Gas Chromatography with Flame-Ionization Detection-Based Analysis of Sugar Contents in Korean Agricultural Products for Patients with Galactosemia
Abstract
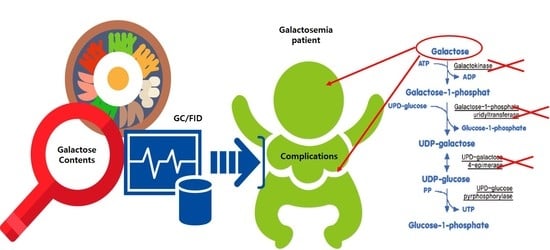
:1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Standards and Reagents
2.3. Sample Preparation
2.4. TMSO Derivatization
2.5. GC/FID Analysis Conditions
3. Results and Discussion
3.1. Resolution of GC for TMSO Sugar Derivatives
3.2. Analysis of Free Sugars in Agro-Food Resources
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohn, Y.B. A Diagnostic Algorithm of Newborn Screening for Galactosemia. J. Korean Soc. Inher. Metab. Dis. 2015, 15, 101–109. [Google Scholar]
- Lee, J.S. Treatment and management of patients with inherited metabolic diseases. Korean J. Pediatr. 2006, 49, 1152–1157. [Google Scholar] [CrossRef]
- Kerckhove, K.V.; Diels, M.; Vanhaesebrouck, S.; Luyten, K.; Pyck, N.; De Meyer, A.; Van Driessche, M.; Robert, M.; Corthouts, K.; Caris, A.; et al. Consensus on the guidelines for the dietary management of classical galactosemia. Clin. Nutr. ESPEN 2015, 10, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Mattews, R.; Pehrsson, P.; Farhat-Sabet, M. Sugar content of selected foods: Individual and total sugars. In Home Economics Research Report No. 48; U.S. Department of Agriculture (USDA): Hyattsville, MD, USA, 1987; p. 1. [Google Scholar]
- Havel, P.J. Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr. Rev. 2005, 63, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Seo, J.S.; Kang, H.Y.; Lee, Y.S.; Choi, Y.; Lee, H.K.; Park, I.T. Rapid Quantitative Analysis for Sugars of Agricultural Products by HPLC. Food Eng. Prog. 2016, 20, 406–410. [Google Scholar] [CrossRef]
- Jeong, D.U.; Im, J.; Kim, C.H.; Kim, Y.K.; Park, Y.J.; Jeong, Y.H.; Om, A.S. Sugar Contents Analysis of Retort Foods. J. Korean Soc. Food. Sci. Nutr. 2015, 44, 1666–1671. [Google Scholar] [CrossRef]
- Harvey, D.J. Derivatization of carbohydrates for analysis by chromatography; electrophoresis and mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2011, 879, 1196–1225. [Google Scholar] [CrossRef]
- Ruiz-Matute, A.I.; Hernandez-Hernandez, O.; Rodriguez-Sanchez, S.; Sanz, M.L.; Martinez-Castro, I. Derivation of carbohydrates for GC and GC-MS analyses. J. Chomatogr. B Analyt. Technol. Biomed. Life. Sci. 2011, 879, 1226–1240. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.C.; Acosta, P.B. Fruits and Vegetables are a Source of Galactose: Implications in Planning the Diets of Patients with Galactosaemia. J. Inher. Metab. Dis. 1991, 14, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Al-Mhanna, N.M.; Huebner, H.; Buchholz, R. Analysis of the Sugar Content in Food Products by Using Gas Chromatography Mass Spectrometry and Enzymatic Methods. Foods 2018, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.O.; Hartnett, C.; Scaman, C.H. Free Galactose Content in Selected Fresh Fruits and Vegetables and Soy Beverages. J. Agric. Food Chem. 2007, 55, 8133–8137. [Google Scholar] [CrossRef] [PubMed]
