Relative Bioavailability of Cadmium in Rice: Assessment, Modeling, and Application for Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Rice Samples
2.2. Chemical Analysis of Rice Samples
2.3. Relative Bioavailability of Cd in Rice
2.4. Prediction Model of Cd-RBA in Rice
2.5. Calculation of Weekly Dietary Cd Intake
2.6. Statistical Analysis
3. Results
3.1. Chemical Compositions in Rice
3.2. Relative Bioavailability of Cd in Rice
3.3. Using Rice Compositions to Predict Cd-RBA in Rice
3.4. Health Risk Assessment Based on Total Cd and Bioavailable Cd in Rice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and health risk assessment of heavy metals in China’s lead-zinc mine tailings: A meta-analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.; Williams, D.J.; Moore, M.R. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol. Lett. 2003, 137, 65–83. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Adams, S.V.; Quraishi, S.M.; Shafer, M.M.; Passarelli, M.N.; Freney, E.P.; Chlebowski, R.T.; Luo, J.; Meliker, J.R.; Mu, L.; Neuhouser, M.L.; et al. Dietary cadmium exposure and risk of breast, endometrial, and ovarian cancer in the Women’s Health Initiative. Environ. Health Perspect. 2014, 122, 594–600. [Google Scholar] [CrossRef] [Green Version]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, P.; Zhao, F.-J. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2022, 53, 939–963. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92–93, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Kobayashi, E.; Suwazono, Y.; Uetani, M.; Inaba, T.; Oishi, M.; Kido, T.; Nakagawa, H.; Nogawa, K. Association between lifetime cadmium intake and cadmium concentration in individual urine. Bull. Environ. Contam. Toxicol. 2005, 74, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, K.; Sakurai, M.; Ishizaki, M.; Kido, T.; Nakagawa, H.; Suwazono, Y. Threshold limit values of the cadmium concentration in rice in the development of itai-itai disease using benchmark dose analysis. J. Appl. Toxicol. 2017, 37, 962–966. [Google Scholar] [CrossRef]
- Li, H.B.; Li, M.Y.; Zhao, D.; Li, J.; Li, S.W.; Juhasz, A.L.; Basta, N.T.; Luo, Y.M.; Ma, L.Q. Oral bioavailability of As, Pb, and Cd in contaminated soils, dust, and foods based on animal bioassays: A review. Environ. Sci. Technol. 2019, 53, 10545–10559. [Google Scholar] [CrossRef]
- Wang, M.Y.; Li, M.Y.; Ning, H.; Xue, R.Y.; Liang, J.H.; Wang, N.; Luo, X.S.; Li, G.; Juhasz, A.L.; Ma, L.Q.; et al. Cadmium oral bioavailability is affected by calcium and phytate contents in food: Evidence from leafy vegetables in mice. J. Hazard. Mater. 2022, 424, 127373. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.Y.; Xiang, P.; Juhasz, A.L.; Huang, L.; Luo, J.; Li, H.B.; Ma, L.Q. Applying cadmium relative bioavailability to assess dietary intake from rice to predict cadmium urinary excretion in nonsmokers. Environ. Sci. Technol. 2017, 51, 6756–6764. [Google Scholar] [CrossRef] [PubMed]
- Amzal, B.; Julin, B.; Vahter, M.; Wolk, A.; Johanson, G.; Akesson, A. Population toxicokinetic modeling of cadmium for health risk assessment. Environ. Health Perspect. 2009, 117, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.; Wang, Y.; Deng, Z.; Wu, Q.; Fang, M.; Wu, Y.; Gong, Z. Study on the bioaccessibility and bioavailability of Cd in contaminated rice in vitro and in vivo. J. Food Sci. 2021, 86, 3730–3742. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Sun, S.; Zhou, X.; Mao, P.; McBride, M.B.; Zhang, C.; Li, Y.; Xia, H.; Li, Z. Bioavailability and bioaccessibility of cadmium in contaminated rice by in vivo and in vitro bioassays. Sci. Total Environ. 2020, 719, 137453. [Google Scholar] [CrossRef]
- Li, H.B.; Li, M.Y.; Zhao, D.; Li, J.; Li, S.W.; Xiang, P.; Juhasz, A.L.; Ma, L.Q. Arsenic, lead, and cadmium bioaccessibility in contaminated soils: Measurements and validations, Crit. Rev. Environ. Sci. Technol. 2020, 50, 1303–1338. [Google Scholar] [CrossRef]
- Ma, R.; Shen, J.; Wu, J.; Tang, Z.; Shen, Q.; Zhao, F.J. Impact of agronomic practices on arsenic accumulation and speciation in rice grain. Environ. Pollut. 2014, 194, 217. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, P.; Zhang, S.; Dai, J.; Chen, H.P.; Lombi, E.; Howard, D.L.; van der Ent, A.; Zhao, F.J.; Kopittke, P.M. Chemical speciation and distribution of cadmium in rice grain and implications for bioavailability to humans. Environ. Sci. Technol. 2020, 54, 12072–12080. [Google Scholar] [CrossRef]
- GB 5009.153-2016; National Food Safety Standard-Determination of Phytic Acid in Foods. Standard Press of China: Beijing, China, 2016.
