Antioxidant and In Vivo Hypoglycemic Activities of Ethanol Extract from the Leaves of Engelhardia roxburghiana Wall, a Comparative Study of the Extract and Astilbin
Abstract
:1. Introduction
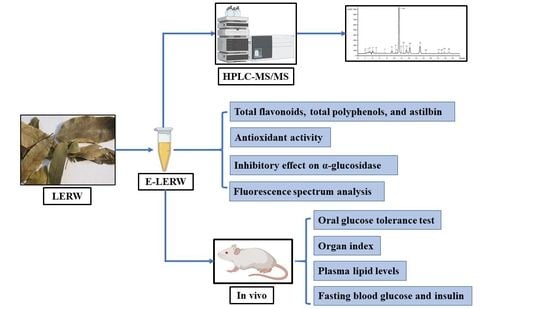
2. Materials and Methods
2.1. Materials
2.2. Preparation of E-LERW
2.3. Identification by HPLC-MS/MS
2.4. Determination of Active Ingredients in E-LERW
2.4.1. Total Flavonoids
2.4.2. Total Phenols
2.4.3. Astilbin
2.5. Antioxidant Activity
2.5.1. Scavenging DPPH Free Radicals
2.5.2. Scavenging ABTS+ Radicals
2.5.3. Ferric Reducing Activity of Power
2.5.4. Inhibition of Lipid Membrane Oxidation
2.6. Inhibitory Effect on α-Glucosidase
Kinetic Analysis on the Inhibition of α-Glucosidase
2.7. Hypoglycemic Activity In Vivo
2.7.1. Animal Experiment Design
2.7.2. Oral Glucose Tolerance Test
2.7.3. Blood Sample Analysis
2.7.4. Organ Index
2.8. Data Analysis
3. Results
3.1. HPLC-MS/MS Analysis
3.2. Determination of Active Components
3.3. Antioxidant Activity
3.4. Inhibitory Effects on α-Glucosidase
3.4.1. Inhibition on α-Glucosidase
3.4.2. Inhibitory Kinetic Analysis
3.5. Hypoglycemic Activity In Vivo
3.5.1. Body Weight, Food Intake, Water Intake and Excretion
3.5.2. Fasting Blood Glucose and Insulin
3.5.3. Oral Glucose Tolerance Test
3.5.4. Blood Lipid Analysis
3.5.5. Effects of E-LERW on Organ Indexes of Liver and Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, H.; He, L.S.; Zhou, Z.T.; Yuan, M. Antioxidant constituents from leaves of Engelhardtia roxburghiana. Chem. Nat. Compd. 2012, 48, 679–680. [Google Scholar] [CrossRef]
- Xin, W.B.; Huang, H.Q.; Yu, L.; Shi, H.M.; Sheng, Y.; Wang, T.T.Y.; Yu, L.L. Three new flavanonol glycosides from leaves of Engelhardtia roxburghiana, and their anti-inflammation, antiproliferative and antioxidant properties. Food Chem. 2012, 2, 788–798. [Google Scholar] [CrossRef]
- Haraguchi, H.; Ohmi, l.; Fukuda, A.; Tamura, Y.; Mizutani, K.; Tanaka, O.; Chou, W.H. Inhibition of aldose reductase and sorbitol accumulation by astilbin and taxifolin dihydroflavonols in Engelhardtia chrysolepis. Biosci. Biotech. Biochem. 1997, 61, 651–654. [Google Scholar] [CrossRef]
- Wirasathien, L.; Pengsuparp, T.; Suttisri, R.; Ueda, H.; Moriyasu, M.; Kawanishi, K. Inhibitors of aldose reductase and advanced glycation end-products formation from the leaves of Stelechocarpus cauliflorus RE Fr. Phytomedicine 2007, 14, 546–550. [Google Scholar] [CrossRef]
- Patel, D.K. Therapeutic potential of astilbin on diabetes and related secondary complication ‘diabetic nephropathy’: Therapeutic potential and scientific data analysis of current research work. Bone Rep. 2021, 14, 21–22. [Google Scholar] [CrossRef]
- Motoyashiki, T.; Miyake, M.; Morita, T.; Mizutani, K.; Masuda, H.; Ueki, H. Enhancement of the vanadate stimulated release of lipoprotein lipase activity by astilbin from the leaves of Engelhardtia chrysolepis. Biol. Pharm. Bull. 1998, 21, 517–519. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Gou, X.J.; Guo, X.Q.; Wang, S.H.; Li, L.; Yao, Q. Investigation on encapsulation efficiency for oleuropein liposome. Chin. Hosp. Pharm. J. 2015, 35, 1286–1289. (In Chinese) [Google Scholar]
