Sub-Regional Variation and Characteristics of Cabernet Sauvignon Wines in the Eastern Foothills of the Helan Mountain: A Perspective from Phenolics, Visual Properties and Mouthfeel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Samples
2.2. Chemicals and Standards
2.3. Analysis of Phenolic Compounds
2.3.1. Analysis of Non-Anthocyanin Phenolic Compounds [21]
2.3.2. Analysis of Anthocyanins [23]
2.3.3. Analysis of Anthocyanin Derivatives [24]
2.4. Color Measurement
2.5. Sensory Analysis
2.5.1. CATA
2.5.2. QDA
2.6. Statistical Analysis
3. Results and Discussion
3.1. Basic Wine Compositions
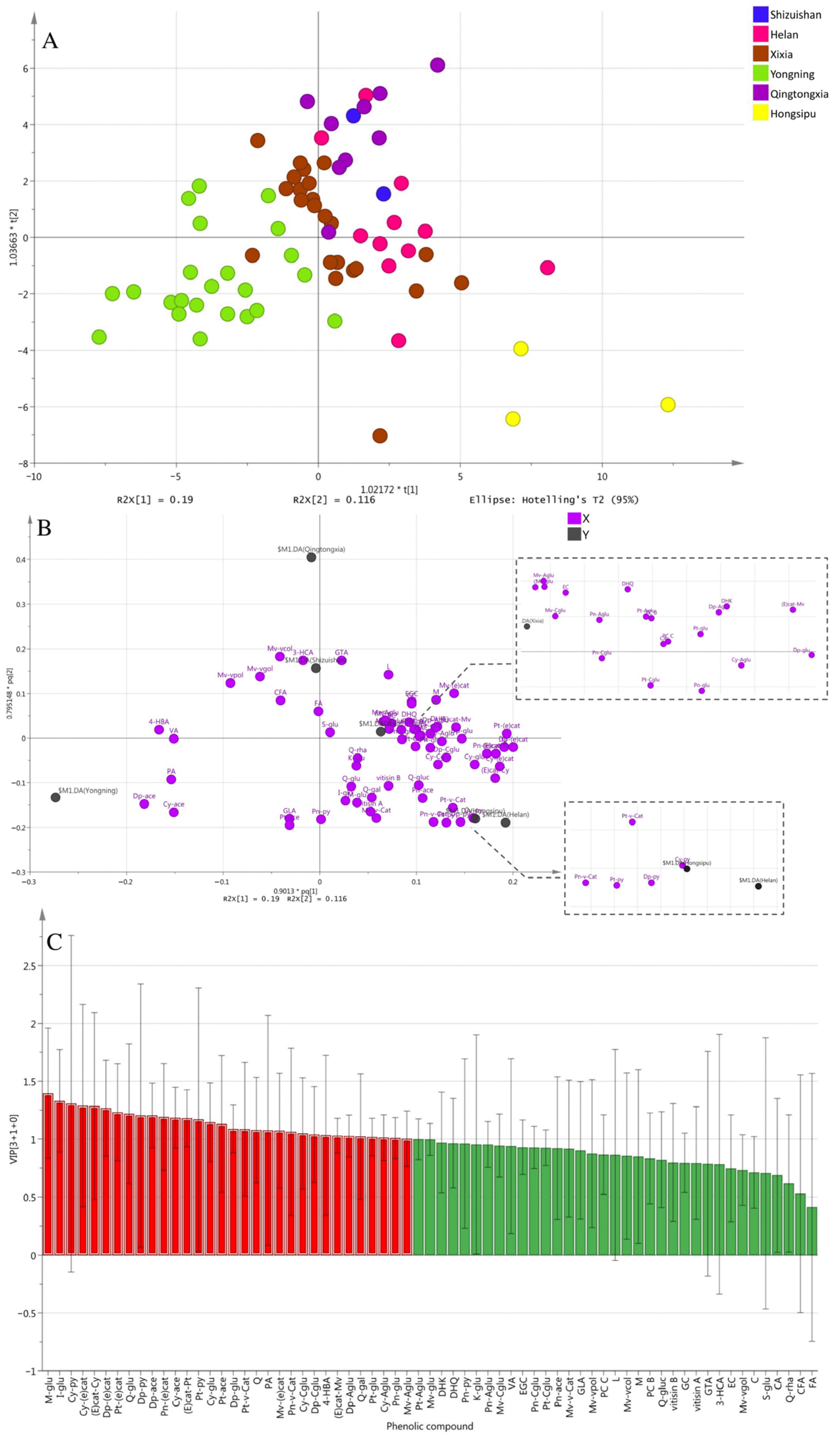
3.2. OPLS-DA Analysis
3.3. Sub-Regional Variation of Phenolic Compounds
3.3.1. Comparison of Non-Anthocyanin Phenolic Compounds
3.3.2. Comparison of Anthocyanins
3.3.3. Comparison of Anthocyanin Derivatives
3.4. Hierarchical Cluster Analysis
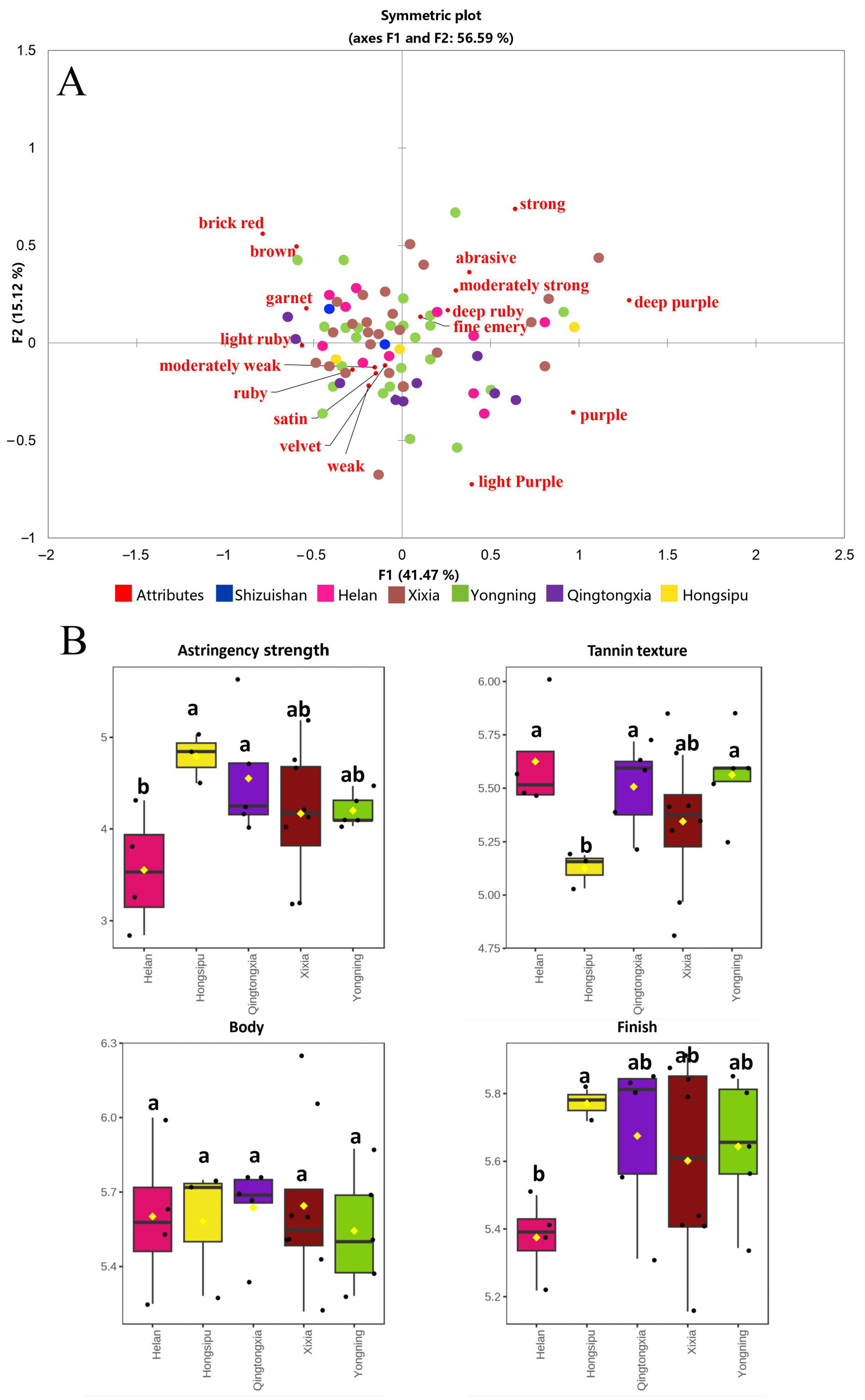
3.5. Comparison of Color of Wines
3.6. Sensory Characteristics of Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whalen, P. ‘Insofar as the Ruby Wine Seduces Them’: Cultural Strategies for Selling Wine in Inter-war Burgundy. Contemp. Eur. Hist. 2009, 18, 67–98. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Seguin, G. The concept of terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Li, Y.-D.; Zhang, J.-X.; Wang, Z.-D.; Cai, X.-Q.; Yu, H.-M.; Ma, Y.-M.; Wang, F.-Q.; Zhang, G.-L. Grape Vintages and Climate at the Eastern Foothills of Helan Mountain in Ningxia. Vitic. Winemak. 2004, 2, 54–57. (In Chinese) [Google Scholar]
- Qi, Y.; Wang, R.; Qin, Q.; Sun, Q. Soil affected the variations in grape and wine properties along the eastern foot of Helan Mountain, China. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2019, 69, 494–502. [Google Scholar] [CrossRef]
- Bowers, J.E.; Meredith, C.P. The parentage of a classic wine grape, Cabernet Sauvignon. Nat. Genet. 1997, 16, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Harding, J. The Oxford Companion to Wine; American Chemical Society: Washington, DC, USA, 2015. [Google Scholar]
- Gatti, M.; Civardi, S.; Ferrari, F.; Fernandes, N.; Goncaves, M.V.Z.D.M.B.; Bavaresco, L. Viticultural performances of different ‘cabernet sauvignon’ clones. Acta Hortic. 2014, 1046, 659–664. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Gil-Muñoz, R.; López-Roca, J.; Martínez-Cutillas, A.; Fernández-Fernández, J. Phenolic Compounds and Color Stability of Red Wines: Effect of Skin Maceration Time. Am. J. Enol. Vitic. 2001, 52, 266–270. [Google Scholar] [CrossRef]