- Bruno, E.; Choi, Y.S.; Chung, I.K.; Kim, K.M. QTLs and analysis of the candidate gene for amylose, protein, and moisture content in rice (Oryza sativa L.). 3 Biotech 2017, 7, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Juhasz, A.L.; Luo, J.; Huang, L.; Luo, X.S.; Li, H.B.; Ma, L.Q. Mineral dietary supplement to decrease cadmium relative bioavailability in rice based on a mouse bioassay. Environ. Sci. Technol. 2017, 51, 12123–12130. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Y.; Mao, W.; Sui, H.; Yong, L.; Yang, D.; Jiang, D.; Zhang, L.; Gong, Y. Dietary cadmium exposure assessment among the Chinese population. PLoS ONE 2017, 12, 0177978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Wang, J.Y.; Tang, N.; Yin, D.X.; Luo, J.; Xiang, P.; Juhasz, A.L.; Li, H.B.; Ma, L.Q. Coupling bioavailability and stable isotope ratio to discern dietary and non-dietary contribution of metal exposure to residents in mining-impacted areas. Environ. Int. 2018, 120, 563–571. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Nutritional status affects the absorption and whole-body and organ retention of cadmium in rats fed rice-based diets. Environ. Sci. Technol. 2002, 36, 2684–2692. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Bioavailability as an issue in risk assessment and management of food cadmium: A review. Sci. Total Environ. 2008, 398, 13–19. [Google Scholar] [CrossRef]
- Reeves, P.G.; Chaney, R.L. Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets. Environ. Res. 2004, 96, 311–322. [Google Scholar] [CrossRef]
- Tallkvist, J.; Bowlus, C.L.; Lonnerdal, B. DMT1 gene expression and cadmium absorption in human absorptive enterocytes. Toxicol. Lett. 2001, 122, 171–177. [Google Scholar] [CrossRef]
