Impact of Cavitation Jet on the Structural, Emulsifying Features and Interfacial Features of Soluble Soybean Protein Oxidized Aggregates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation of Soluble Soybean Protein Oxidized Accumulates
2.3. Formulation of Samples for Cavitation Jet Treatment
2.4. Measuring the Particle Size Dispersal
2.5. Measurement of the Molecular Weight Circulation
2.6. Measurement of the Fourier Transform Infrared Spectroscopy (FTIR) Spectroscopy
2.7. Measuring of the Fluorescence Emission Spectra
2.8. Measurement of the Sulfhydryl Content
2.9. Measuring of the Transmission Electron Microscopy (TEM)
2.10. Measuring of the Emulsifying Activity Index (EAI) and Emulsion Solidity Index (ESI)
2.11. Measurement of the Confocal Laser Scanning Microscope (CLSM)
2.12. Measuring of the Quantity of Adsorbed Proteins at Interface (AP%)
2.13. Measurement of the Interfacial Tension
2.14. Measurement of the Viscoelastic Properties
2.15. Measurement of the Apparent Viscosity
2.16. Statistical Analysis
3. Results
3.1. Particle Size Distribution and Molecular Weight Circulation
3.2. FTIR Spectroscopy
3.3. Fluorescence Emission Spectra
3.4. Sulfhydryl Content
3.5. Transmission Electron Microscopy (TEM)
3.6. Emulsion Capacity and Stability
3.7. Confocal Laser Scanning Microscope (CLSM)
3.8. Quantity of Adsorbed Proteins at Interface (AP%)
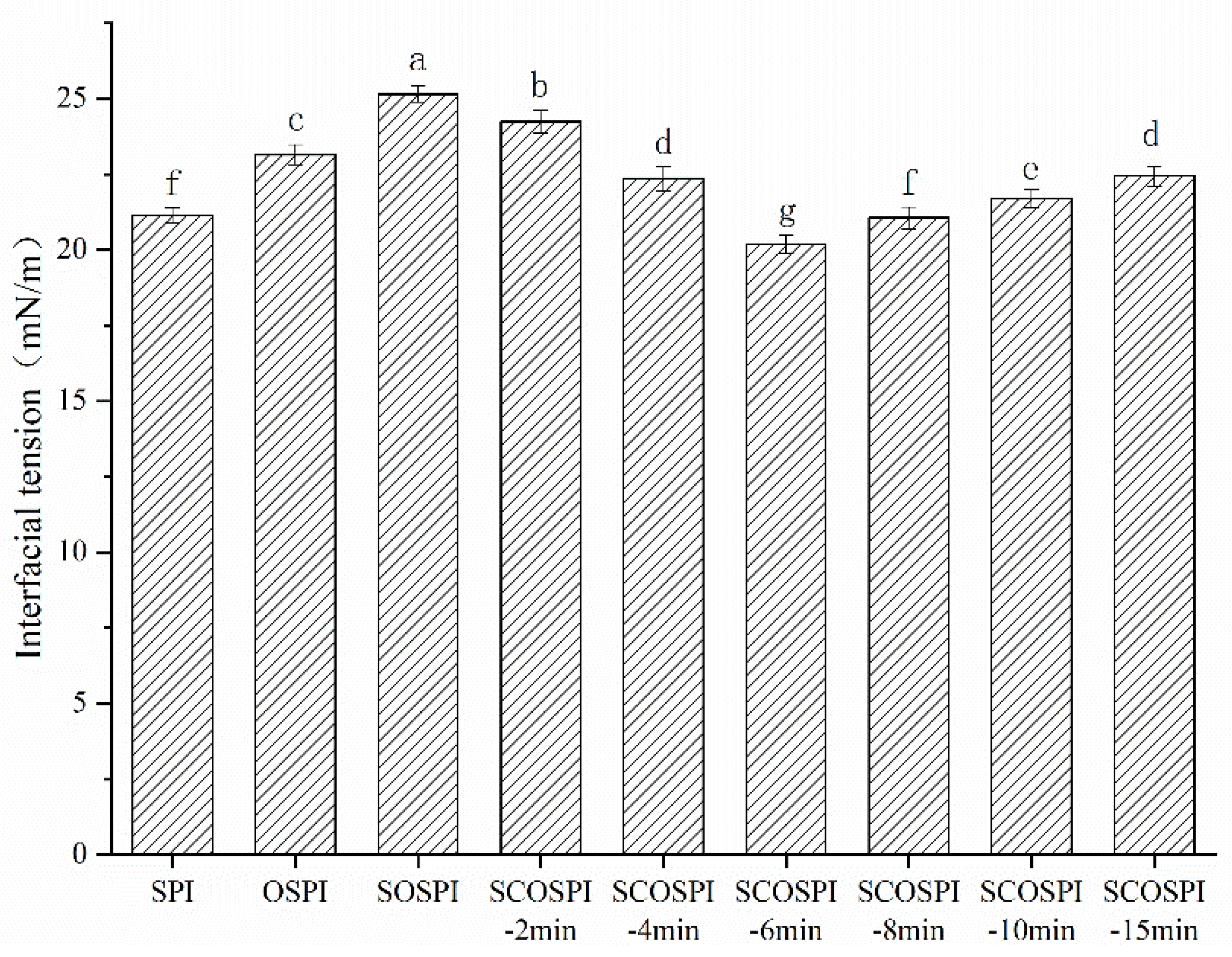
3.9. Interfacial Tension
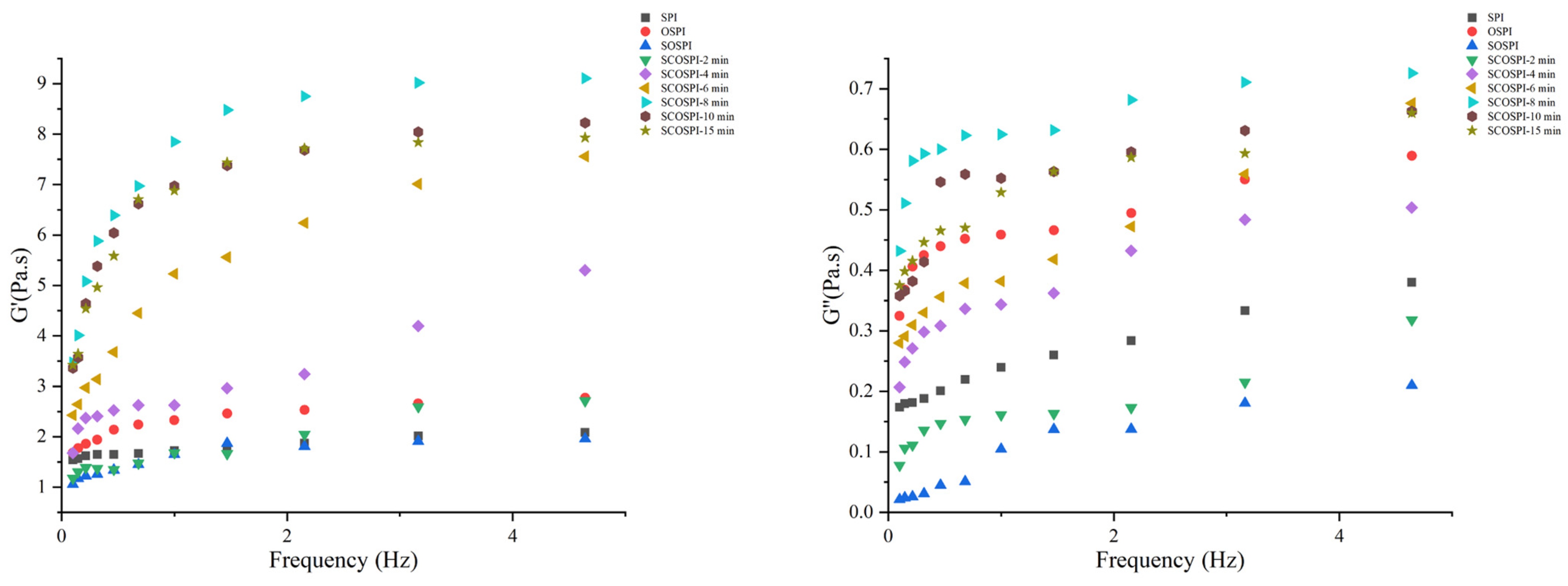
3.10. Viscoelastic Properties
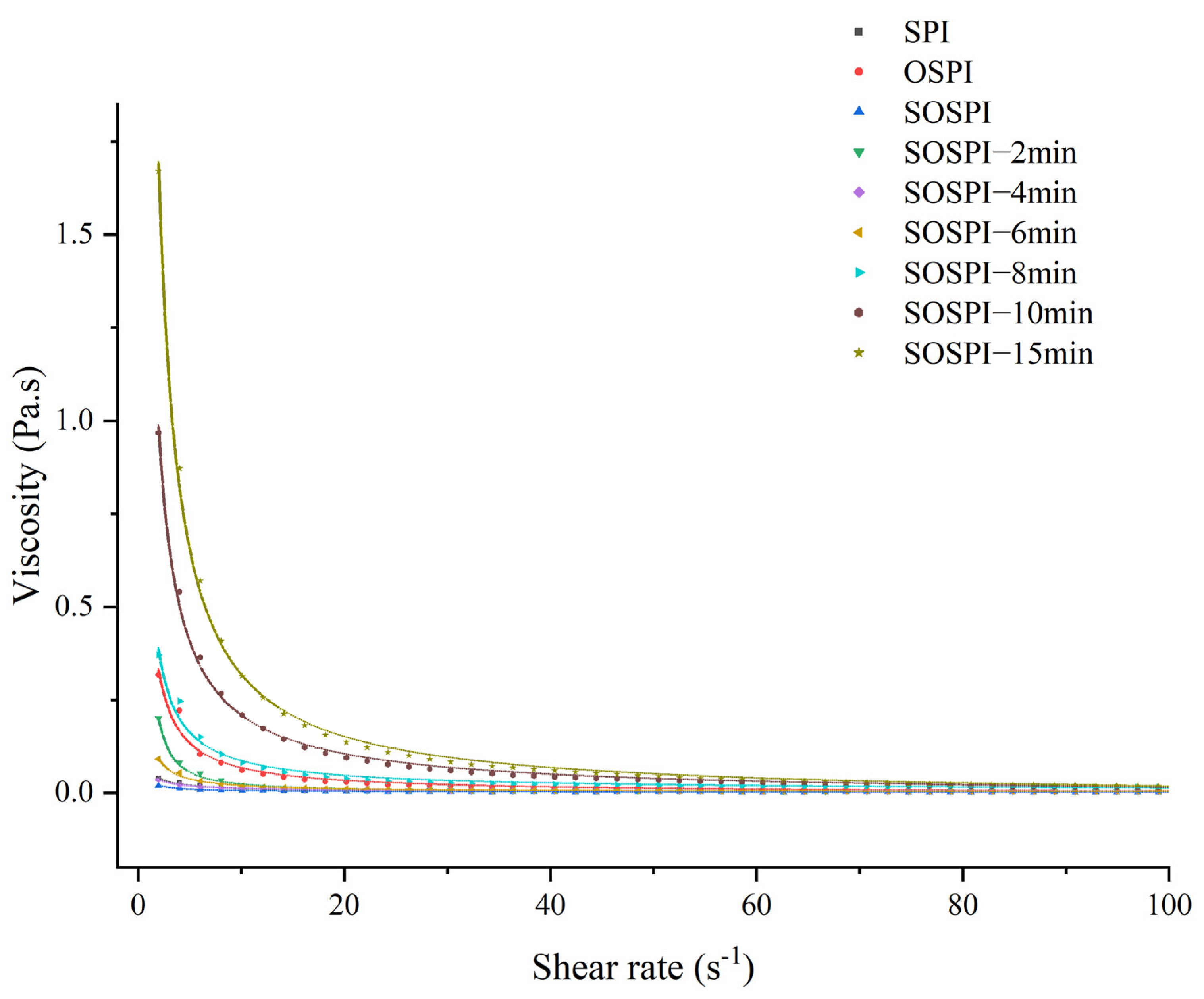
3.11. Apparent Viscosity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boerma, H.R. Managing Inputs for Peak Production; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2004; pp. 15–20. [Google Scholar]
- Caponio, G.R.; Wang, H.; Ciaula, A.D.; Angelis, M.; Portincasa, P. Molecular Sciences Regulation of Cholesterol Metabolism by Bioactive Components of Soy Proteins: Novel Translational Evidence. Int. J. Mol. Sci. 2020, 22, 227. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Han, M.; Qiao, S.; He, P.; Li, D.; Li, N.; Ma, X. Soybean Antigen Proteins and their Intestinal Sensitization Activities. Curr. Protein Pept. Sci. 2015, 16, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Botta, G.F.; Antille, D.L.; Nardon, G.F.; Rivero, D.; Bienvenido, F.; Contessotto, E.E.; Ezquerra-Canalejo, A.; Ressia, J.M. Zero and controlled traffic improved soil physical conditions and soybean yield under no-tillage. Soil Tillage Res. 2022, 215, 105235. [Google Scholar] [CrossRef]
- Yuan, C.A.; Yuan, C.B.; Xg, A.; Yc, A. Effect of 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) induced oxidation on the physicochemical properties, in vitro digestibility, and nutritional value of egg white protein. LWT Food Sci. Technol. 2021, 143, 111103. [Google Scholar]
- Hinderink, E.; Schrder, A.; Sagis, L.; Schron, K.; Berton-Carabin, C.C. Physical and oxidative stability of food emulsions prepared with pea protein fractions. LWT Food Sci. Technol. 2021, 146, 111424. [Google Scholar] [CrossRef]
- Shi, R.; Li, T.; Li, M.; Munkh-Amgalan, G.; Jiang, Z. Consequences of dynamic high-pressure homogenization pretreatment on the physicochemical and functional characteristics of citric acid-treated whey protein isolate. LWT Food Sci. Technol. 2021, 136, 110303. [Google Scholar] [CrossRef]
- Keerati-U-Rai, M.; Corredig, M. Effect of Dynamic High Pressure Homogenization on the Aggregation State of Soy Protein. J. Agric. Food Chem. 2009, 57, 3556–3562. [Google Scholar] [CrossRef]
- Cao, H.; Sun, R.; Shi, J.; Li, M.; Guan, X.; Liu, J.; Huang, K.; Zhang, Y. Effect of ultrasonic on the structure and quality characteristics of quinoa protein oxidation aggregates. Ultrason. Sonochemistry 2021, 77, 105685. [Google Scholar] [CrossRef]
- Zhang, Y.; Di, R.; Zhang, H.; Zhang, W.; Yang, C. Effective recovery of casein from its aqueous solution by ultrasonic treatment assisted foam fractionation: Inhibiting molecular aggregation. J. Food Eng. 2020, 284, 110042. [Google Scholar] [CrossRef]