- Lorenzo, C.; Pardo, F.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of Red Grapes Co-winemaking in Polyphenols and Color of Wines. J. Agric. Food Chem. 2005, 53, 7609–7616. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and astringency of flavan-3-ol monomers, dimers and trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Gawel, R. Red wine astringency: A review. Aust. J. Grape Wine Res. 1998, 4, 74–95. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Cao, L.; Lu, J. Phenolic compounds and antioxidant properties of different grape cultivars grown in China. Food Chem. 2010, 119, 1557–1565. [Google Scholar] [CrossRef]
- Mazza, G.; Fukumoto, L.; Delaquis, P.; Girard, B.; Ewert, B. Anthocyanins, Phenolics, and Color of Cabernet Franc, Merlot, and Pinot Noir Wines from British Columbia. J. Agric. Food Chem. 1999, 47, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Influence of climatic conditions on the phenolic composition of Vitis vinifera L. cv. Graciano. Anal. Chim. Acta 2012, 732, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Gamboa, G.; Carrasco-Quiroz, M.; Verdugo-Vásquez, N.; Díaz-Gálvez, I.; Garde-Cerdán, T.; Moreno-Simunovic, Y. Characterization of grape phenolic compounds of ‘Carignan’ grapevines grafted onto ‘País’ rootstock from Maule Valley (Chile): Implications of climate and soil conditions. Chil. J. Agric. Res. 2018, 78, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Douglas, D.; Cliff, M.A.; Reynolds, A.G. Canadian terroir: Characterization of Riesling wines from the Niagara Peninsula. Food Res. Int. 2001, 34, 559–563. [Google Scholar] [CrossRef]
- Galgano, F.; Caruso, M.; Perretti, G.; Favati, F. Authentication of Italian red wines on the basis of the polyphenols and biogenic amines. Eur. Food Res. Technol. 2011, 232, 889–897. [Google Scholar] [CrossRef]
- Pavloušek, P.; Kumšta, M. Authentication of Riesling wines from the Czech Republic on the basis of the non-flavonoid phenolic compounds. Czech J. Food Sci. 2013, 31, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Pan, Q.; Jin, Z.; Mu, L.; Duan, C. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 2011, 125, 77–83. [Google Scholar] [CrossRef]
- Lan, Y.; Liu, M.; Zhang, X.; Li, S.; Shi, Y.; Duan, C. Regional Variation of Chemical Characteristics in Young Marselan (Vitis vinifera L.) Red Wines from Five Regions of China. Foods 2022, 11, 787. [Google Scholar] [CrossRef]
- Li, S.-Y.; He, F.; Zhu, B.-Q.; Wang, J.; Duan, C.-Q. Comparison of phenolic and chromatic characteristics of dry red wines made from native Chinese grape species and vitis vinifera. Int. J. Food Prop. 2017, 20, 2134–2146. [Google Scholar] [CrossRef]
- Li, S.-Y.; He, F.; Zhu, B.-Q.; Xing, R.-R.; Reeves, M.J.; Duan, C.-Q. A systematic analysis strategy for accurate detection of anthocyanin pigments in red wines. Rapid Commun. Mass Spectrom. 2016, 30, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-K.; Li, S.-Y.; Zhao, X.; Pan, Q.-H.; Shi, Y.; Duan, C.-Q. HPLC-MS/MS-based targeted metabolomic method for profiling of malvidin derivatives in dry red wines. Food Res. Int. 2020, 134, 109226. [Google Scholar] [CrossRef] [PubMed]
