The Extract of Perilla frutescens Seed Residue Attenuated the Progression of Aberrant Crypt Foci in Rat Colon by Reducing Inflammatory Processes and Altered Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Determination of Phenolic Compound
2.3. HPLC Fingerprint Analysis
2.4. Animal Model
2.5. Inhibitory Activities of PCE on Aberrant Crypt Foci Progression
2.6. Aberrant Crypt Foci Analysis
2.7. Gut Microbiome Analysis
2.8. Induction of Short-Term Colonic Inflammation and PCE Administration
2.9. Evaluation of Inflammatory Related Cytokines
2.10. Cell Lines and Culturing
2.11. Inflammation-Induced Cell Proliferation
2.12. Inflammatory Cytokine Production
2.13. Statistical Analysis
3. Results
3.1. The Extraction Yield of Perilla Seed Residue
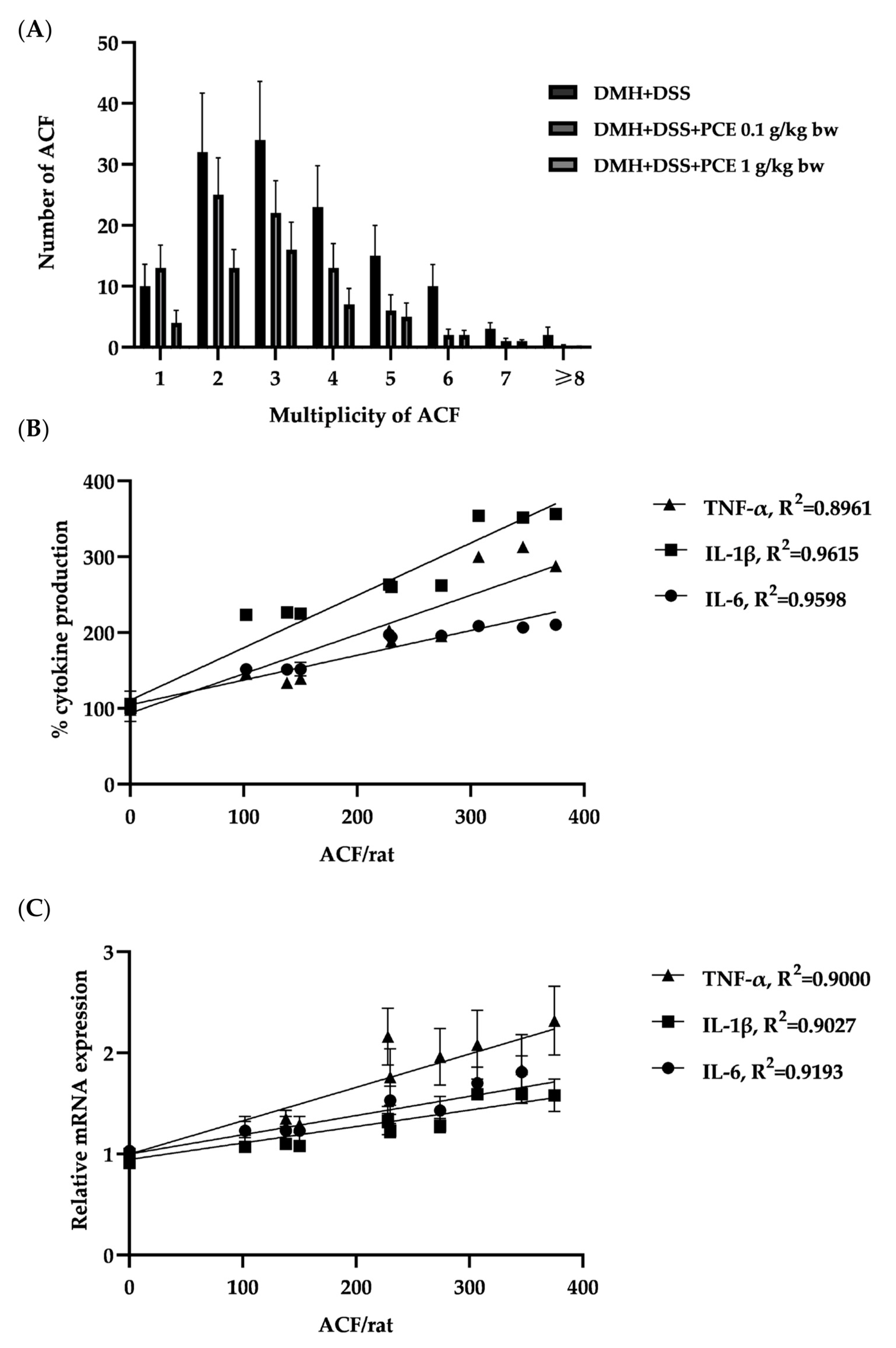
3.2. Effect of PCE on DMH and DSS Induced Rat ACF Progression
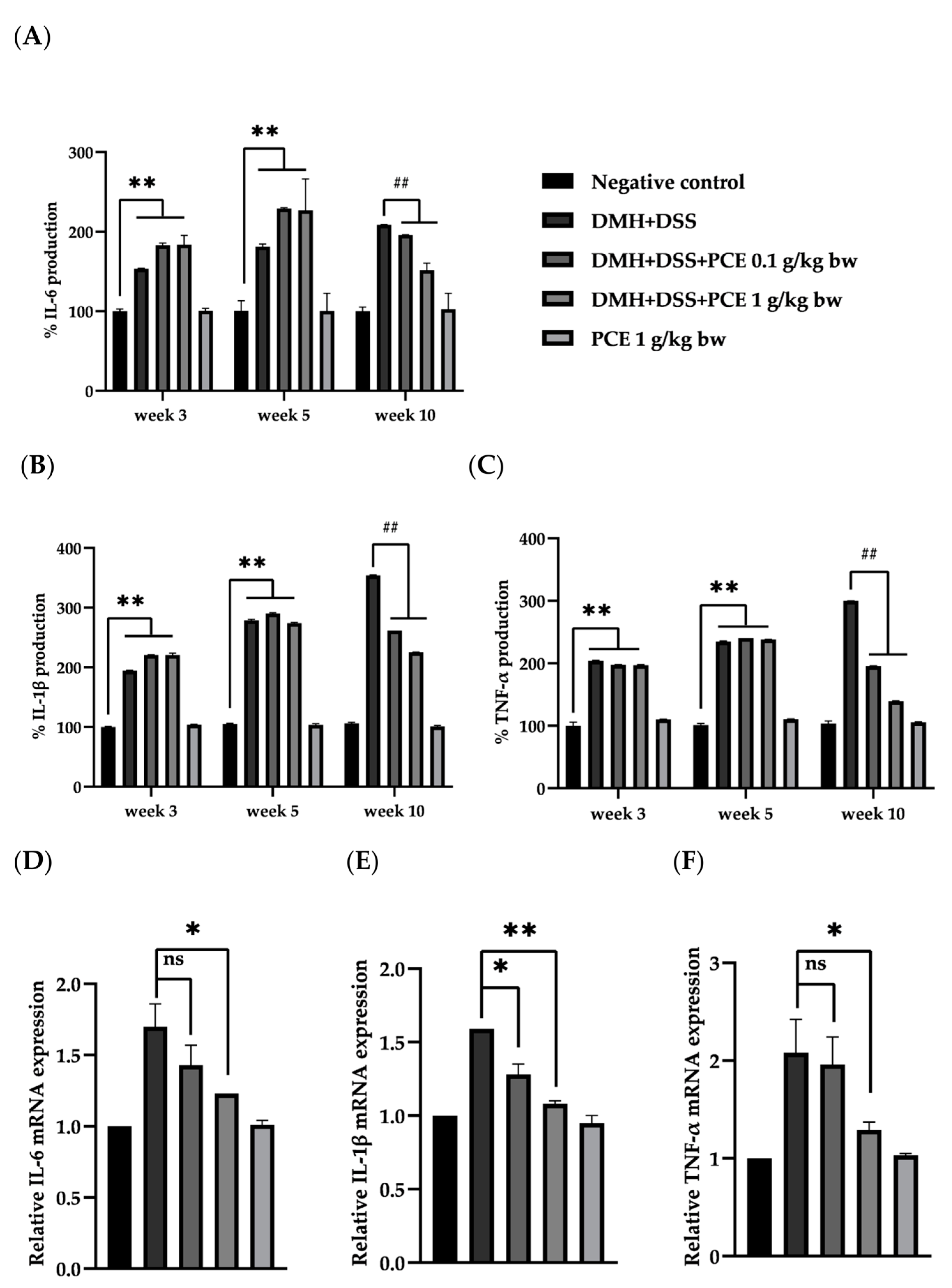