- Chi, H.; Li, G.; Liao, H.; Tian, S.; Song, X. Effects of parameters of self-propelled multi-orifice nozzle on drilling capability of water jet drilling technology. Int. J. Rock Mech. Min. Sci. 2016, 86, 23–28. [Google Scholar] [CrossRef]
- Mitroglou, N.; Stamboliyski, V.; Karathanassis, I.K.; Nikas, K.S.; Gavaises, M. Cloud cavitation vortex shedding inside an injector nozzle. Exp. Therm. Fluid Sci. 2017, 84, 179–189. [Google Scholar] [CrossRef]
- He, M.; Wu, C.; Li, L.; Zheng, L.; Teng, F. Effects of Cavitation Jet Treatment on the Structure and Emulsification Properties of Oxidized Soy Protein Isolate. Foods 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, X.; Hua, Y. Structural modification of soy protein by the lipid peroxidation product acrolein. LWT Food Sci. Technol. 2010, 43, 133–140. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J.; Xu, X.; Qin, L.; Wu, C.; Du, M. Ultrasound treatment improved the physicochemical characteristics of cod protein and enhanced the stability of oil-in-water emulsion. Food Res. Int. 2019, 121, 247–256. [Google Scholar] [CrossRef]
- Tang, C.H.; Ma, C.Y. Effect of high pressure treatment on aggregation and structural properties of soy protein isolate. LWT Food Sci. Technol. 2009, 42, 606–611. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Wu, D.; Wu, C.; Wang, Z.; Fan, F.; Chen, H.; Ma, W.; Du, M. Effects of high pressure homogenize treatment on the physicochemical and emulsifying properties of proteins from scallop (Chlamys farreri). Food Hydrocoll. 2019, 94, 537–545. [Google Scholar] [CrossRef]
- Keppler, J.K.; Heyn, T.R.; Meissner, P.M.; Schrader, K.; Schwarz, K. Protein oxidation during temperature-induced amyloid aggregation of beta-lactoglobulin. Food Chem. 2019, 289, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Pearc, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Liang, H.N.; Tang, C.H. pH-dependent emulsifying properties of pea [Pisum sativum (L.)] proteins. Food Hydrocoll. 2013, 33, 309–319. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation properties of myofibrillar/pea protein mixtures induced by transglutaminase crosslinking. Food Hydrocoll. 2012, 27, 394–400. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Shao, G.; Qu, D.; Zhao, H.; Yang, L.; Zhu, L.; He, Y.; Liu, H.; Zhu, D. Soy protein isolated-soy hull polysaccharides stabilized O/W emulsion: Effect of polysaccharides concentration on the storage stability and interfacial rheological properties. Food Hydrocoll. 2020, 101, 105490. [Google Scholar] [CrossRef]
- Ye, L.; Liao, Y.; Zhao, M.; Sun, W. Effect of Protein Oxidation on the Conformational Properties of Peanut Protein Isolate. J. Chem. 2013, 2013, 423254. [Google Scholar] [CrossRef]
- Shen, L.; Tang, C.H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012, 48, 108–118. [Google Scholar] [CrossRef]
- Oliete, B.; Potin, F.; Cases, E.; Saurel, R. Modulation of the emulsifying properties of pea globulin soluble aggregates by dynamic high-pressure fluidization. Innov. Food Sci. Emerg. Technol. 2018, 47, 292–300. [Google Scholar] [CrossRef]
- Guo, Y.; Li, B.; Cheng, T.; Hu, Z.; Liu, S.; Liu, J.; Sun, F.; Guo, Z.; Wang, Z. Effect of cavitation jet on the structural, emulsifying properties and rheological properties of soybean protein-oxidised aggregates. Int. J. Food Sci. Technol. 2022, 58, 343–354. [Google Scholar] [CrossRef]
- Sarroukh, R.; Goormaghtigh, E.; Ruysschaert, J.M.; Raussens, V. ATR-FTIR: A “rejuvenated” tool to investigate amyloid proteins. BBA Biomembr. 2013, 1828, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhang, H.; Chen, J.; Hu, H.; Rasulov, F.; Bi, D.; Huang, X.; Pan, S. Formation of amyloid fibrils from soy protein hydrolysate: Effects of selective proteolysis on β-conglycinin. Food Res. Int. 2017, 100, 268–276. [Google Scholar] [CrossRef]