- Cruz, R.G.D.; Beney, L.; Gervais, P.; Lira, S.P.; Vieira, T.M.F.S.; Dupont, S. Comparison of the antioxidant property of acerola extracts with synthetic antioxidants using an in vivo method with yeasts. Food Chem. 2019, 277, 698–705. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Schneider, R.G. Quinoa flavonoids and their bioaccessibility during in vitro gastrointestinal digestion. J. Cereal Sci. 2020, 95, e103070. [Google Scholar] [CrossRef]
- Yang, S.J.; Paudel, P.; Shrestha, S.; Seong, S.H.; Jung, H.A.; Choi, J.S. In vitro protein tyrosine phosphatase 1B inhibition and antioxidant property of different onion peel cultivars: A comparative study. Food Sci. Nutr. 2018, 1, 205–215. [Google Scholar] [CrossRef]
- Maseko, I.; Mabhaudhi, T.; Ncube, B.; Tesfay, S.; Araya, H.T.; Fessehazion, M.K.; Chimonyo, V.G.P.; Ndhlala, A.R.; Plooy, C.P.D. Postharvest drying maintains phenolic, flavonoid and gallotannin content of some cultivated African leafy vegetables. Sci. Hortic. 2019, 20, 70–76. [Google Scholar] [CrossRef]
- Yao, Q.; Shen, Y.; Bu, L.; Yang, P.; Xu, Z.; Guo, X. Ultrasound-assisted aqueous extraction of total flavonoids and hydroxytyrosol from olive leaves optimized by response surface methodology. Prep. Biochem. Biotechnol. 2019, 4, 837–845. [Google Scholar] [CrossRef]
- Dirar, A.I.; Alsaadi, D.H.M.; Wada, M.; Mohamed, M.A.; Watanabe, T.; Devkota, H.P. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S. Afr. J. Bot. 2019, 120, 261–267. [Google Scholar] [CrossRef]
- Makgatho, M.E.; Nxumalo, W.; Raphoko, L.A. Anti-mycobacterial, -oxidative, -proliferative and -inflammatory activities of dichloromethane leaf extracts of Gymnosporia senegalensis (Lam.) Loes. S. Afr. J. Bot. 2018, 114, 217–222. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Extractable and macromolecular antioxidants of Opuntia ficus-indica cladodes: Phytochemical profiling, antioxidant and antibacterial activities. S. Afr. J. Bot. 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Hao, G.X.; Cao, W.Q.; Li, T.; Chen, J.; Zhang, J.L.; Weng, W.V.; Osako, K.; Ren, H.F. Effect of temperature on chemical properties and antioxidant activities of abalone viscera subcritical water extract. J. Supercrit. Fluids 2019, 147, 17–23. [Google Scholar] [CrossRef]
- Vamanu, E.; Nita, S. Bioactive compounds, antioxidant and anti-inflammatory activities of extracts from Cantharellus cibarius. Rev. Chim. 2014, 65, 372–380. [Google Scholar]
- Broholm, S.L.; Gramsbergen, S.M.; Nyberg, N.T.; Jager, A.K.; Staerk, D. Potential of Sorbus berry extracts for management of type 2 diabetes: Metabolomics investigation of 1H NMR spectra, α-amylase and α-glucosidase inhibitory activities, and in vivo anti-Hyperglycaemic activity of S. norvegica. J. Ethnopharmacol. 2019, 242, e112061. [Google Scholar] [CrossRef]
- Yan, J.K.; Zhang, G.W.; Pan, J.H.; Wang, Y.J. alpha-Glucosidase inhibition by luteolin: Kinetics, interaction and molecular docking. Int. J. Biol. Macromol. 2014, 64, 213–223. [Google Scholar] [CrossRef]
