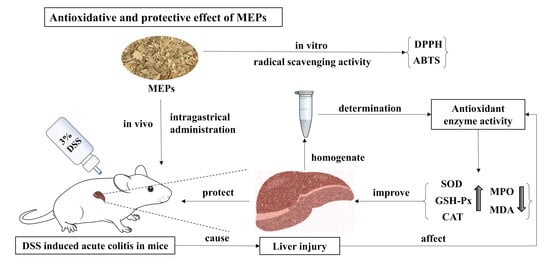
Antioxidative and Protective Effect of Morchella esculenta against Dextran Sulfate Sodium-Induced Alterations in Liver
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the MEPs
2.3. Molecular Weight Determination and Monosaccharide Composition Analysis
2.4. In Vitro Radical Scavenging Activities
2.5. Animal Experiment and Sample Collection
2.6. Histological Evaluation
2.7. Determination of the SOD, GSH-Px and CAT Activities
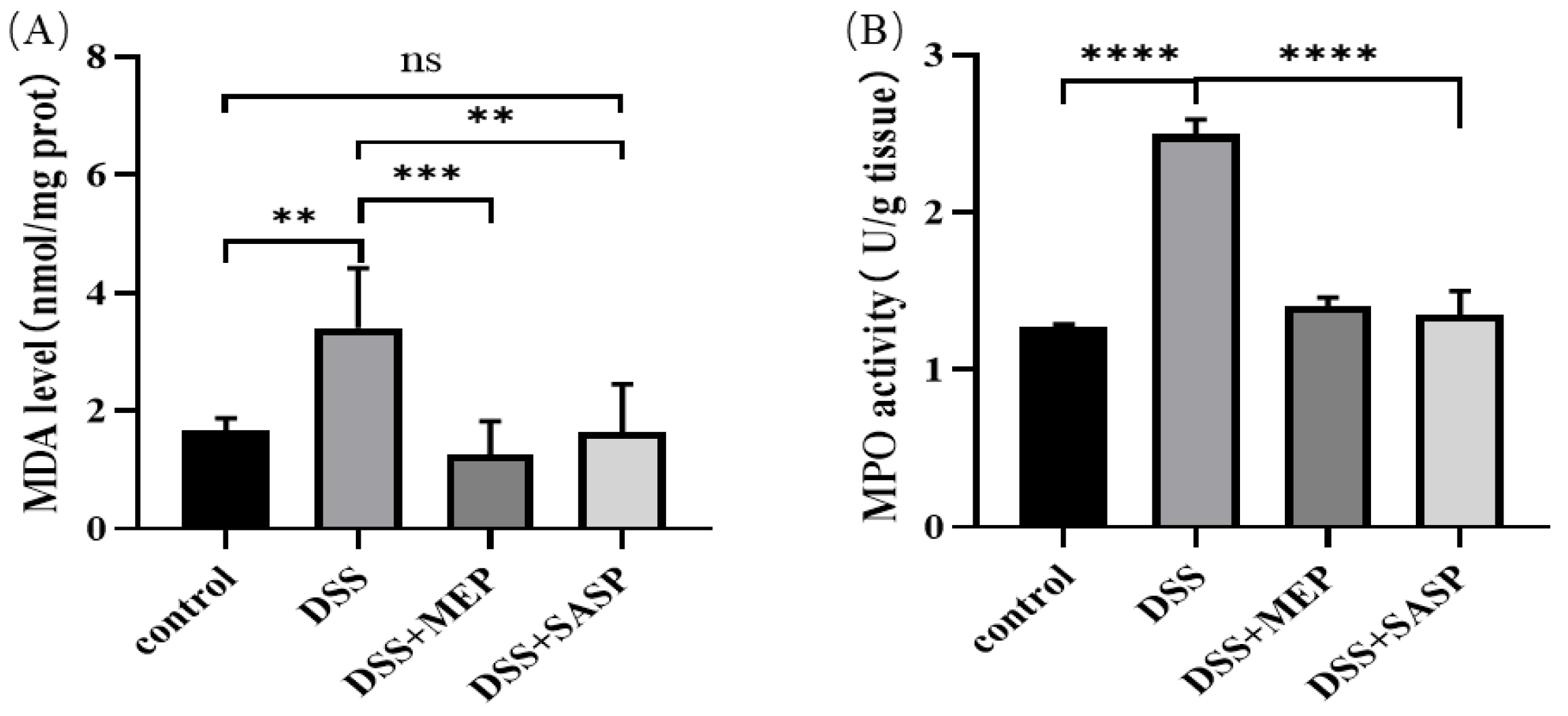
2.8. MPO Activity and MDA Content
2.9. Statistical Analysis
3. Results and Discussion
3.1. Molecular Weight and Monosaccharide Composition of MEPs
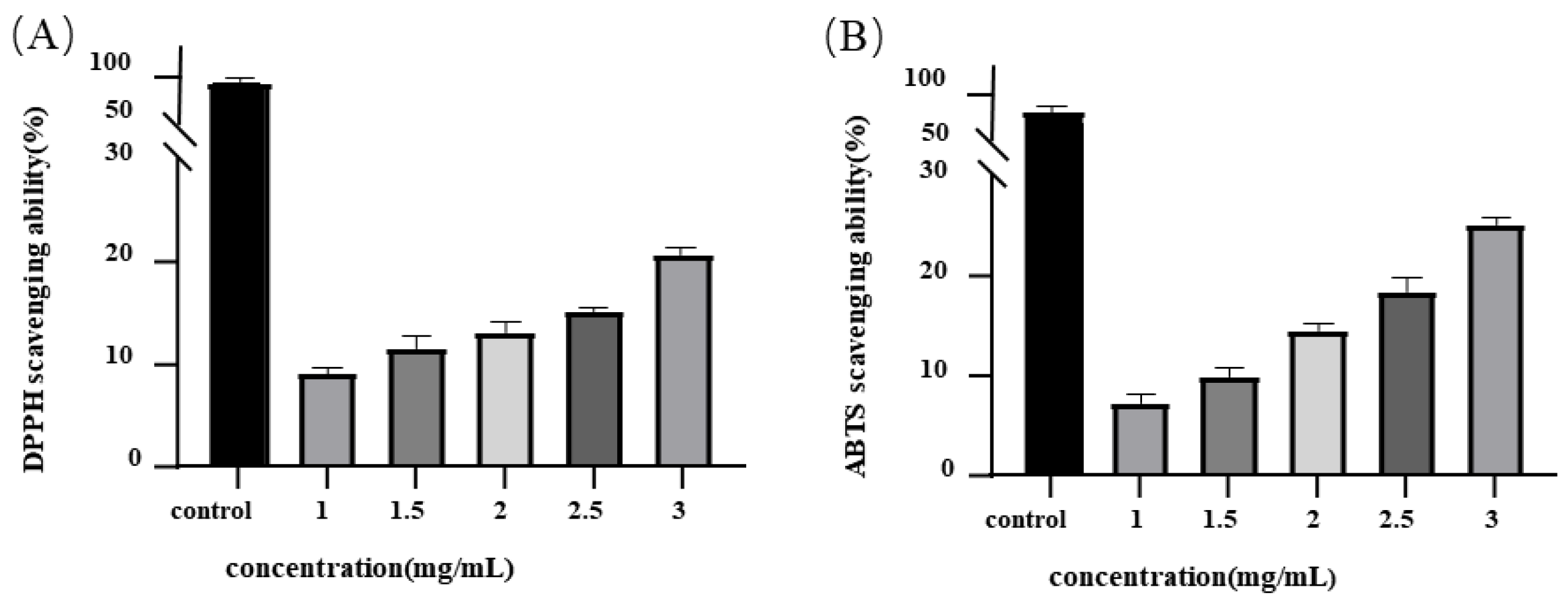
3.2. Effects of MEP Antioxidant Properties In Vitro
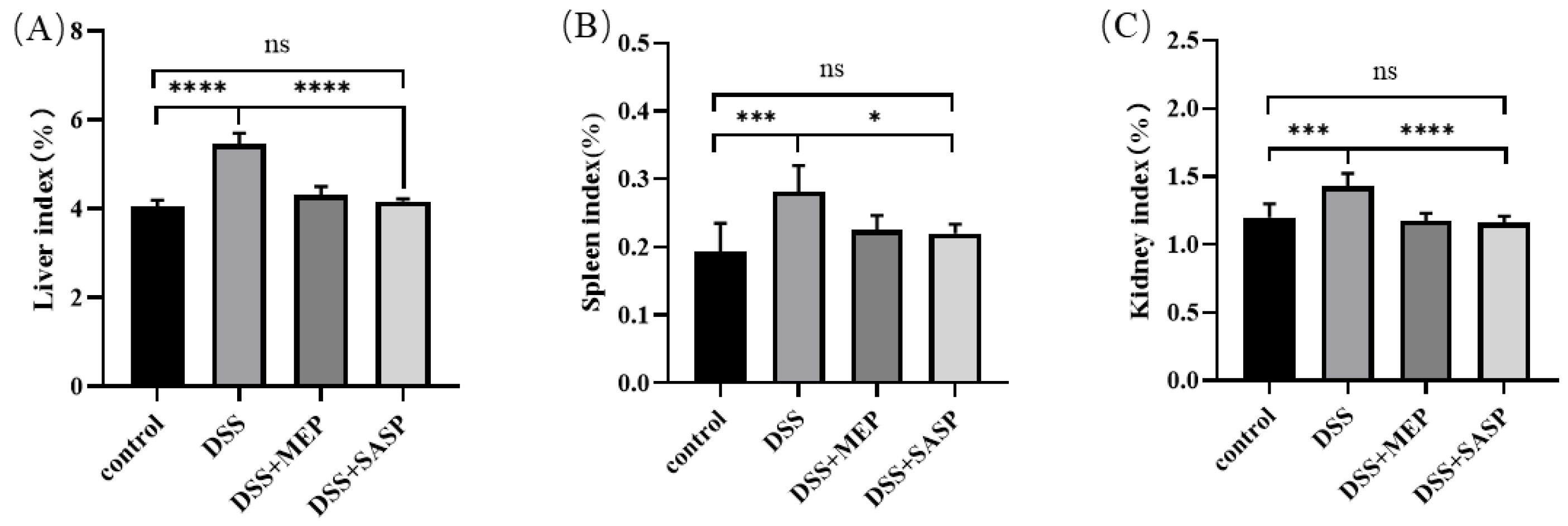
3.3. Effects of MEPs on Organ Indices in Mice
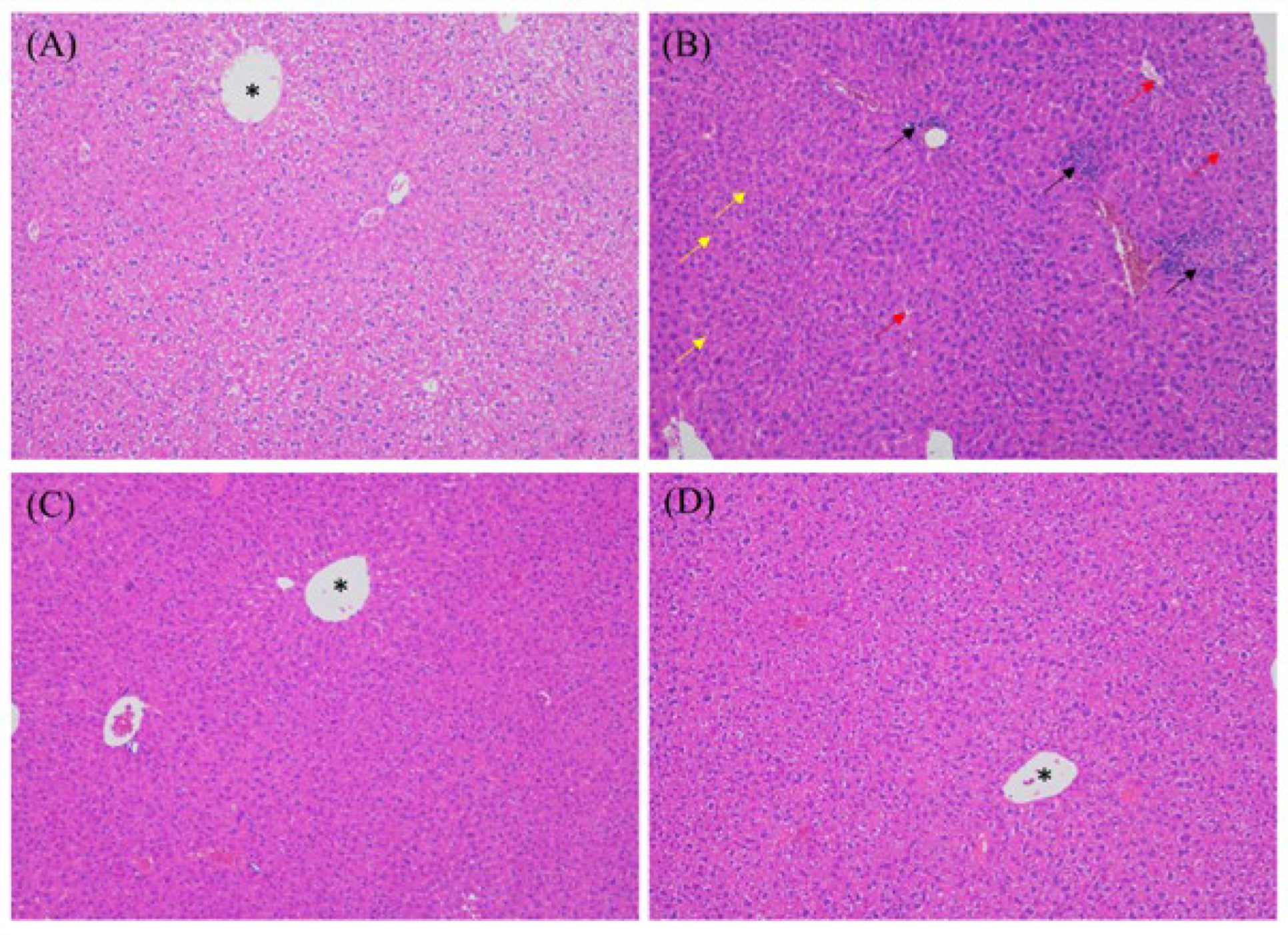
3.4. MEP Treatment Attenuated DSS-Induced Hepatitis
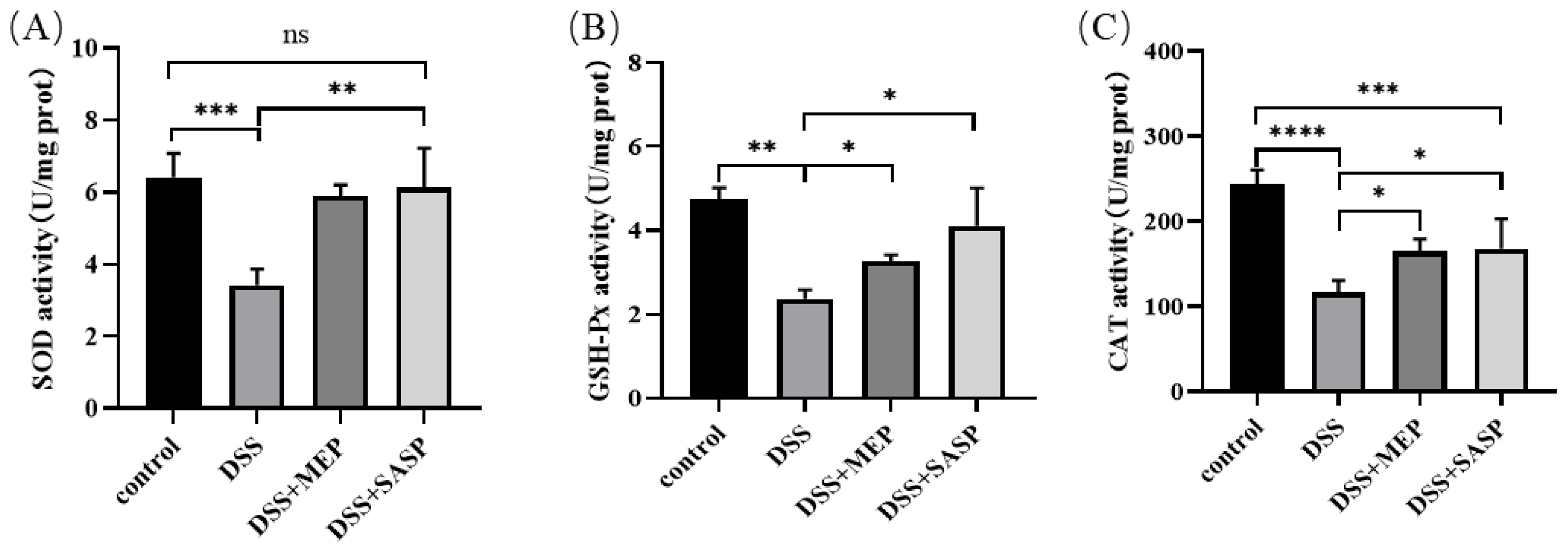
3.5. Anti-Oxidative Effects Contribute to MEP-Mediated Hepatoprotection
3.6. MEP Suppressed DSS-Induced Oxidative Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef]
- Fridovich, I. Fundamental aspects of reactive oxygen species, or what’s the matter with oxygen? Ann. N. Y. Acad. Sci. 1999, 893, 13–18. [Google Scholar] [CrossRef]
- Pereira, C.; Grácio, D.; Teixeira, J.P.; Magro, F. Oxidative stress and DNA damage: Implications in inflammatory bowel disease. Inflamm. Bowel Dis. 2015, 21, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract. Res. Clin. Gastroenterol. GA. 2010, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Du, X.H.; Zhao, Q.; Yang, Z.L. A review on research advances, issues, and perspectives of morels. Mycology 2015, 6, 78–85. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. Mycochemical profile and health-promoting effects of morel mushroom Morchella esculenta (L.)—A review. Food Res. Int. 2022, 159, 111571. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Chiang, L.H.; Wu, S.Y.; Shih, Y.C.; Chen, C.C. Nutrition profile and animal-tested safety of Morchella esculenta mycelia produced by fermentation in bioreactors. Foods 2022, 11, 1385. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: Current state, issues and perspectives. Crit. Rev. Biotechnol. 2018, 38, 259–271. [Google Scholar] [CrossRef]
- Badshah, S.L.; Riaz, A.; Muhammad, A.; Tel Çayan, G.; Çayan, F.; Emin Duru, M.; Ahmad, N.; Emwas, A.-H.; Jaremko, M. Isolation, characterization, and medicinal potential of polysaccharides of Morchella esculenta. Molecules 2021, 26, 1459. [Google Scholar] [CrossRef]
- Li, W.; Cai, Z.N.; Mehmood, S.; Liang, L.L.; Liu, Y.; Zhang, H.Y.; Chen, Y.; Lu, Y.M. Anti-inflammatory effects of Morchella esculenta polysaccharide and its derivatives in fine particulate matter-treated NR8383 cells. Int. J. Biol. Macromol. 2019, 129, 904–915. [Google Scholar] [CrossRef]
- Zhang, N.N.; Ma, H.; Zhang, Z.F.; Zhang, W.N.; Chen, L.; Pan, W.J.; Wu, Q.X.; Lu, Y.M.; Chen, Y. Characterization and immunomodulatory effect of an alkali-extracted galactomannan from Morchella esculenta. Carbohydr. Polym. 2022, 278, 118960. [Google Scholar] [CrossRef]