- Fan, S.; Guo, J.; Wang, X.; Liu, X.; Chen, Z.; Zhou, P. Effects of lipoxygenase/linoleic acid on the structural characteristics and aggregation behavior of pork myofibrillar protein under low salt concentration. LWT Food Sci. Technol. 2022, 161, 113359. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Kong, B.; Shi, S.; Xia, X. Decreased gelling properties of protein in mirror carp (Cyprinus carpio) are due to protein aggregation and structure deterioration when subjected to freeze-thaw cycles. Food Hydrocoll. 2019, 97, 105223. [Google Scholar] [CrossRef]
- Xu, Y.; Silva, W.; Qian, Y.; Gray, S.M. An aromatic amino acid and associated helix in the C-terminus of the potato leafroll virus minor capsid protein regulate systemic infection and symptom expression. PLoS Pathog. 2018, 14, e1007451. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.G. Introduction to Functional Properties of Proteins. In Structure–Function Properties of Food Proteins; Academic Press: Cambridge, MA, USA, 1994; pp. 107–109. [Google Scholar]
- Boora, K.A.; Amarjeet, K.; Kumar, K.S.; Nitin, M. Characterization of heat-stable whey protein: Impact of ultrasound on rheological, thermal, structural and morphological properties. Ultrason. Sonochemistry 2018, 49, 333–342. [Google Scholar]
- Viseu, M.I.; Carvalho, T.I.; Costa, S. Conformational Transitions in β-Lactoglobulin Induced by Cationic Amphiphiles: Equilibrium Studies. Biophys. J. 2004, 86, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, R.; Zhang, W.; Hu, Z.; Zhao, W. Structural characterization and physicochemical properties of protein extracted from soybean meal assisted by steam flash-explosion with dilute acid soaking. Food Chem. 2017, 219, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Siddique, M.A.B.; Maresca, P.; Pataro, G.; Ferrari, G. Influence of pulsed light treatment on the aggregation of whey protein isolate. Food Res. Int. 2017, 99, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Evangelho, J.A.; Vanier, N.L.; Pinto, V.Z.; Berrios, J.J.; Dias, A.R.; Zavareze, E.R. Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem. 2017, 214, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, X.; Wu, W. Effects of protein oxidation induced by rice bran rancidity on the structure and functionality of rice bran glutelin. LWT Food Sci. Technol. 2021, 149, 111874. [Google Scholar] [CrossRef]
- Platt, A.A.; Gieseg, S.P. Inhibition of protein hydroperoxide formation by protein thiols. Redox Rep. 2003, 8, 81–86. [Google Scholar] [CrossRef]
- Morzel, M.; Gatellier, P.; Sayd, T.; Renerre, M.; Laville, E. Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci. 2006, 73, 536–543. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, Z.; Guo, Y.; Li, B.; Yu, W.; Zhou, L.; Jiang, L.; Teng, F.; Wang, Z. Effects of high-pressure homogenization on structural and emulsifying properties of thermally soluble aggregated kidney bean (Phaseolus vulgaris L.) proteins. Food Hydrocoll. 2021, 119, 106835. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhong, J.Z.; Liu, W.; Tu, Z.C.; Wan, J.; Cai, X.F.; Song, X.Y. Relationship between Functional Properties and Aggregation Changes of Whey Protein Induced by High Pressure Microfluidization. J. Food Sci. 2011, 76, 341–347. [Google Scholar] [CrossRef]
- Chen, N.; Zhao, M.; Sun, W.; Ren, J.; Cui, C. Effect of oxidation on the emulsifying properties of soy protein isolate. Food Res. Int. 2013, 52, 26–32. [Google Scholar] [CrossRef]
- Jing, X.; Zijing, C.; Dong, H.; Yangyang, L.; Xiaotong, S.; Zhongjiang, W.; Hua, J. Structural and Functional Properties Changes of β-Conglycinin Exposed to Hydroxyl Radical-Generating Systems. Molecules 2017, 22, 1893. [Google Scholar]
- Zhang, H.J.; Zhang, H.; Wang, L.; Guo, X.N. Preparation and functional properties of rice bran proteins from heat-stabilized defatted rice bran. Food Res. Int. 2012, 47, 359–363. [Google Scholar] [CrossRef]