- Li, C.; Gan, H.; Tan, X.L.; Hu, Z.X.; Deng, B.; Sullivan, M.A.; Gilbert, R.G. Effects of active ingredients from traditional Chinese medicines on glycogen molecular structure in diabetic mice. Eur. Polym. J. 2019, 112, 67–72. [Google Scholar] [CrossRef]
- Wang, T.T.; Li, X.; Zhou, B.; Li, H.F.; Zeng, J.; Gao, W.Y. Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.). J. Funct. Foods 2015, 13, 276–288. [Google Scholar] [CrossRef]
- Abdel-Haleem, S.A.; Ibrahim, A.Y.; Ismail, R.F.; Shaffie, N.M.; Hendawy, S.F.; Omer, E.A. In-vivo hypoglycemic and hypolipidemic properties of tagetes lucida ethanolic extract in streptozotocin-induced hyperglycemic Wistar albino rats. Ann. Agr. Sci. 2017, 62, 169–181. [Google Scholar] [CrossRef]
- Feng, Y.L.; Lin, J.L.; He, G.; Liang, L.; Liu, Q.J.; Yan, J.; Yao, Q. Compositions and biological activities of pomegranate peel polyphenols extracted by different solvents. Molecules 2022, 27, 4796. [Google Scholar] [CrossRef]
- Zhou, S.X.; Shao, Y.; Fu, J.H.; Xiang, L.; Zheng, Y.N.; Li, W. Characterization and quantification of taxifolin related flavonoids in Larix olgensis Henry Var. koreana Nakai extract analysis and its antioxidant activity assay. Int. J. Pharmacol. 2018, 14, 534–545. [Google Scholar] [CrossRef] [Green Version]
- AliAbadi, M.H.S.; Karami-Osboo, R.; Kobarfard, F.; Jahani, R.; Nabi, M.; Yazdanpanah, H.; Mahboubi, A.; Nasiri, A. Detection of lime juice adulteration by simultaneous determination of main organic acids using liquid chromatography-tandem mass spectrometry. J. Food. Compos. Anal. 2021, 105, e104223. [Google Scholar] [CrossRef]
- Zhang, A.; Wan, L.; Wu, C.Y.; Fang, Y.L.; Han, G.M.; Li, H.; Zhang, Z.W.; Wang, H. Simultaneous determination of 14 phenolic compounds in grape canes by HPLC-DAD-UV using wavelength switching detection. Molecules 2013, 18, 14241–14257. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.; Tajuddin, N.; Shaikh, A. Retrospective case series of patients with diabetes or prediabetes who were switched from omega-3-acid ethyl esters to icosapent ethyl. Cardiol Ther. 2015, 4, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Nájera, V.C.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Hidalgo-Figueroa, S.; Del-Toro-Sánchez, C.L.; Salazar-Olivo, L.A.; Lugo-Cervantes, E. Smilax aristolochiifolia root extract and its compounds chlorogenic acid and astilbin inhibit the activity of α-amylase and α-glucosidase enzymes. Evid. Based Complement Alternat. Med. 2018, 2018, e6247306. [Google Scholar] [CrossRef] [Green Version]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Hossain, M.K.; Dayem, A.A.; Han, J.; Yin, Y.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mole. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [Green Version]
- Mia, M.A.; Mosaib, M.G.; Khalil, M.I.; Islam, M.A.; Gan, S.H. Potentials and safety of date palm fruit against diabetes: A critical review. Foods 2020, 11, 1557. [Google Scholar] [CrossRef]
- Ghorbani, A.; Rashidi, R.; Shafiee-Nick, R. Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomed. Pharmacother. 2019, 11, 947–957. [Google Scholar] [CrossRef]