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. A New Simplified Method for Measuring the Color of Wines. I. Red and Rosé Wines. Am. J. Enol. Vitic. 1997, 48, 357–363. [Google Scholar] [CrossRef]
- Dooley, L.; Lee, Y.-S.; Meullenet, J.-F. The application of check-all-that-apply (CATA) consumer profiling to preference mapping of vanilla ice cream and its comparison to classical external preference mapping. Food Qual. Prefer. 2010, 21, 394–401. [Google Scholar] [CrossRef]
- Gawel, R.; Oberholster, A.; Francis, I.L. A ‘Mouth-feel Wheel’: Terminology for communicating the mouth-feel characteristics of red wine. Aust. J. Grape Wine Res. 2000, 6, 203–207. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.; Oliver, S.; Woolsey, A.; Singleton, R.C. Sensory evaluation by quantitative descriptive analysis. In Descriptive Sensory Analysis in Practice; Gacular, M.C., Jr., Ed.; Food & Nutrition Press, Inc.: Trumbull, CT, USA, 2004; pp. 23–34. ISBN 9780917678370. [Google Scholar]
- Ng, M.; Lawlor, J.; Chandra, S.; Chaya, C.; Hewson, L.; Hort, J. Using quantitative descriptive analysis and temporal dominance of sensations analysis as complementary methods for profiling commercial blackcurrant squashes. Food Qual. Prefer. 2012, 25, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Krumbein, A.; Peters, P.; Brückner, B. Flavour compounds and a quantitative descriptive analysis of tomatoes (Lycopersicon esculentum Mill.) of different cultivars in short-term storage. Postharvest Biol. Technol. 2004, 32, 15–28. [Google Scholar] [CrossRef]
- Chapman, K.; Lawless, H.; Boor, K. Quantitative Descriptive Analysis and Principal Component Analysis for Sensory Characterization of Ultrapasteurized Milk. J. Dairy Sci. 2001, 84, 12–20. [Google Scholar] [CrossRef]
- Castiñeira, M.D.M.; Feldmann, I.; Jakubowski, N.; Andersson, J.T. Classification of German White Wines with Certified Brand of Origin by Multielement Quantitation and Pattern Recognition Techniques. J. Agric. Food Chem. 2004, 52, 2962–2974. [Google Scholar] [CrossRef]
- Li, S.-Y.; Zhu, B.-Q.; Reeves, M.J.; Duan, C.-Q. Phenolic Analysis and Theoretic Design for Chinese Commercial Wines’ Authentication. J. Food Sci. 2017, 83, 30–38. [Google Scholar] [CrossRef]