- Wang, D.; Yin, Z.; Ma, L.; Han, L.; Chen, Y.; Pan, W.; Gong, K.; Gao, Y.; Yang, X.; Chen, Y.; et al. Polysaccharide MCP extracted from Morchella esculenta reduces atherosclerosis in LDLR-deficient mice. Food Funct. 2021, 12, 4842–4854. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Nan, J.; Park, H.J.; Chen, Y.; Yang, L. The chemical structure and immunomodulatory activity of an exopolysaccharide produced by Morchella esculenta under submerged fermentation. Food Funct. 2021, 12, 9327–9338. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Qiu, H.M.; Cheong, K.L.; Zhong, S. Advances in anti-cancer effects and underlying mechanisms of marine algae polysaccharides. Int. J. Biol. Macromol. 2022, 221, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.L.; Yu, B.; Chen, J.; Zhong, S. A comprehensive review of the cardioprotective effect of marine algae polysaccharide on the gut microbiota. Foods 2022, 11, 3550. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Zhang, Y.; Jin, X.; Zhu, Y.; Li, L.; Huang, X.; Wang, D.; Lin, Z. Structure and hepatoprotective activity of Usp10/NF-kappaB/Nrf2 pathway-related Morchella esculenta polysaccharide. Carbohydr. Polym. 2023, 303, 120453. [Google Scholar] [CrossRef]
- Dong, Y.; Qi, Y.; Liu, M.; Song, X.; Zhang, C.; Jiao, X.; Wang, W.; Zhang, J.; Jia, L. Antioxidant, anti-hyperlipidemia and hepatic protection of enzyme-assisted Morehella esculenta polysaccharide. Int. J. Biol. Macromol. 2018, 120, 1490–1499. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; An, L.Y.; Liu, W.; Hu, Y.C.; Wang, S.P.; Zou, L. In vitro fecal fermentation properties of polysaccharides from Tremella fuciformis and related modulation effects on gut microbiota. Food Res. Int. 2022, 156, 111185. [Google Scholar] [CrossRef]
- Li, R.; Zhou, Q.L.; Chen, S.T.; Tai, M.R.; Cai, H.Y.; Ding, R.; Liu, X.F.; Chen, J.P.; Luo, L.X.; Zhong, S.Y. Chemical characterization and immunomodulatory activity of fucoidan from Sargassum hemiphyllum. Mar. Drugs 2022, 21, 18. [Google Scholar] [CrossRef]
- Hui, Y.; Jun-Li, H.; Chuang, W. Anti-oxidation and anti-aging activity of polysaccharide from Malus micromalus Makino fruit wine. Int. J. Biol. Macromol. 2019, 121, 1203–1212. [Google Scholar] [CrossRef]
- Cheong, K.L.; Li, J.K.; Zhong, S. Preparation and structure characterization of high-value Laminaria digitata oligosaccharides. Front. Nutr. 2022, 9, 945804. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.M.; Xu, S.Y.; Liu, Y.; Cheong, K.L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Veeraperumal, S.; Zhong, S.; Cheong, K.L. Fucoidan-derived functional oligosaccharides: Recent developments, preparation, and potential applications. Foods 2023, 12, 878. [Google Scholar] [CrossRef]
- Zheng, L.X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.L. Preparation methods, biological activities, and potential applications of marine algae oligosaccharides: A review. Food Sci. Hum. Well. 2023, 12, 359–370. [Google Scholar] [CrossRef]