- Leme, J.A.C.A.; Castellar, A.; Remedio, R.N. Effects in short-term of alloxan application to diabetes induction in Wistar rats. Biosci. J. 2010, 26, 451–456. [Google Scholar]
- Vahid, H.; Rakhshandeh, H.; Ghorbani, A. Antidiabetic properties of Capparis spinosa L. and its components. Biomed. Pharmacother. 2017, 92, 293–302. [Google Scholar] [CrossRef]
- Oršolić, N.; Gajski, G.; Garaj-Vrhovac, V. DNA-protective effects of quercetin or naringenin in alloxan-induced diabetic mice. Eur. J. Pharmacol. 2011, 656, 110–118. [Google Scholar] [CrossRef]
- Kim, J.; Shon, E.; Kim, C.S. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp. Diabetes Res. 2012, 2012, e210821. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Wang, J.; Duan, C. Aqueous extracts of se-enriched auricularia auricular exhibits antioxidant capacity and attenuate liver damage in high-fat diet/streptozotocin-induced diabetic mice. J. Med. Food 2020, 23, 153–160. [Google Scholar] [CrossRef]
- Malekinejad, H.; Rezabakhsh, A.; Rahmani, F.; Hobbenaghi, R. Silyrnarin regulates the cytochrome P450 3A2 and glutathione peroxides in the liver of streptozotocin-induced diabetic rats. Phytomedicine 2012, 19, 583–590. [Google Scholar] [CrossRef]
- Fernandes, A.A.H.; Novelli, E.L.B.; Okoshi, K. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed. Pharmacother. 2010, 64, 214–219. [Google Scholar] [CrossRef]





| No. | RT (min) | Molecular Ion m/z | Molecular Weight | Fragment | λmax | Formula | Compound | |
|---|---|---|---|---|---|---|---|---|
| m/z | Abundance (%) | |||||||
| 1 | 2.84 | 360.1497 (M+NH4)+, 365.1050 (M+Na)+ | 342.1158 | 85.0289 127.0390 145.0494 | 100 65.63 68.75 | C12H22O11 | α-Lactose | |
| 2 | 3.26 | 133.0129 (M−H)− | 134.0202 | 115.0023 71.0125 | 100 64.76 | C4H6O5 | Malic acid | |
| 3 | 4.42 | 277.0325 (M−H)− | 278.0398 | 96.9682 78.9576 | 100 36.52 | C9H6N6O5 | 3,5-Dinitro-n-(4H-1,2,4-triazole-4-yl)-benzamide | |
| 4 | 9.38 | 303.0497 (M+H)+ | 302.0426 | 303.0497 285.0385 257.0442 238.9389 183.0285 | 100 1.18 2.35 29.41 32.35 | 234 291 360 | C15H10O7 | Quercetin |
| 5 | 11.30 | 449.1083 (M−H)− | 450.1155 | 303.0607 285.0400 178.9975 151.0024 | 19.23 50.00 0.12 100 | 228 291 | C21H22O11 | Astilbin |
| 6 | 13.36 | 465.1024 (M+H)+ | 464.0951 | 229 290 | C21H20O12 | Bractein | ||
| 7 | 14.27 | 287.0548 (M+H)+ | 286.0473 | 295 338 | C15H10O6 | Maritimetin | ||
| 8 | 17.08 | 433.1133 (M−H)− | 434.1206 | 433.1133 269.0452 178.9975 152.0103 | 68.29 78.05 97.56 100 | 293 | C21H22O10 | Engeletin |
| 9 | 23.73 | 303.0508 (M−H)− | 304.0579 | 285.0401 125.0231 | 58.33 100 | 291 | C15H12O7 | Taxifolin |
| 10 | 24.97 | 191.0188 (M−H)− | 192.0260 | 111.0074 87.0074 | 100 52.17 | C6H8O7 | Citric acid | |
| Group | Body Weight (g) | Food Intake (g) | Water Intake (mL) | Excretion (g) | ||||
|---|---|---|---|---|---|---|---|---|
| 1st Day | 28th Day | 1st Day | 28th Day | 1st Day | 28th Day | 1st Day | 28th Day | |
| NC | 22.72 ± 0.69 | 41.08 ± 1.23 a | 5.76 ± 0.53 | 6.68 ± 0.28 a | 5.49 ± 0.36 | 6.42 ± 0.42 a | 5.38 ± 0.63 | 5.14 ± 0.57 a |
| MC | 22.83 ± 0.48 | 23.56 ± 0.41 e | 6.57 ± 0.26 | 12.02 ± 1.57 d | 18.12 ± 1.63 | 40.47 ± 2.36 f | 16.84 ± 1.58 | 36.03 ± 2.14 f |