- Wu, C.; He, M.; Zheng, L.; Tian, T.; Teng, F.; Li, Y. Effect of cavitation jets on the physicochemical properties and structural characteristics of the okara protein. J. Food Sci. 2021, 86, 4566–4576. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Chen, Z.; Sun, D.; Li, Y. Electron beam irradiation induced aggregation behaviour, structural and functional properties changes of rice proteins and hydrolysates. Food Hydrocoll. 2019, 97, 105192. [Google Scholar] [CrossRef]
- Fu, Z.; Popov, V. The ACA–BEM approach with a binary-key mosaic partitioning for modelling multiple bubble dynamics. Eng. Anal. Bound. Elem. 2015, 50, 169–179. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Yi, C.; Hua, Y.; Yang, C.; Cui, S. Effect of ionic strength on the heat-induced soy protein aggregation and the phase separation of soy protein aggregate/dextran mixtures. Food Hydrocoll. 2009, 23, 1015–1023. [Google Scholar] [CrossRef]
- Sha, L.; Koosis, A.O.; Wang, Q.; True, A.D.; Xiong, Y.L. Interfacial dilatational and emulsifying properties of ultrasound-treated pea protein. Food Chem. 2021, 350, 129271. [Google Scholar] [CrossRef]
- Cheng, Y.; Donkor, P.O.; Ren, X.; Wu, J.; Agyemang, K.; Ayim, I.; Ma, H. Effect of ultrasound pretreatment with mono-frequency and simultaneous dual frequency on the mechanical properties and microstructure of whey protein emulsion gels. Food Hydrocoll. 2019, 89, 434–442. [Google Scholar] [CrossRef]
- Hu, H.; Wu, J.; Li-Chan, E.C.Y.; Zhu, L.; Zhang, F.; Xu, X.; Fan, G.; Wang, L.; Huang, X.; Pan, S. Effects of ultrasound on structural and physical properties of soy protein isolate (SPI) dispersions. Food Hydrocoll. 2013, 30, 647–655. [Google Scholar] [CrossRef]
- Stathopulos, P.B.; Scholz, G.A.; Hwang, Y.M.; Rumfeldt, J.A.O.; Lepock, J.R.; Meiering, E.M. Sonication of proteins causes formation of aggregates that resemble amyloid. Protein Sci. 2010, 13, 3017–3027. [Google Scholar] [CrossRef] [PubMed]
- Izmailova, V.N.; Yampolskaya, G.P.; Tulovskaya, Z.D. Development of the Rehbinder’s concept on structure-mechanical barrier in stability of dispersions stabilized with proteins. Colloids Surf. A Physicochem. Eng. Asp. 1999, 160, 89–106. [Google Scholar] [CrossRef]
- Graham, D.E.; Phillips, M.C. Proteins at liquid interfaces: III. Molecular structures of adsorbed films. J. Colloid Interface Sci. 1979, 70, 427–439. [Google Scholar] [CrossRef]
- Damodaran, S.; Razumovsky, L. Role of surface area-to-volume ratio in protein adsorption at the air–water interface. Surf. Sci. 2008, 602, 307–315. [Google Scholar] [CrossRef]
- Delahaije, R.; Wierenga, P.A.; Nieuwenhuijzen, N.V.; Giuseppin, M.; Gruppen, H. Protein concentration and protein-exposed hydrophobicity as dominant parameters determining the flocculation of protein-stabilized oil-in-water emulsions. Langmuir ACS J. Surf. Colloids 2013, 29, 11567–11574. [Google Scholar] [CrossRef]
- Peng, L.P.; Xu, Y.T.; Li, X.T.; Tang, C.H. Improving the emulsification of soy β-conglycinin by alcohol-induced aggregation. Food Hydrocoll. 2019, 98, 105307. [Google Scholar] [CrossRef]
- Nilsson, L.; Leeman, M.; Wahlund, K.G.; Bergenståhl, B. Competitive adsorption of a polydisperse polymer during emulsification: Experiments and modeling. Langmuir ACS J. Surf. Colloids 2007, 23, 2346–2351. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Xia, N.; Yang, X.Q.; Yin, S.W.; Qi, J.R.; He, X.T.; Yuan, D.B.; Wang, L.J. Adsorption and dilatational rheology of heat-treated soy protein at the oil-water interface: Relationship to structural properties. J. Agric. Food Chem. 2012, 60, 3302–3310. [Google Scholar] [CrossRef]
- Alizadeh, V.; Amirkhizi, A. Modeling of Cavitation Erosion Resistance in Polymeric Materials Based on Strain Accumulation. 2019, Volume 2. Available online: https://www.researchgate.net/publication/326959965_Modeling_of_Cavitation_Erosion_Resistance_in_Polymeric_Materials_Based_on_Strain_Accumulation (accessed on 5 February 2023).