- Wagay, J.A.; Nayik, G.A.; Wani, S.A.; Mir, R.A.; Ahmad, M.A.; Rahman, Q.I.; Vyas, D. Phenolic profiling and antioxidant capacity of Morchella esculenta L. by chemical and electrochemical methods at multiwall carbon nanotube paste electrode. J. Food Meas. Charact. 2019, 13, 1805–1819. [Google Scholar] [CrossRef]
- Cai, Z.N.; Li, W.; Mehmood, S.; Pan, W.J.; Wang, Y.; Meng, F.J.; Wang, X.F.; Lu, Y.M.; Chen, Y. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohydr. Polym. 2018, 195, 29–38. [Google Scholar] [CrossRef]
- Li, S.; Sang, Y.; Zhu, D.; Yang, Y.; Lei, Z.; Zhang, Z. Optimization of fermentation conditions for crude polysaccharides by Morchella esculenta using soybean curd residue. Ind. Crops Prod. 2013, 50, 666–672. [Google Scholar] [CrossRef]
- Yuan, Y.; Xu, X.; Jing, C.; Zou, P.; Zhang, C.; Li, Y. Microwave assisted hydrothermal extraction of polysaccharides from Ulva prolifera: Functional properties and bioactivities. Carbohydr. Polym. 2018, 181, 902–910. [Google Scholar] [CrossRef]
- Wang, M.; Cheong, K.L. Preparation, structural characterisation, and bioactivities of fructans: A review. Molecules 2023, 28, 1613. [Google Scholar] [CrossRef]
- Restellini, S.; Chazouillères, O.; Frossard, J.L. Hepatic manifestations of inflammatory bowel diseases. Liver Int. 2017, 37, 475–489. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Zhao, L.; Ye, G.; Shi, F.; Li, Y.; Zou, Y.; Song, X.; Zhao, X.; Yin, Z.; et al. Sanhuang xiexin decoction ameliorates secondary liver injury in DSS-induced colitis involve regulating inflammation and bile acid metabolism. J. Ethnopharmacol. 