± 1.21 | 1.26 ± 0.23 | ND | |
| 8 | Benzene,1,3-bis(1,1-dimethylethyl)- | 14.783 | 1014-60-4 90 | C14H22 | 1420 | 3.22 ± 0.27 | 3.05 ± 0.32 | 3.00 ± 0.28 | ||
| Nitrogen-containing compounds | 1 | Isobutyramide | 8.94 | 563-83-7 | C4H9NO | 1009 | ND | 6.43 ± 1.07 | ND | |
| Phenols | 1 | Phenol | 21.421 | 108-95-2 | C6H6O | 2008 | 1988 | 1.75 ± 0.91 | 0.86 ± 0.40 | 1.55 ± 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, N.; Chi, X.; Ye, Q.; Liu, H.; Zheng, N. Analysis of Volatile Organic Compounds in Milk during Heat Treatment Based on E-Nose, E-Tongue and HS-SPME-GC-MS. Foods 2023, 12, 1071. https://doi.org/10.3390/foods12051071
Yuan N, Chi X, Ye Q, Liu H, Zheng N. Analysis of Volatile Organic Compounds in Milk during Heat Treatment Based on E-Nose, E-Tongue and HS-SPME-GC-MS. Foods. 2023; 12(5):1071. https://doi.org/10.3390/foods12051071
Chicago/Turabian StyleYuan, Ning, Xuelu Chi, Qiaoyan Ye, Huimin Liu, and Nan Zheng. 2023. "Analysis of Volatile Organic Compounds in Milk during Heat Treatment Based on E-Nose, E-Tongue and HS-SPME-GC-MS" Foods 12, no. 5: 1071. https://doi.org/10.3390/foods12051071