- Yang, J.; Liu, G.; Zeng, H.; Chen, L. Effects of high pressure homogenization on faba bean protein aggregation in relation to solubility and interfacial properties. Food Hydrocoll. 2018, 83, 275–286. [Google Scholar] [CrossRef]
- Qiu, H.; Chen, X.; Wei, X.; Liang, J.; Zhou, D.; Wang, L. The Emulsifying Properties of Hydrogenated Rosin Xylitol Ester as a Biomass Surfactant for Food: Effect of pH and Salts. Molecules 2020, 25, 302. [Google Scholar] [CrossRef]
- Dapueto, N.; Troncoso, E.; Mella, C.; Zúñiga, R. The effect of denaturation degree of protein on the microstructure, rheology and physical stability of oil-in-water (O/W) emulsions stabilized by whey protein isolate. J. Food Eng. 2019, 263, 253–261. [Google Scholar] [CrossRef]
- Fu, L.; Tang, C.H. Soy glycinin as food-grade Pickering stabilizers: Part. I. Structural characteristics, emulsifying properties and adsorption/arrangement at interface. Food Hydrocoll. 2016, 60, 606–619. [Google Scholar]
- Sakuno, M.M.; Matsumoto, S.; Kawai, S.; Taihei, K.; Matsumura, Y. Adsorption and structural change of beta-lactoglobulin at the diacylglycerol-water interface. Langmuir 2008, 24, 11483–11488. [Google Scholar] [CrossRef] [PubMed]
- Nurazwa, I.; Ahmad, L.; Rosfarizan, M.; Arbakariya, A.; Mohd, M.; Murni, H.; Helmi, W. Kinetics and Optimization of Lipophilic Kojic Acid Derivative Synthesis in Polar Aprotic Solvent Using Lipozyme RMIM and Its Rheological Study. Molecules 2018, 23, 501. [Google Scholar]
- Antonio, L.; Annalisa, C.; Nunzio, D.; Mara, P.; Rosa, I.; Elisabetta, F.; Angela, L.; Nicoletta, D.; Modesto, D.C.; Massimo, F. Delivery of Proapoptotic Agents in Glioma Cell Lines by TSPO Ligand–Dextran Nanogels. Int. J. Mol. Sci. 2018, 19, 1155. [Google Scholar]
- Toledo, L.; Ramos, M.; Silva, P.; Rodero, C.F.; Bauab, T.M. Improved in vitro and in vivo Anti-Candida albicans Activity of Cymbopogon nardus Essential Oil by Its Incorporation into a Microemulsion System. Int. J. Nanomed. 2020, 15, 10481–10497. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. A Study on the Rheology and Fiber Extrusion of Polyacrylonitrile Gel; Auburn University: Auburn, AL, USA, 2003. [Google Scholar]