- Brzoska, M.M.; Moniuszko-Jakoniuk, J. The influence of calcium content in diet on cumulation and toxicity of cadmium in the organism. Arch. Toxicol. 1998, 72, 63–73. [Google Scholar] [CrossRef]
- Larsson, S.E.; Piscator, M. Effect of cadmium on skeletal tissue in normal and calcium-deficient rats. Isr. J. Med. Sci. 1971, 7, 495–498. [Google Scholar]
- Omori, M.; Muto, Y. Effects of dietary protein, calcium, phosphorus and fiber on renal accumulation of exogenous cadmium in young rats. J. Nutr. Sci. Vitaminol. 1977, 23, 361–373. [Google Scholar] [CrossRef]
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Lee, H.H.; Loh, S.P.; Bong, C.F.; Sarbini, S.R.; Yiu, P.H. Impact of phytic acid on nutrient bioaccessibility and antioxidant properties of dehusked rice. J. Food Sci. Technol. 2015, 52, 7806–7816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turecki, T.; Ewan, R.C.; Stahr, H.M. Effect of phytic acid and calcium on the intestinal absorption of cadmium in vitro. Bull. Environ. Contam. Toxicol. 1994, 53, 464–470. [Google Scholar] [CrossRef] [PubMed]
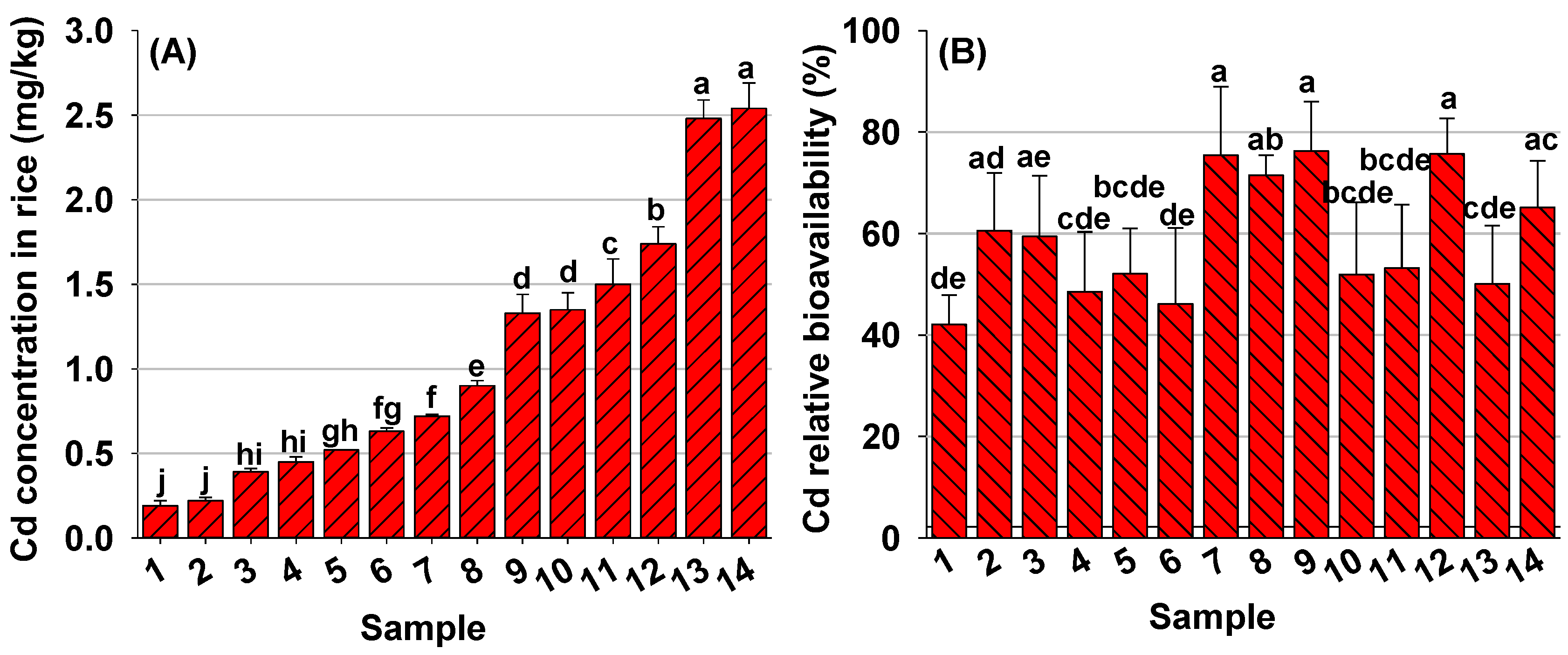
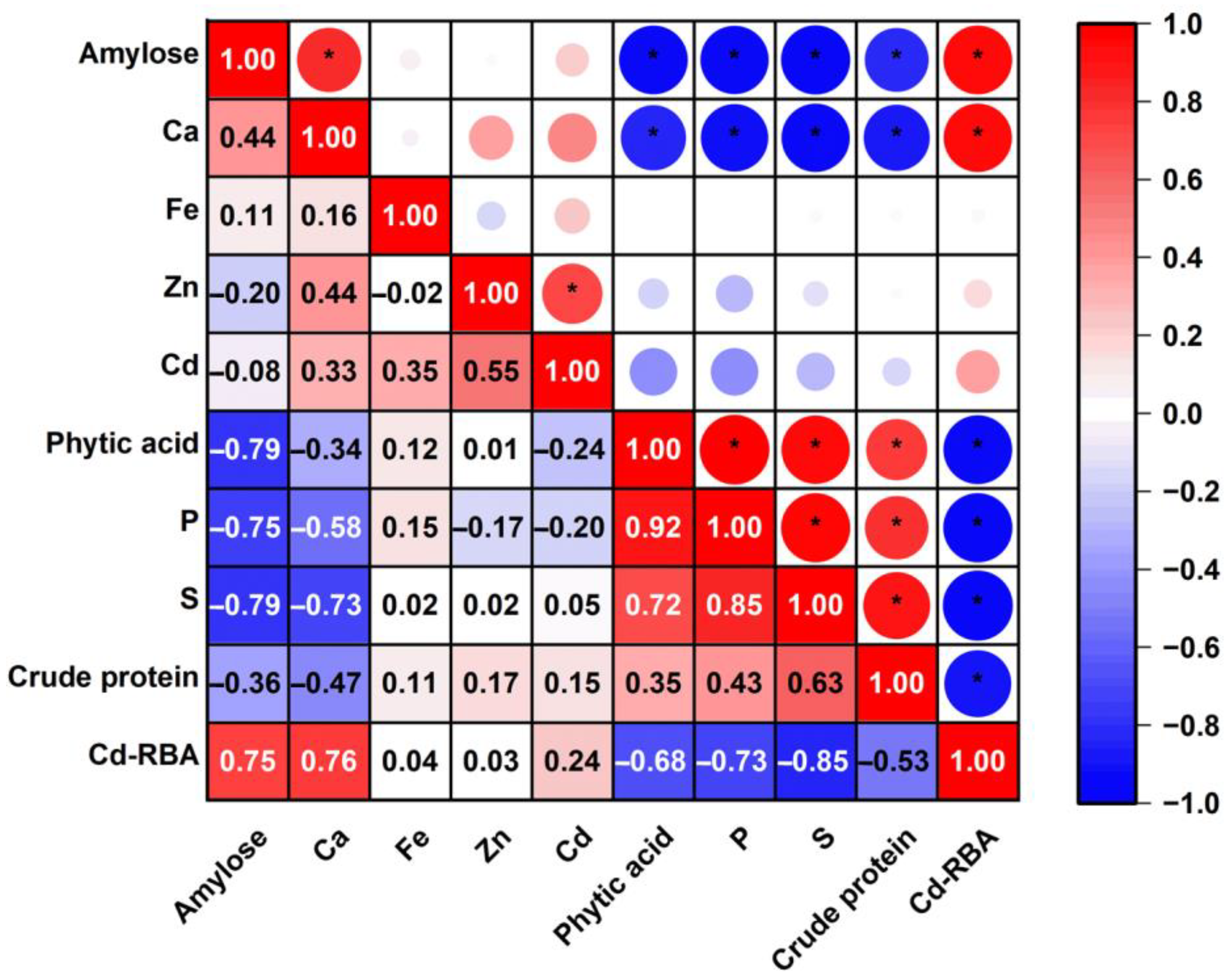
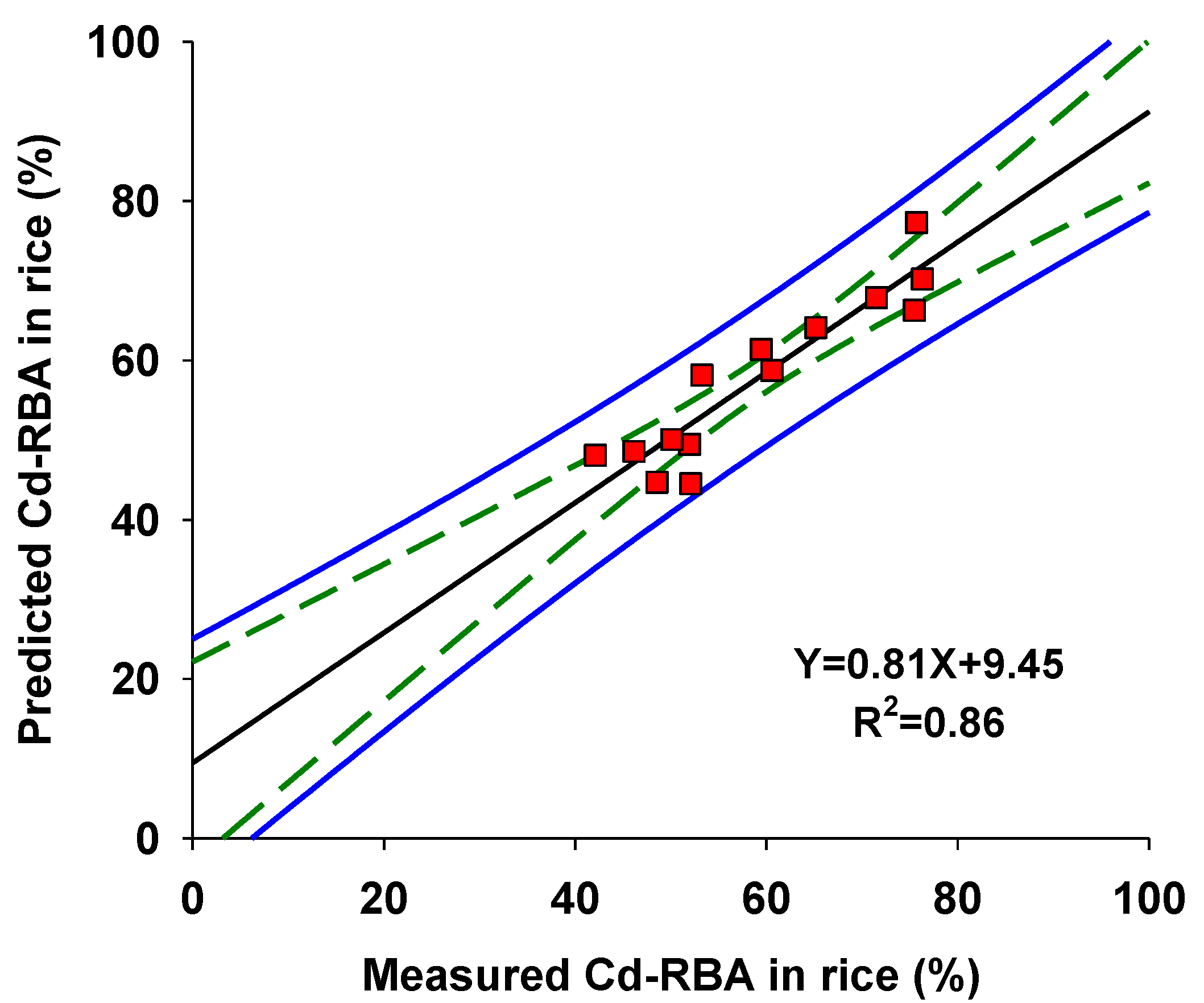
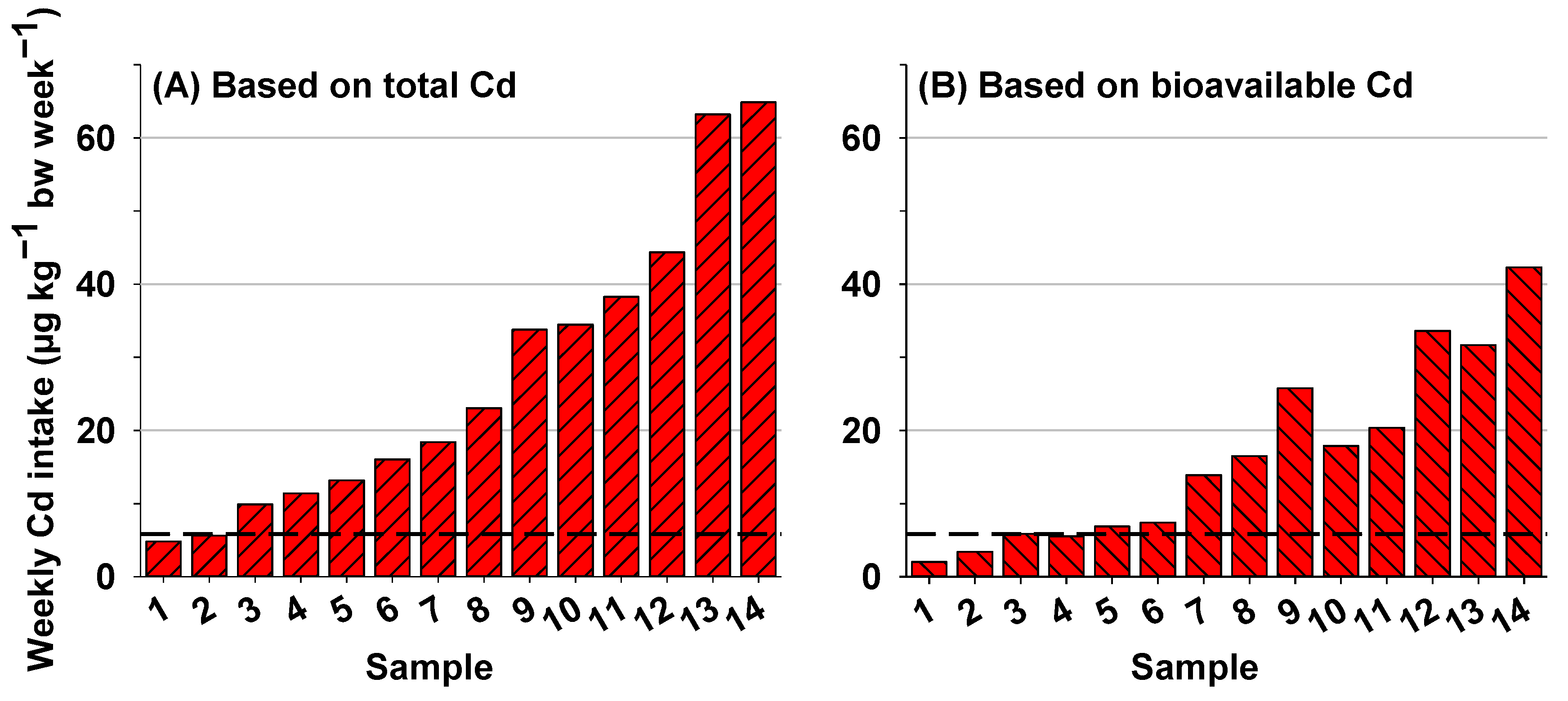
| Compositions | Mean ± SD | Range |
|---|---|---|
| Cd (mg/kg) | 1.07 ± 0.78 | 0.19–2.54 |
| Ca (mg/kg) | 120.16 ± 31.20 | 79.49–165.71 |
| Fe (mg/kg) | 5.07 ± 1.32 | 2.60–7.37 |
| Zn (mg/kg) | 14.33 ± 2.88 | 11.35–21.22 |
| S (mg/kg) | 1499.72 ± 197.52 | 1183.81–1813.53 |
| P (mg/kg) | 1393.48 ± 434.01 | 878.93–2345.57 |
| Crude protein (%) | 10.66 ± 0.70 | 9.31–12.36 |
| Amylose (%) | 26.32 ± 4.62 | 18.55–32.64 |
| Phytic acid (g/kg) | 1.59 ± 0.56 | 0.91–2.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, X.; Zhao, D.; Wang, P.; Zhao, F. Relative Bioavailability of Cadmium in Rice: Assessment, Modeling, and Application for Risk Assessment. Foods 2023, 12, 984. https://doi.org/10.3390/foods12050984
Yang L, Zhang X, Zhao D, Wang P, Zhao F. Relative Bioavailability of Cadmium in Rice: Assessment, Modeling, and Application for Risk Assessment. Foods. 2023; 12(5):984. https://doi.org/10.3390/foods12050984
Chicago/Turabian StyleYang, Likun, Xiaoyue Zhang, Di Zhao, Peng Wang, and Fangjie Zhao. 2023. "Relative Bioavailability of Cadmium in Rice: Assessment, Modeling, and Application for Risk Assessment" Foods 12, no. 5: 984. https://doi.org/10.3390/foods12050984
APA StyleYang, L., Zhang, X., Zhao, D., Wang, P., & Zhao, F. (2023). Relative Bioavailability of Cadmium in Rice: Assessment, Modeling, and Application for Risk Assessment. Foods, 12(5), 984. https://doi.org/10.3390/foods12050984