- Chong, I.-G.; Jun, C.-H. Performance of some variable selection methods when multicollinearity is present. Chemom. Intell. Lab. Syst. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Prado, R.D.A.-D.; Yuste-Rojas, M.; Sort, X.; Andrés-Lacueva, C.; Torres, M.; Lamuela-Raventós, R.M. Effect of Soil Type on Wines Produced from Vitis vinifera L. Cv. Grenache in Commercial Vineyards. J. Agric. Food Chem. 2007, 55, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.; Roby, J.-P.; De Rességuier, L. Soil-related terroir factors: A review. OENO One 2018, 52, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Sun, P.; Wang, Z. Effects of different soil condition on physiology and fruit quality of wine grapes. J. Fruit Sci. 2012, 29, 837–842. (In Chinese) [Google Scholar]
- Wang, L.; Zhang, Z.J.N.H. Research on quality of wine grape of main cultivated in Ningxia in 2009. North. Hortic. 2011, 3, 4–8. (In Chinese) [Google Scholar]
- Wang, R.; Sun, Q.; Chang, Q. Soil Types Effect on Grape and Wine Composition in Helan Mountain Area of Ningxia. PLoS ONE 2015, 10, e0116690. [Google Scholar] [CrossRef]
- Liang, N.-N.; He, F.; Bi, H.-Q.; Duan, C.-Q.; Reeves, M.J.; Wang, J. Evolution of flavonols in berry skins of different grape cultivars during ripening and a comparison of two vintages. Eur. Food Res. Technol. 2012, 235, 1187–1197. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Cadenas, E.; Packer, L. Handbook of Antioxidants; Marcel Dekker: New York, NY, USA, 2002; Volume 712. [Google Scholar]
- Cantos, E.; Espín, J.C.; Tomás-Barberán, F.A. Varietal Differences among the Polyphenol Profiles of Seven Table Grape Cultivars Studied by LC−DAD−MS−MS. J. Agric. Food Chem. 2002, 50, 5691–5696. [Google Scholar] [CrossRef]
- Magro, L.D.; Goetze, D.; Ribeiro, C.T.; Paludo, N.; Rodrigues, E.; Hertz, P.; Klein, M.P.; Rodrigues, R.C. Identification of Bioactive Compounds From Vitis labrusca L. Variety Concord Grape Juice Treated With Commercial Enzymes: Improved Yield and Quality Parameters. Food Bioprocess. Technol. 2015, 9, 365–377. [Google Scholar] [CrossRef]
- Azuma, A.; Yakushiji, H.; Koshita, Y.; Kobayashi, S. Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 2012, 236, 1067–1080. [Google Scholar] [CrossRef]
- Gao-Takai, M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. A low temperature promotes anthocyanin biosynthesis but does not accelerate endogenous abscisic acid accumulation in red-skinned grapes. Plant Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cascales, I.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. The maceration process during winemaking extraction of anthocyanins from grape skins into wine. Eur. Food Res. Technol. 2005, 221, 163–167. [Google Scholar] [CrossRef]
- Wang, H.; Race, E.J.; Shrikhande, A.J. Anthocyanin Transformation in Cabernet Sauvignon Wine during Aging. J. Agric. Food Chem. 2003, 51, 7989–7994. [Google Scholar] [CrossRef]