2022, 299, 115682. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Zhang, Y.; Wang, Z.; Ding, Q.; Song, J.; Wang, D. Hepatoprotective effects of Morchella esculenta against alcohol-induced acute liver injury in the C57BL/6 mouse related to Nrf-2 and NF-κB signaling. Oxid. Med. Cell. Longev. 2019, 2019, 6029876. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, S.; Hu, Y.; Yang, Y.; Yuan, J.; Wu, Y.; Li, S.; Lin, J.; He, L.; Hou, S.; et al. Protective roles and mechanisms of Dendrobium officinal polysaccharides on secondary liver injury in acute colitis. Int. J. Biol. Macromol. 2018, 107, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. BBA—Gen. Subjects 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Dziąbowska-Grabias, K.; Sztanke, M.; Zając, P.; Celejewski, M.; Kurek, K.; Szkutnicki, S.; Korga, P.; Bulikowski, W.; Sztanke, K. Antioxidant therapy in inflammatory bowel diseases. Antioxidants 2021, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Erzurum, S.C.; Hay, J.G.; Lemarchand, P.; Crystal, R.G. Vulnerability of the human airway epithelium to hyperoxia: Constitutive expression of the catalase gene in human bronchial epithelial cells despite oxidant stress. J. Clin. Investig. 1994, 93, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef]

| Indices | RT (min) | Relative Molecular Weight (kDa) | Relative Percentage of Peak Area (%) | ||
|---|---|---|---|---|---|
| Mw | Mn | Mp | |||
| Molecular weight | (1) 33.298 | 504.275 | 289.188 | 354.204 | 15.739 |
| (2) 38.700 | 48.229 | 32.837 | 39.183 | 19.984 | |