- Korea Rural Economic Institute (KREI). Food Balance Sheet 2020; KREI: Naju, Republic of Korea, 2021. [Google Scholar]
- Sanz, M.L.; Sanz, J.; Martínez-Castro, I. Gas chromatographic-mass spectrometric method for the qualitative and quantitative determination of disaccharides and trisaccharides in honey. J. Chromatogr. A 2004, 1059, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, H.; Won, M.; Yoo, S. Germination effect of soybean on its contents of isoflavones and oligosaccharides. Food Sci. Biotechnol. 2005, 14, 489–502. [Google Scholar]
- Islam, M.A.; Lee, J.; Yoo, S. Effect of oximation reagents on gas chromatographic separation of eight different kinds of mono- and di-saccharides. Food Chem. 2022, 386, 132797. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Elison, S.L.R.; Wood, R. Harmonized guideline for single-laboratory validation of methods of analysis. Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Jim, V.J.W. Analysis of Free Galactose Contents during Cold Storage of Four Apple Cultivars, in Thermally Treated Apples and Green Beans, and in Clear Apple Juices Produced Using Different Enzymatic Aids. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2002. [Google Scholar]


| Item | Condition | ||||
|---|---|---|---|---|---|
| Instrument | GC-FID (Agilent 7890A) | ||||
| Column | Agilent HP-5 (30 m × 0.25 mm × 0.25 μm) | ||||
| Gradient | Rate | Value | Hold Time | Run Time | |
| Initial | 180 °C | 5 min | 5 min | ||
| Ramp 1 | 1 °C/min | 195 °C | 0 min | 20 min | |
| Ramp 2 | 20 °C/min | 280 °C | 10 min | 34.25 min | |
| Ramp 3 | 50 °C/min | 295 °C | 3 min | 34.75 min | |
| Ramp 4 | 30 °C/min | 180 °C | 3 min | 44.383 min | |
| Flow rate | 0.9 mL/min | ||||
| Inlet temp. | 280 °C | ||||
| Detector | Flame Ionization Detector (FID) | ||||
| Injection vol. | 2 μL | ||||
| Split ratio | 10:1 | ||||
| Free Sugar | Standard Deviation (σ) | Slope of the Calibration Curve (S) | Limit of Detection (LOD) (mg/100 g) | Limit of Quantitation (LOQ (mg/100 g) |
|---|---|---|---|---|
| Fructose | 3.3011 | 5.0964 | 0.2137 | 0.6477 |
| Galactose | 7.0147 | 6.7199 | 0.3445 | 1.0439 |
| Glucose | 6.5372 | 7.2594 | 0.2972 | 0.9005 |
| Sucrose | 2.5364 | 6.8827 | 0.1216 | 3.68520 |
| Lactose | 0.6032 | 5.5205 | 0.0361 | 0.1093 |
| Maltose | 0.8429 | 5.3172 | 0.0523 | 0.1585 |
| Food Group | No. | Sample | Galactose Content (mg/100 g FW) | No. | Sample | Galactose Content (mg/100 g FW) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cereals and their products | 1 | flour (cake flour) | 4.73 | ± | 0.11 | 10 | corn, steamed | 5.04 | ± | 0.40 |
| 2 | flour (bread flour) | 3.99 | ± | 0.28 | 11 | Baek-seolgi | 1.94 | ± | 0.46 | |
| 3 | non-glutinous rice, white rice | nd | 12 | Songpyeon, sesame | 0.61 | ± | 0.87 | |||