- Gao, L.; Girard, B.; Mazza, G.; Reynolds, A.G. Changes in Anthocyanins and Color Characteristics of Pinot Noir Wines during Different Vinification Processes. J. Agric. Food Chem. 1997, 45, 2003–2008. [Google Scholar] [CrossRef]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P.; Hermosín-Gutiérrez, I. Formation of Hydroxyphenyl-pyranoanthocyanins in Grenache Wines: Precursor Levels and Evolution during Aging. J. Agric. Food Chem. 2007, 55, 4883–4888. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, K.; Gu, X.; Sun, X.; Jin, G.; Zhang, J.; Ma, W. Flavor Chemical Profiles of Cabernet Sauvignon Wines: Six Vintages from 2013 to 2018 from the Eastern Foothills of the Ningxia Helan Mountains in China. Foods 2021, 11, 22. [Google Scholar] [CrossRef]
- Han, F.-L.; Zhang, W.-N.; Pan, Q.-H.; Zheng, C.-R.; Chen, H.-Y.; Duan, C.-Q. Principal Component Regression Analysis of the Relation Between CIELAB Color and Monomeric Anthocyanins in Young Cabernet Sauvignon Wines. Molecules 2008, 13, 2859–2870. [Google Scholar] [CrossRef] [Green Version]
- Czibulya, Z.; Horváth, I.; Kollár, L.; Pour Nikfardjam, M.; Kunsági-Máté, S. The effect of temperature, pH, and ionic strength on color stability of red wine. Tetrahedron 2015, 71, 3027–3031. [Google Scholar] [CrossRef]
- Sims, C.A.; Morris, J.R. Effects of pH, Sulfur Dioxide, Storage Time, and Temperature on the Color and Stability of Red Muscadine Grape Wine. Am. J. Enol. Vitic. 1984, 35, 35–39. [Google Scholar] [CrossRef]
- Greenacre, M. Correspondence analysis in medical research. Stat. Methods Med. Res. 1992, 1, 97–117. [Google Scholar] [CrossRef]
- Huang, R.; Xu, C. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1036–1074. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Hernández-Hierro, J.M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Sensory evaluation of bitterness and astringency sub-qualities of wine phenolic compounds: Synergistic effect and modulation by aromas. Food Res. Int. 2014, 62, 1100–1107. [Google Scholar] [CrossRef] [Green Version]
- Fontoin, H.; Saucier, C.; Teissedre, P.-L.; Glories, Y. Effect of pH, ethanol and acidity on astringency and bitterness of grape seed tannin oligomers in model wine solution. Food Qual. Prefer. 2008, 19, 286–291. [Google Scholar] [CrossRef]
- González-Muñoz, B.; Garrido-Vargas, F.; Pavez, C.; Osorio, F.; Chen, J.; Bordeu, E.; A O’Brien, J.; Brossard, N. Wine astringency: More than just tannin–protein interactions. J. Sci. Food Agric. 2021, 102, 1771–1781. [Google Scholar] [CrossRef] [PubMed]


| Phenolic Compounds | Abbreviation | MRM Transition Ions (m/z) | Retention Time (Min) | Quantitative Standards | Calibration Curves (mg/L) | R2 |
|---|---|---|---|---|---|---|