| PC | 23.55 ± 0.61 | 35.13 ± 0.74 b | 6.42 ± 0.54 | 7.57 ± 0.83 b | 17.32 ± 1.34 | 17.89 ± 1.25 b | 16.80 ± 1.27 | 16.50 ± 1.16 b |
| AC | 23.41 ± 0.43 | 27.87 ± 0.37 d | 6.21 ± 0.68 | 9.24 ± 0.76 c | 19.77 ± 2.31 | 28.13 ± 1.57 e | 16.90 ± 1.93 | 32.37 ± 1.38 e |
| E-LERW[H] | 23.39 ± 0.57 | 32.47 ± 0.26 c | 6.31 ± 0.36 | 7.88 ± 0.33 b | 18.68 ± 0.93 | 21.38 ± 0.73 c | 17.37 ± 0.83 | 20.18 ± 1.11 c |
| E-LERW[M] | 23.36 ± 0.27 | 29.52 ± 0.52 d | 6.12 ± 0.72 | 8.74 ± 0.63 c | 19.83 ± 1.35 | 25.84 ± 1.24 d | 17.50 ± 0.92 | 28.49 ± 1.39 d |
| E-LERW[L] | 22.85 ± 0.36 | 23.07 ± 0.27 e | 6.27 ± 0.63 | 11.64 ± 1.14 d | 18.85 ± 0.83 | 39.56 ± 2.34 f | 18.23 ± 1.46 | 36.44 ± 2.12 f |
| Group | TG (mmol/L) | TC (mmol/L) | HDL (mmol/L) | LDL (mmol/L) |
|---|---|---|---|---|
| NC | 0.727 ± 0.13 a | 0.409 ± 0.09 a | 5.673 ± 1.21 a | 0.526 ± 0.14 a |
| MC | 1.484 ± 0.52 c | 1.162 ± 0.11 d | 2.211 ± 0.35 e | 2.436 ± 0.25 e |
| PC | 0.734 ± 0.23 a | 0.411 ± 0.17 a | 4.521 ± 0.56 b | 0.885 ± 0.22 b |
| AC | 1.174 ± 0.2 b | 0.848 ± 0.24 c | 2.982 ± 0.28 d | 2.045 ± 0.31 c |
| E-LERW[H] | 0.786 ± 0.15 a | 0.573 ± 0.16 b | 3.897 ± 0.51 c | 0.961 ± 0.13 b |
| E-LERW[M] | 1.293 ± 0.03 b | 0.782 ± 0.22 c | 2.794 ± 0.17 d | 1.876 ± 0.47 c |
| E-LERW[L] | 1.423 ± 0.21 c | 1.149 ± 0.12 d | 2.355 ± 0.33 e | 2.378 ± 0.36 e |
| Group | Liver (%) | Kidney (%) |
|---|---|---|
| NC | 4.76 ± 0.34 a | 1.47 ± 0.25 a |
| MC | 6.33 ± 0.41 f | 2.45 ± 0.37 d |
| PC | 5.16 ± 0.18 b | 1.59 ± 0.48 a |
| AC | 6.06 ± 0.07 e | 2.22 ± 0.28 c |
| E-LERW[H] | 5.32 ± 0.16 c | 1.87 ± 0.23 b |
| E-LERW[M] | 5.78 ± 0.37 d | 2.11 ± 0.14 c |
| E-LERW[L] | 6.37 ± 0.73 f | 2.42 ± 0.21 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zhou, T.; Xing, H.; Zhang, Y.; Fang, J.; Kang, T.; Yao, C.; Yan, J.; Huang, Y.; Yao, Q. Antioxidant and In Vivo Hypoglycemic Activities of Ethanol Extract from the Leaves of Engelhardia roxburghiana Wall, a Comparative Study of the Extract and Astilbin. Foods 2023, 12, 927. https://doi.org/10.3390/foods12050927
Guo X, Zhou T, Xing H, Zhang Y, Fang J, Kang T, Yao C, Yan J, Huang Y, Yao Q. Antioxidant and In Vivo Hypoglycemic Activities of Ethanol Extract from the Leaves of Engelhardia roxburghiana Wall, a Comparative Study of the Extract and Astilbin. Foods. 2023; 12(5):927. https://doi.org/10.3390/foods12050927
Chicago/Turabian StyleGuo, Xiaoqiang, Ting Zhou, Hongxia Xing, Yucheng Zhang, Jingmei Fang, Tairan Kang, Caimei Yao, Jun Yan, Yaxuan Huang, and Qian Yao. 2023. "Antioxidant and In Vivo Hypoglycemic Activities of Ethanol Extract from the Leaves of Engelhardia roxburghiana Wall, a Comparative Study of the Extract and Astilbin" Foods 12, no. 5: 927. https://doi.org/10.3390/foods12050927
APA StyleGuo, X., Zhou, T., Xing, H., Zhang, Y., Fang, J., Kang, T., Yao, C., Yan, J., Huang, Y., & Yao, Q. (2023). Antioxidant and In Vivo Hypoglycemic Activities of Ethanol Extract from the Leaves of Engelhardia roxburghiana Wall, a Comparative Study of the Extract and Astilbin. Foods, 12(5), 927. https://doi.org/10.3390/foods12050927