- Bos, M.A.; Vliet, T.V. Interfacial rheological properties of adsorbed protein layers and surfactants: A review. Adv. Colloid Interface Sci. 2001, 91, 437–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Regenstein, J.M.; Zhou, P.; Yang, Y. Effects of high intensity ultrasound modification on physicochemical property and water in myofibrillar protein gel. Ultrason. Sonochemistry 2017, 34, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.S.; Chen, S.S.; Li, X.J.; Luo, S.Z.; Zhong, X.Y.; Jiang, S.T.; Zhao, Y.Y.; Zheng, Z. Gelation Properties of Transglutaminase-Induced Soy Protein Isolate and Wheat Gluten Mixture with Ultrahigh Pressure Pretreatment. Food Bioprocess Technol. 2017, 10, 866–874. [Google Scholar] [CrossRef]
- Pal, R.; Yan, Y.; Masliyah, J. Advances in Food Rheology and Its Applications. Rheol. Emuls. 1992, 12, 437–457. [Google Scholar]
- Walstra, P. Food emulsions: Principles, practice, and techniques. Trends Food Sci. Technol. 2001, 36, 223–224. [Google Scholar] [CrossRef]
- Guo, Z.; Teng, F.; Huang, Z.; Lv, B.; Lv, X.; Babich, O.; Yu, W.; Li, Y.; Wang, Z.; Jiang, L. Effects of material characteristics on the structural characteristics and flavor substances retention of meat analogs. Food Hydrocoll. 2020, 105, 105752. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Hu, Z.; Yang, Z.; Liu, J.; Tan, B.; Guo, Z.; Li, B.; Li, H. The temporal evolution mechanism of structure and function of oxidized soy protein aggregates. Food Hydrocoll. 2022, 15, 100382. [Google Scholar] [CrossRef] [PubMed]
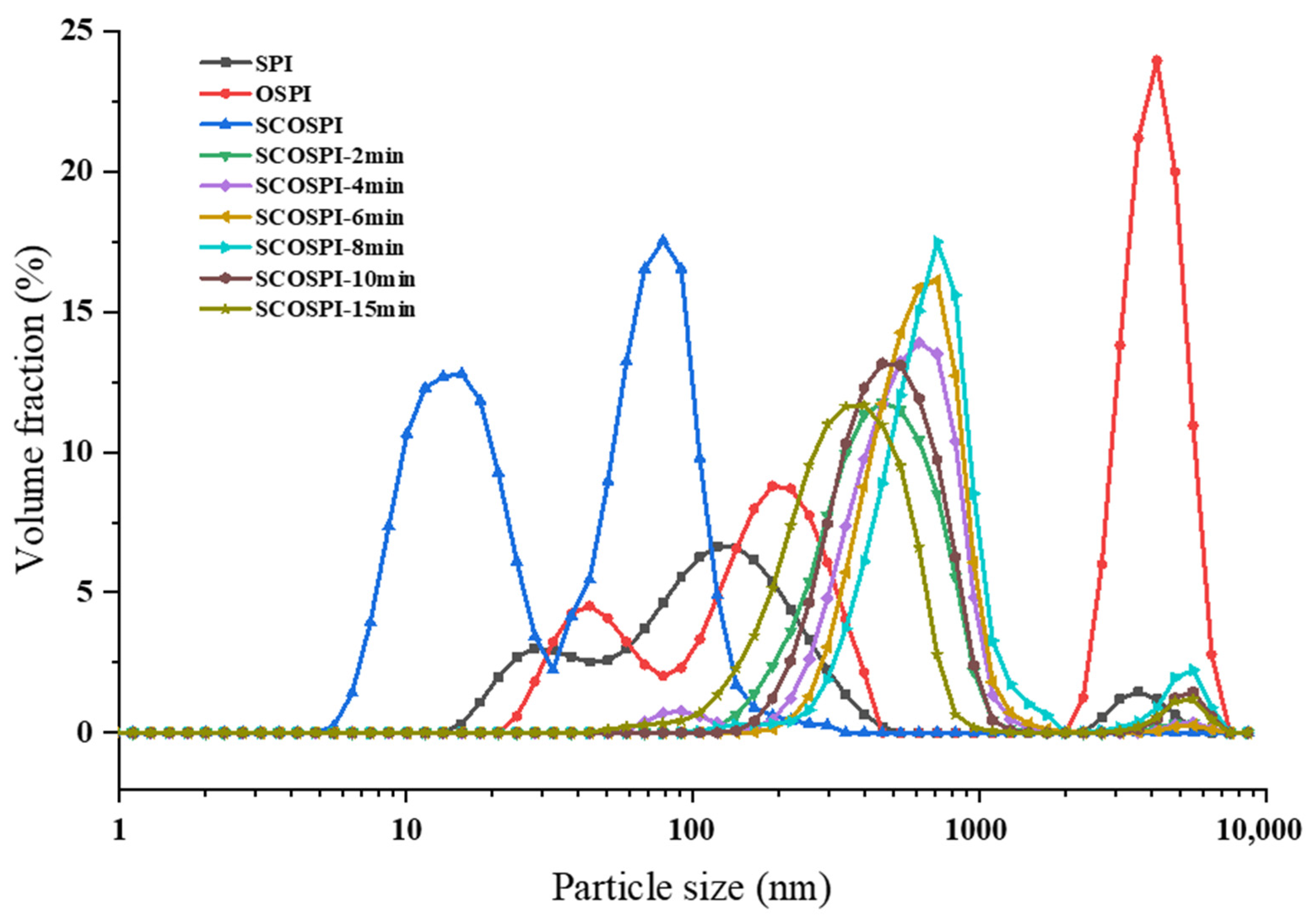


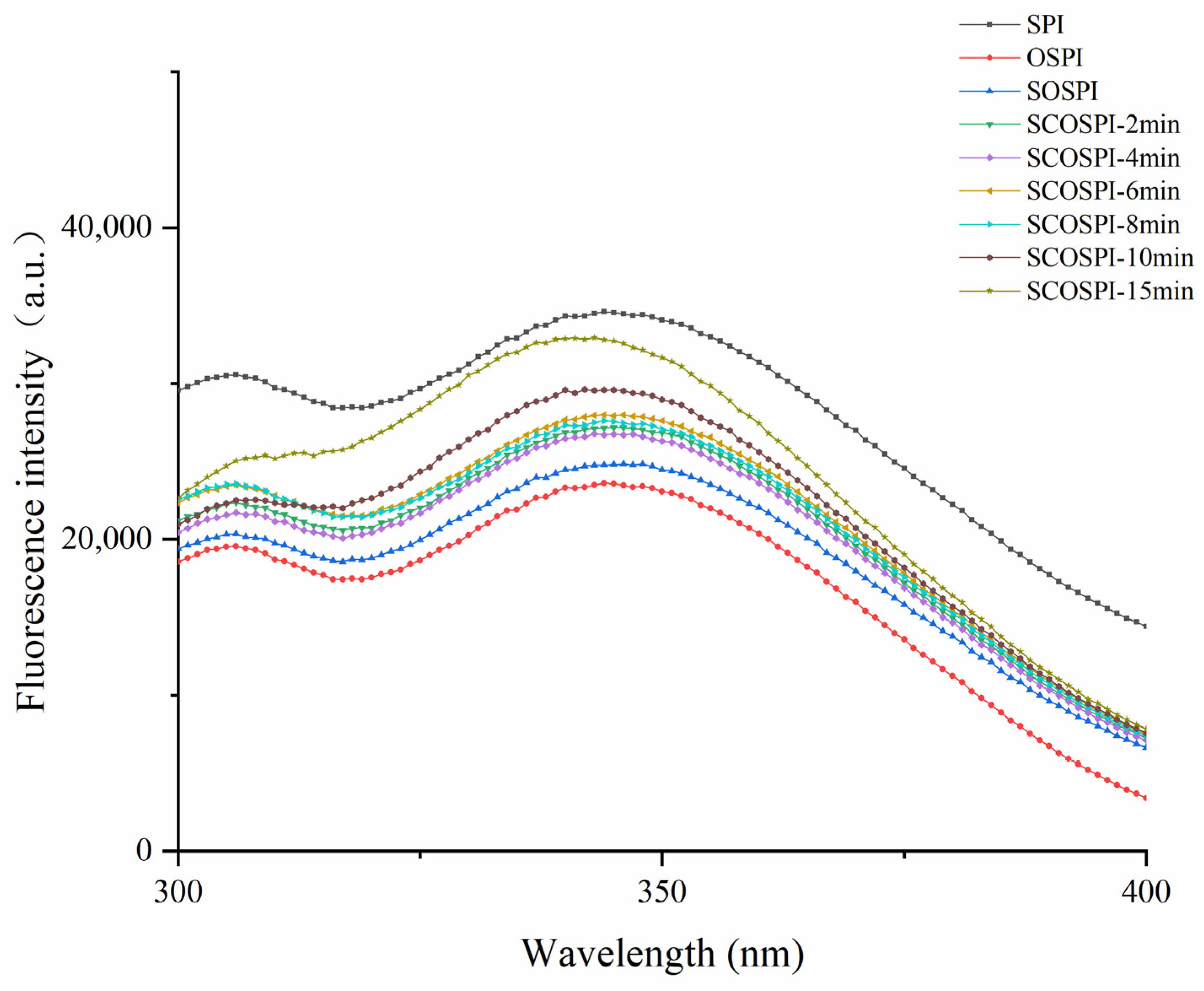
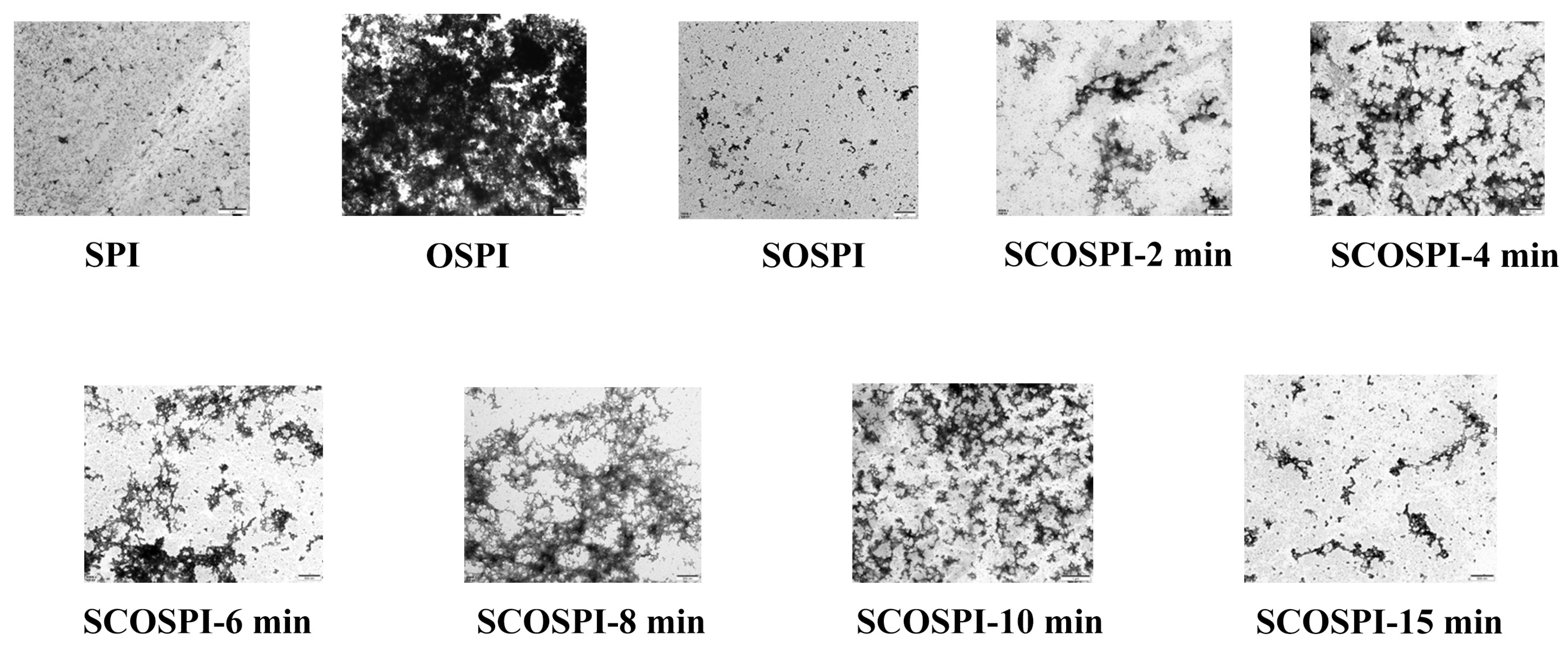

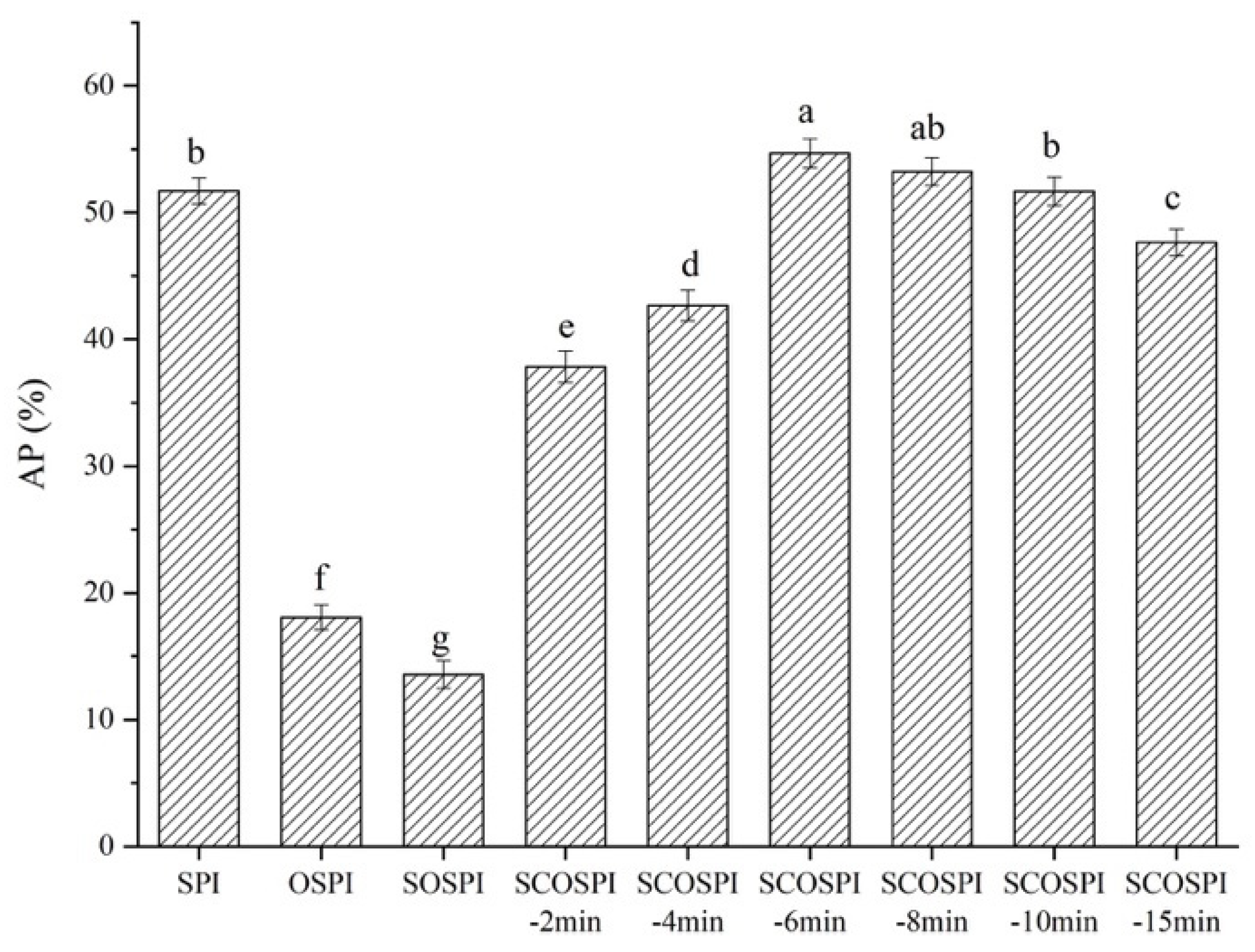
| Samples | Particle Size (nm) | PDI |
|---|---|---|
| SPI | 181.59 ± 2.77 h | 0.76 ± 0.11 a |
| OSPI | 3836.18 ± 66.82 a | 0.19 ± 0.03 d |
| SOSPI | 97.38 ± 1.63 i | 0.17 ± 0.01 d |
| SCOSPI-2 min | 551.03 ± 5.07 e | 0.14 ± 0.02 ef |
| SCOSPI-4 min | 639.27 ± 18.31 d | 0.11 ± 0.04 f |
| SCOSPI-6 min | 705.81 ± 15.33 c | 0.29 ± 0.04 c |
| SCOSPI-8 min | 776.14 ± 11.91 b | 0.15 ± 0.01 e |