| 4 | glutinous rice, steamed | nd | 13 | Jeungpyeon | 3.94 | ± | 0.50 | |||
| 5 | pearl barley, steamed | 5.57 | ± | 0.34 | 14 | wheat Tteok-bokki rice cake (boiled) | 3.49 | ± | 0.55 | |
| 6 | brown rice, steamed | 2.00 | ± | 0.12 | 15 | rice cake soup (boiled) | nd | |||
| 7 | spaghetti, boiled | 3.21 | ± | 0.03 | 16 | Sirutteock | 12.63 | ± | 0.91 | |
| 8 | somyeon, boiled | 6.76 | ± | 5.22 | 17 | Injeolmi | nd | |||
| 9 | buckwheat noodles, boiled | 0.53 | ± | 0.24 | ||||||
| Potatoes and starches | 1 | superior, steamed | 4.63 | ± | 0.03 | 5 | sweet potato starch | nd | ||
| 2 | superior, roasted | 5.51 | ± | 0.22 | 6 | potato starch | nd | |||
| 3 | Japanese sweet potato, steamed | 12.75 | ± | 0.29 | 7 | corn starch | nd | |||
| 4 | Garnet sweet potato, steamed | 36.01 | ± | 0.38 | ||||||
| Beans, nuts, and seeds | 1 | soybean, boiled | 1.26 | ± | 0.04 | 7 | almond, roasted | nd | ||
| 2 | green flesh black bean, boiled | 2.07 | ± | 0.06 | 8 | chestnut, boiled | 16.79 | ± | 0.18 | |
| 3 | red bean, boiled | 0.16 | ± | 0.09 | 9 | peanut, roasted | 3.63 | ± | 0.49 | |
| 4 | red bean paste | 58.34 | ± | 2.58 | 10 | sesame, white sesame, roasted | nd | |||
| 5 | soybean sprout, blanched | 1.13 | ± | 0.01 | 11 | perilla, roasted | nd | |||
| 6 | soybean sprout, steamed | 0.98 | ± | 0.10 | ||||||
| Mushrooms | 1 | oyster mushroom, blanched | 0.19 | ± | 0.27 | 4 | enoki mushroom, blanched | 0.08 | ± | 0.12 |
| 2 | shiitake mushroom, blanched | 3.15 | ± | 0.38 | 5 | matsutake, grilled | 3.54 | ± | 0.06 | |
| 3 | white button mushroom, grilled | 0.57 | ± | 0.12 | ||||||
| Vegetables | 1 | napa cabbage, boiled | 2.18 | ± | 0.26 | 9 | perilla leaf | 10.48 | ± | 0.25 |
| 2 | napa cabbage, ugeoji, boiled | nd | 10 | spinach, blanched | 1.89 | ± | 0.16 | |||
| 3 | garlic, parboiled | 1.27 | ± | 0.18 | 11 | zucchini, blanched | 23.09 | ± | 1.16 | |
| 4 | green onion, blanched | 4.74 | ± | 0.05 | 12 | broccoli, blanched | 5.89 | ± | 0.50 | |
| 5 | carrot, blanched | 11.36 | ± | 0.51 | 13 | paprika, roasted | 10.61 | ± | 0.46 | |
| 6 | tomato | 6.36 | ± | 0.02 | 14 | cucumber | 4.30 | ± | 0.09 | |
| 7 | lettuce | 3.96 | ± | 0.03 | 15 | eggplant, blanched | 5.27 | ± | 0.18 | |
| 8 | iceberg lettuce | 29.08 | ± | 1.74 | 16 | Kabocha squash, steamed | 61.56 | ± | 4.41 | |
| Fruits | 1 | tangerine (citrus unshiu) | nd | 14 | Cherry | 6.90 | ± | 0.66 | ||
| 2 | tangerine (dekopon) | 3.88 | ± | 0.52 | 15 | sweet persimmon | 13.41 | ± | 3.37 | |
| 3 | tangerine (setoka) | 6.30 | ± | 1.40 | 16 | dried persimmon | 132.09 | ± | 3.71 | |
| 4 | apple (Fuji) | 5.42 | ± | 0.11 | 17 | ripe persimmon | 7.06 | ± | 0.48 | |
| 5 | aori apple | nd | 18 | plum | 7.47 | ± | 0.25 | |||
| 6 | banana | 6.47 | ± | 0.45 | 19 | kiwifruit | 21.05 | ± | 0.99 | |
| 7 | peach (nectarine) | 3.99 | ± | 0.51 | 20 | Golden kiwifruit | 11.45 | ± | 0.49 | |