| (3) 40.759 | 19.714 | 14.330 | 16.930 | 38.874 | |
| (4) 41.697 | 13.115 | 9.822 | 11.551 | 18.435 | |
| (5) 43.322 | 6.473 | 5.105 | 5.957 | 6.967 | |
| Monosaccharide | Molar ratio | ||||
| Monosaccharide composition | GlcN | 0.009 | |||
| Gal | 0.053 | ||||
| Glc | 0.900 | ||||
| Man | 0.037 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Wang, M.; Veeraperumal, S.; Teng, B.; Li, R.; Qian, Z.; Chen, J.; Zhong, S.; Cheong, K.-L. Antioxidative and Protective Effect of Morchella esculenta against Dextran Sulfate Sodium-Induced Alterations in Liver. Foods 2023, 12, 1115. https://doi.org/10.3390/foods12051115
Chen S, Wang M, Veeraperumal S, Teng B, Li R, Qian Z, Chen J, Zhong S, Cheong K-L. Antioxidative and Protective Effect of Morchella esculenta against Dextran Sulfate Sodium-Induced Alterations in Liver. Foods. 2023; 12(5):1115. https://doi.org/10.3390/foods12051115
Chicago/Turabian StyleChen, Shutong, Min Wang, Suresh Veeraperumal, Bo Teng, Rui Li, Zhengming Qian, Jianping Chen, Saiyi Zhong, and Kit-Leong Cheong. 2023. "Antioxidative and Protective Effect of Morchella esculenta against Dextran Sulfate Sodium-Induced Alterations in Liver" Foods 12, no. 5: 1115. https://doi.org/10.3390/foods12051115
APA StyleChen, S., Wang, M., Veeraperumal, S., Teng, B., Li, R., Qian, Z., Chen, J., Zhong, S., & Cheong, K.-L. (2023). Antioxidative and Protective Effect of Morchella esculenta against Dextran Sulfate Sodium-Induced Alterations in Liver. Foods, 12(5), 1115. https://doi.org/10.3390/foods12051115