| SCOSPI-10 min | 538.15 ± 9.52 f | 0.28 ± 0.03 c |
| SCOSPI-15 min | 443.96 ± 22.28 g | 0.33 ± 0.01 b |
| Content (%) | Anti-Parallel Intermolecular β-Sheet (β1) | Parallel Intermolecular β-Sheet (β2) | Intramolecular β-Sheet | ɑ-Helix | β-Turn | γ-Random Coil |
|---|---|---|---|---|---|---|
| Wavenumber (cm−1) | 1608–1622 | 1682–1700 | 1622–1637 | 1646–1662 | 1662–1681 | 1637–1645 |
| SPI | 9.27 ± 0.28 d | 15.95 ± 0.23 a | 18.41 ± 0.18 a | 26.35 ± 0.18 c | 21.33 ± 0.17 f | 8.69 ± 0.15 e |
| OSPI | 13.58 ± 0.19 a | 7.16 ± 0.18 f | 17.18 ± 0.13 b | 17.82 ± 0.19 d | 26.66 ± 0.20 e | 17.41 ± 0.20 a |
| SOSPI | 9.05 ± 0.26 d | 9.74 ± 0.22 b | 13.58 ± 0.25 f | 27.84 ± 0.19 a | 28.28 ± 0.18 d | 10.51 ± 0.22 b |
| SCOSPI-2 min | 11.28 ± 0.08 c | 8.54 ± 0.26 d | 13.38 ± 0.07 g | 26.27 ± 0.08 c | 30.61 ± 0.13 b | 9.92 ± 0.17 c |
| SCOSPI-4 min | 12.70 ± 0.10 b | 7.84 ± 0.19 e | 13.80 ± 0.26 e | 24.52 ± 0.19 e | 31.82 ± 0.02 a | 9.32 ± 0.18 d |
| SCOSPI-6 min | 12.77 ± 0.01 b | 9.02 ± 0.25 c | 13.58 ± 0.05 f | 23.36 ± 0.04 f | 31.92 ± 0.22 a | 9.35 ± 0.06 d |
| SCOSPI-8 min | 11.42 ± 0.19 c | 9.83 ± 0.01 b | 15.80 ± 0.19 c | 22.72 ± 0.14 g | 29.66 ± 0.22 c | 10.57 ± 0.16 b |
| SCOSPI-10 min | 12.78 ± 0.06 b | 6.78 ± 0.22 g | 13.70 ± 0.03 ef | 26.68 ± 0.20 b | 30.73 ± 0.24 b | 9.33 ± 0.30 d |
| SCOSPI-15 min | 13.51 ± 0.13 a | 6.76 ± 0.05 g | 15.36 ± 0.15 d | 25.09 ± 0.06 d | 29.79 ± 0.15 c | 9.49 ± 0.18 d |
| Samples | Free Sulfhydryl (nmol/mg) | Total Sulfhydryl (nmol/mg) | Disulfide Bond (nmol mg−1) |
|---|---|---|---|
| SPI | 11.72 ± 0.15 a | 15.64 ± 0.32 a | 1.96 ± 0.11 c |
| OSPI | 4.05 ± 0.23 h | 10.78 ± 0.14 g | 3.37 ± 0.13 a |
| SOSPI | 8.06 ± 0.22 e | 10.99 ± 0.12 f | 1.47 ± 0.16 d |
| SCOSPI-2 min | 8.65 ± 0.17 c | 11.95 ± 0.16 e | 1.65 ± 0.17 cd |
| SCOSPI-4 min | 8.92 ± 0.21 b | 12.56 ± 0.18 c | 1.82 ± 0.13 c |
| SCOSPI-6 min | 8.87 ± 0.19 bc | 13.29 ± 0.22 b | 2.21 ± 0.15 b |
| SCOSPI-8 min | 8.21 ± 0.14 d | 12.15 ± 0.19 d | 1.97 ± 0.16 c |
| SCOSPI-10 min | 7.65 ± 0.16 f | 10.69 ± 0.15 g | 1.52 ± 0.16 d |
| SCOSPI-15 min | 7.15 ± 0.18 g | 10.07 ± 0.16 h | 1.46 ± 0.19 d |