| 8 | peach (white peach) | 3.12 | ± | 0.19 | 21 | grapefruit | nd | |||
| 9 | pear | 8.52 | ± | 0.17 | 22 | watermelon | 4.95 | ± | 0.25 | |
| 10 | strawberry (sulhyang) | 2.46 | ± | 0.15 | 23 | lemon | 6.67 | ± | 1.19 | |
| 11 | avocado | 10.05 | ± | 0.21 | 24 | pineapple | 2.45 | ± | 0.19 | |
| 12 | blueberries | 13.43 | ± | 0.70 | 25 | green grape | nd | |||
| 13 | mango | 0.63 | ± | 0.89 | ||||||
| Meat and eggs | 1 | beef, sirloin, grilled | 5.76 | ± | 0.17 | 10 | whole egg, boiled | 3.64 | ± | 0.24 |
| 2 | beef, beef plate, boiled | 2.53 | ± | 0.09 | 11 | whole egg, fried, hard-boiled | 6.64 | ± | 0.46 | |
| 3 | pork, pork belly, grilled | 6.33 | ± | 0.06 | 12 | whole egg, fried, soft-boiled | 7.21 | ± | 0.80 | |
| 4 | pork, sirloin, boiled | 3.12 | ± | 0.34 | 13 | egg yolk, boiled | 5.27 | ± | 0.14 | |
| 5 | chicken, boiled | 0.27 | ± | 0.38 | 14 | egg yolk, fried, hard-boiled | 8.48 | ± | 0.45 | |
| 6 | chicken, leg, grilled | 0.27 | ± | 0.00 | 15 | egg yolk, fried, soft-boiled | 8.12 | ± | 0.51 | |
| 7 | chicken, breast, grilled | nd | 16 | scrambled egg, roasted | 5.55 | ± | 0.16 | |||
| 8 | Tea-smoked duck (grilled) | 6.73 | ± | 0.53 | 17 | quail egg, boiled | 2.87 | ± | 0.28 | |
| 9 | pork liver, boiled | 5.97 | ± | 0.53 | ||||||
| Aquatic products | 1 | mackerel, grilled | 3.24 | ± | 0.16 | 6 | pollack, dried pollack, dried | 2.04 | ± | 0.16 |
| 2 | hairtail, grilled | 3.46 | ± | 0.79 | 7 | mussel, boiled | 0.66 | ± | 0.02 | |
| 3 | squid, blanched | 0.70 | ± | 0.01 | 8 | clam, boiled | 1.25 | ± | 0.04 | |
| 4 | stir-fried anchovy, stir-fried baby anchovy | nd | 9 | abalone, boiled | 5.71 | ± | 0.72 | |||
| 5 | anchovy broth | nd | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.-N.; Kwon, R.H.; Kim, Y.; Yoo, S.-H.; Yoo, S.M.; Wee, C.-D. Gas Chromatography with Flame-Ionization Detection-Based Analysis of Sugar Contents in Korean Agricultural Products for Patients with Galactosemia. Foods 2023, 12, 1104. https://doi.org/10.3390/foods12051104
Jeong H-N, Kwon RH, Kim Y, Yoo S-H, Yoo SM, Wee C-D. Gas Chromatography with Flame-Ionization Detection-Based Analysis of Sugar Contents in Korean Agricultural Products for Patients with Galactosemia. Foods. 2023; 12(5):1104. https://doi.org/10.3390/foods12051104
Chicago/Turabian StyleJeong, Ha-Neul, Ryeong Ha Kwon, Yuri Kim, Sang-Ho Yoo, Seon Mi Yoo, and Chi-Do Wee. 2023. "Gas Chromatography with Flame-Ionization Detection-Based Analysis of Sugar Contents in Korean Agricultural Products for Patients with Galactosemia" Foods 12, no. 5: 1104. https://doi.org/10.3390/foods12051104
APA StyleJeong, H.-N., Kwon, R. H., Kim, Y., Yoo, S.-H., Yoo, S. M., & Wee, C.-D. (2023). Gas Chromatography with Flame-Ionization Detection-Based Analysis of Sugar Contents in Korean Agricultural Products for Patients with Galactosemia. Foods, 12(5), 1104. https://doi.org/10.3390/foods12051104