| Procyanin B | PC B | 577–407 | 7.6 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Procyanin C1 | PC C | 865–407 | 10.36 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Epigallocatechin | EGC | 305–125 | 5.2 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Catechin | C | 289–123 | 5.8 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Epicatechin | EC | 289–123 | 9.3 | EC | y = 0.002x − 2.4286 | R² = 0.9929 |
| Gallo-catechin | GC | 305–125 | 2.5 | C | y = 0.0019x − 1.5545 | R² = 0.9934 |
| Caffeic acid | CFA | 179–135 | 7.2 | CFA | y = 0.00008x + 0.8751 | R² = 0.9921 |
| 3-hydroxycinnamic acid | 3-HCA | 163–119 | 10.58 | 3-HCA | y = 0.000006x + 0.066 | R² = 0.9966 |
| Ferulic acid | FA | 193–134 | 12.72 | FA | y = 0.0003x + 0.3138 | R² = 0.9991 |
| Chlorogenic acid | CA | 353–191 | 6.3 | CA | y = 0.00009x + 0.3061 | R² = 0.9969 |
| Gallic acid | GLA | 169–125 | 1.7 | GLA | y = 0.0002x + 0.4347 | R² = 0.9972 |
| Protocatechuic acid | PA | 153–109 | 3.0 | PA | y = 0.0002x − 0.0369 | R² = 0.9996 |
| 4-hydroxybenzoic acid | 4-HBA | 137–93 | 5.02 | 4-HBA | y = 0.0003x − 0.0372 | R² = 0.9909 |
| Gentisic acid | GTA | 153–109 | 5.0 | GTA | y = 0.0002x + 0.045 | R² = 0.9996 |
| Vanillic acid | VA | 167–152 | 6.9 | GTA | y = 0.0002x + 0.045 | R² = 0.9996 |
| Myricetin-glucoside | M-glu | 479–316 | 13.3 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Dihydro-quercetin | DHQ | 303–125 | 13.5 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Dihydro-kampferol | DHK | 287–259 | 17.13 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin-glucoside | Q-glu | 463–300 | 16.3 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin-galactoside | Q-gal | 463–300 | 15.75 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin-glucuronide | Q-gluc | 477–301 | 15.9 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin | Q | 301–151 | 23.9 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Larictrin | L | 331–151 | 24.8 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Myricetin | M | 317–151 | 19.02 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Isorhamnetin-glucoside | I-glu | 477–314 | 19.77 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Kaempferol-3-O-glucoside | K-glu | 447–255 | 18.9 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Syringetin-glucoside | S-glu | 507–344 | 20.1 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Quercetin-rhamnoside | Q-rha | 447–300 | 19.0 | DHQ | y = 0.0002x + 0.2793 | R² = 0.9965 |
| Cyanidin-3-O-glucoside | Cy-glu | 449–287 | 4.5 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-acetylglucoside | Cy-Aglu | 491–287 | 5.69 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-coumaroylglucoside (cis+trans) | Cy-Cglu | 595–287 | 6.43 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-glucoside | Dp-glu | 465–303 | 4.6 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-acetylglucoside | Dp-Aglu | 