| Samples | EAI/(m2·g−1) | ESI/min |
|---|---|---|
| SPI | 91.61 ± 1.17 a | 186.19 ± 3.89 a |
| OSPI | 46.97 ± 2.24 g | 137.20 ± 3.51 e |
| SOSPI | 89.92 ± 2.37 a | 126.75 ± 3.18 f |
| SCOSPI-f-2 min | 57.17 ± 1.89 f | 149.08 ± 3.15 d |
| SCOSPI-f-4 min | 71.92 ± 1.91 e | 156.24 ± 2.89 c |
| SCOSPI-f-6 min | 86.24 ± 2.39 b | 163.17 ± 2.68 b |
| SCOSPI-f-8 min | 81.64 ± 2.64 c | 152.56 ± 3.37 cd |
| SCOSPI-f-10 min | 76.95 ± 2.19 d | 147.89 ± 2.90 d |
| SCOSPI-f-15 min | 72.68 ± 2.64 e | 140.27 ± 3.64 e |
| Samples | K (Pa·sn) | n | R2 |
|---|---|---|---|
| SPI | 0.066 | 0.271 | 0.99 |
| OSPI | 0.626 | 0.059 | 0.99 |
| SOSPI | 0.021 | 0.169 | 0.99 |
| SCOSPI-2 min | 0.539 | 0.183 | 0.99 |
| SCOSPI-4 min | 1.380 | 0.216 | 0.99 |
| SCOSPI-6 min | 2.673 | 0.219 | 0.99 |
| SCOSPI-8 min | 3.343 | 0.173 | 0.99 |
| SCOSPI-10 min | 3.123 | 0.154 | 0.99 |
| SCOSPI-15 min | 2.676 | 0.152 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, C.; Wang, Y.; Ren, S.; Zheng, X.; Zhang, J.; Cheng, T.; Guo, Z.; Wang, Z. Impact of Cavitation Jet on the Structural, Emulsifying Features and Interfacial Features of Soluble Soybean Protein Oxidized Aggregates. Foods 2023, 12, 909. https://doi.org/10.3390/foods12050909
Guo Y, Liu C, Wang Y, Ren S, Zheng X, Zhang J, Cheng T, Guo Z, Wang Z. Impact of Cavitation Jet on the Structural, Emulsifying Features and Interfacial Features of Soluble Soybean Protein Oxidized Aggregates. Foods. 2023; 12(5):909. https://doi.org/10.3390/foods12050909
Chicago/Turabian StyleGuo, Yanan, Caihua Liu, Yichang Wang, Shuanghe Ren, Xueting Zheng, Jiayu Zhang, Tianfu Cheng, Zengwang Guo, and Zhongjiang Wang. 2023. "Impact of Cavitation Jet on the Structural, Emulsifying Features and Interfacial Features of Soluble Soybean Protein Oxidized Aggregates" Foods 12, no. 5: 909. https://doi.org/10.3390/foods12050909
APA StyleGuo, Y., Liu, C., Wang, Y., Ren, S., Zheng, X., Zhang, J., Cheng, T., Guo, Z., & Wang, Z. (2023). Impact of Cavitation Jet on the Structural, Emulsifying Features and Interfacial Features of Soluble Soybean Protein Oxidized Aggregates. Foods, 12(5), 909. https://doi.org/10.3390/foods12050909