507–303 | 5.39 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-coumaroylglucoside (cis+trans) | Dp-Cglu | 611–303 | 6.16 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside | Pn-glu | 463–301 | 5.07 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-acetylglucoside | Pn-Aglu | 505–301 | 6.08 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-coumaroylglucoside (cis+trans) | Pn-Cglu | 609–301 | 6.76 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside | Pt-glu | 479–317 | 4.7 | Mv-glu | y = 0.00002 x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-acetylglucoside | Pt-Aglu | 521–317 | 5.76 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-coumaroylglucoside (cis + trans) | Pt-Cglu | 625–317 | 6.47 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside | Mv-glu | 493–331 | 5.15 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-acetylglucoside | Mv-Aglu | 535–331 | 6.08 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-coumaroylglucoside (cis + trans) | Mv-Cglu | 639–331 | 6.74 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-(epi)catechin (A type) | Mv-(e)cat | 783–343 | 10.53 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside-(epi)catechin (A type) | Pn-(e)cat | 753–313 | 10.29 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-glucoside-(epi)catechin (A type) | Dp-(e)cat | 755–315 | 8.08 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside-(epi)catechin (A type) | Pt-(e)cat | 769–329 | 9.1 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-glucoside-(epi)catechin (A type) | Cy-(e)cat | 739–299 | 8.9 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| (Epi)catechin-cyanidin-3-O-glucoside (B type) | (E)cat-Cy | 737–575 | 6.39 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| (Epi)catechin-malvidin-3-O-glucoside (B type) | (E)cat-Mv | 781–619 | 6.97 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| (Epi)catechin-petunidin-3-O-glucoside (B type) | (E)cat-Pt | 767–605 | 6.5 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-glucoside-acetaldehyde | Cy-ace | 473–311 | 8.5 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-glucoside-acetaldehyde | Dp-ace | 489–327 | 7.2 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-acetaldehyde | Vitisin B | 517–355 | 10.7 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside-acetaldehyde | Pt-ace | 503–341 | 10.1 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside-acetaldehyde | Pn-ace | 487–325 | 10.14 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Cyanidin-3-O-glucoside-pyruvic acid | Cy-py | 517–355 | 8.36 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Delphinidin-3-O-glucoside-pyruvic acid | Dp-py | 533–371 | 7.6 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-pyruvic acid | Vitisin A | 561–399 | 10.415 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside-pyruvic acid | Pt-py | 547–385 | 8.7 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside-pyruvic acid | Pn-py | 532–369 | 9.86 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-4-vinyl(epi)catechin | Mv-v-Cat | 805–643 | 20.69 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Peonidin-3-O-glucoside-4-vinyl(epi)catechin | Pn-v-Cat | 775–613 | 20.51 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Petunidin-3-O-glucoside-4-vinyl(epi)catechin | Pt-v-Cat | 791–629 | 19.34 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-4-vinylcatechol | Mv-vcol | 625–463 | 20.9 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-4-vinylphenol | Mv-vpol | 609–447 | 21.21 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Malvidin-3-O-glucoside-4-vinylguaiacol | Mv-vgol | 639–477 | 21.31 | Mv-glu | y = 0.00002x + 0.0327 | R² = 0.9954 |
| Shizuishan | Helan | Xixia | Yongning | Qingtongxia | Hongsipu | |
|---|---|---|---|---|---|---|
| L* | 51.38 ± 2.36 a | 44.06 ± 9.66 a | 48.02 ± 10.08 a | 48.25 ± 8.85 a | 50.03 ± 8.95 a | 47.46 ± 12.14 a |
| a* | 40.84 ± 0.34 b | 47.24 ± 7.52 a | 43.92 ± 7.71 ab | 43.09 ± 6.94 ab | 41.98 ± 5.82 ab | 45.22 ± 7.68 ab |
| b* | 28.52 ± 5.22 a | 24.05 ± 4.91 b | 24.36 ± 5.85 b | 21.82 ± 5.11 bc | 22.16 ± 3.60 bc | 19.78 ± 5.15 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.-Y.; Zhang, X.-K.; Lan, Y.-B.; Duan, C.-Q.; Zhu, B.-Q.; Li, D.-M. Sub-Regional Variation and Characteristics of Cabernet Sauvignon Wines in the Eastern Foothills of the Helan Mountain: A Perspective from Phenolics, Visual Properties and Mouthfeel. Foods 2023, 12, 1081. https://doi.org/10.3390/foods12051081
Zhao B-Y, Zhang X-K, Lan Y-B, Duan C-Q, Zhu B-Q, Li D-M. Sub-Regional Variation and Characteristics of Cabernet Sauvignon Wines in the Eastern Foothills of the Helan Mountain: A Perspective from Phenolics, Visual Properties and Mouthfeel. Foods. 2023; 12(5):1081. https://doi.org/10.3390/foods12051081
Chicago/Turabian StyleZhao, Bing-Yan, Xin-Ke Zhang, Yi-Bin Lan, Chang-Qing Duan, Bao-Qing Zhu, and De-Mei Li. 2023. "Sub-Regional Variation and Characteristics of Cabernet Sauvignon Wines in the Eastern Foothills of the Helan Mountain: A Perspective from Phenolics, Visual Properties and Mouthfeel" Foods 12, no. 5: 1081. https://doi.org/10.3390/foods12051081
APA StyleZhao, B.-Y., Zhang, X.-K., Lan, Y.-B., Duan, C.-Q., Zhu, B.-Q., & Li, D.-M. (2023). Sub-Regional Variation and Characteristics of Cabernet Sauvignon Wines in the Eastern Foothills of the Helan Mountain: A Perspective from Phenolics, Visual Properties and Mouthfeel. Foods, 12(5), 1081. https://doi.org/10.3390/foods12051081