3.3. The Effect of PCE on the Level of Srum Pro-Inflammatory Cytokines
3.4. The Effect of PCE on the Expresion of Inflammatory Cytokine in Colonic Epithelial Cells
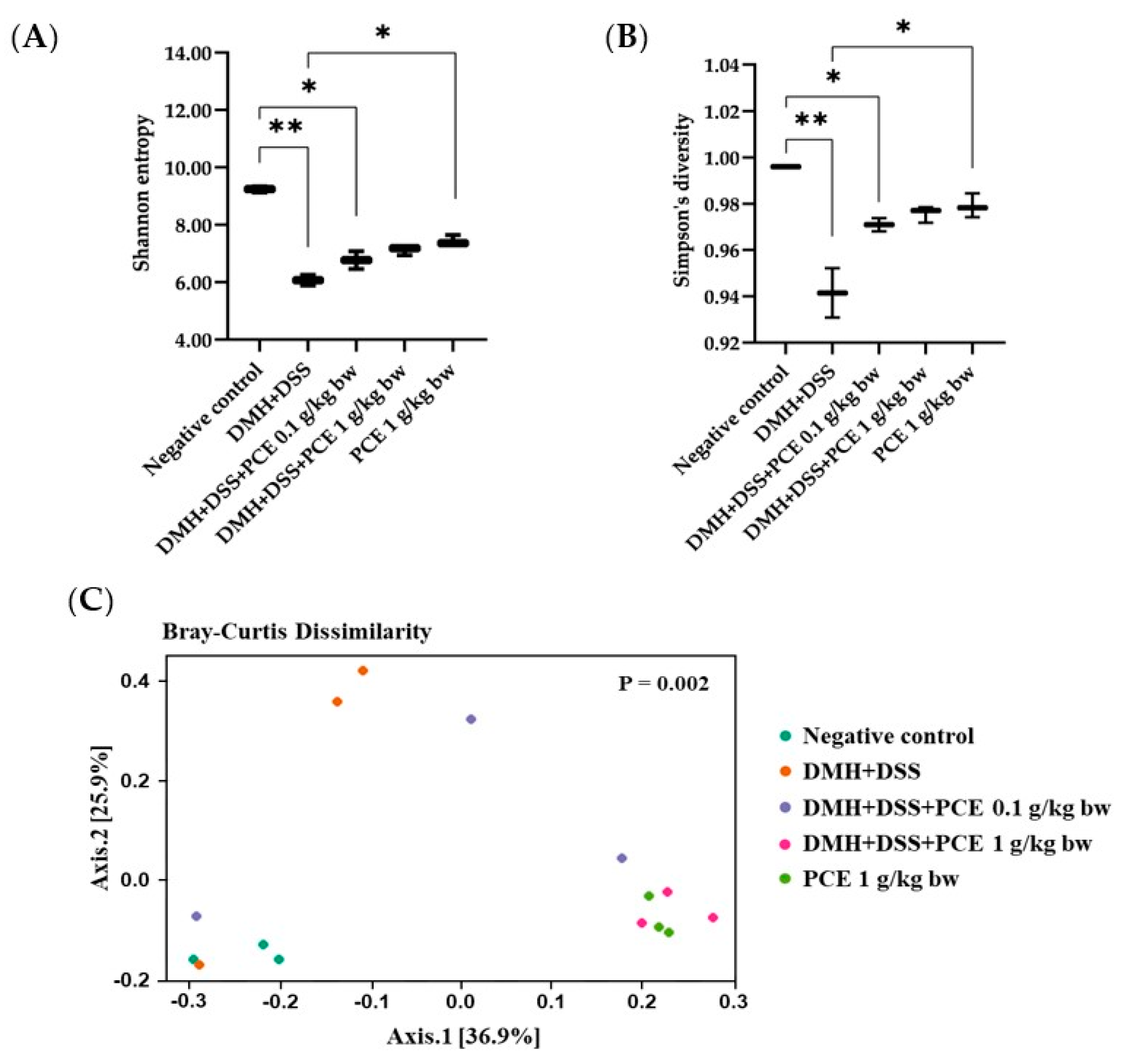
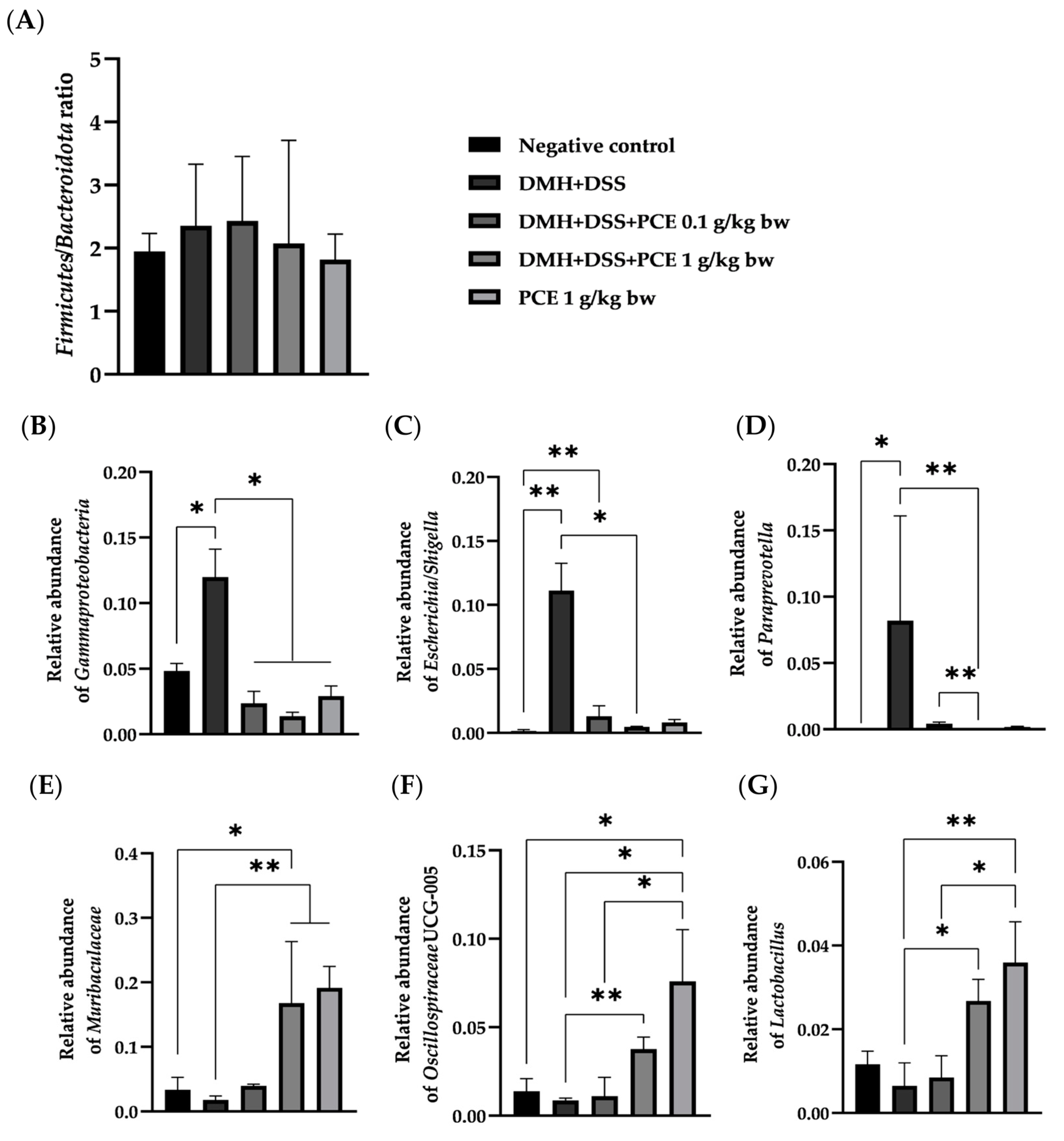
3.5. Altered Rat Gut Microbiota Composition by Reduction of the Dysbiotic Gut Marker in Rats
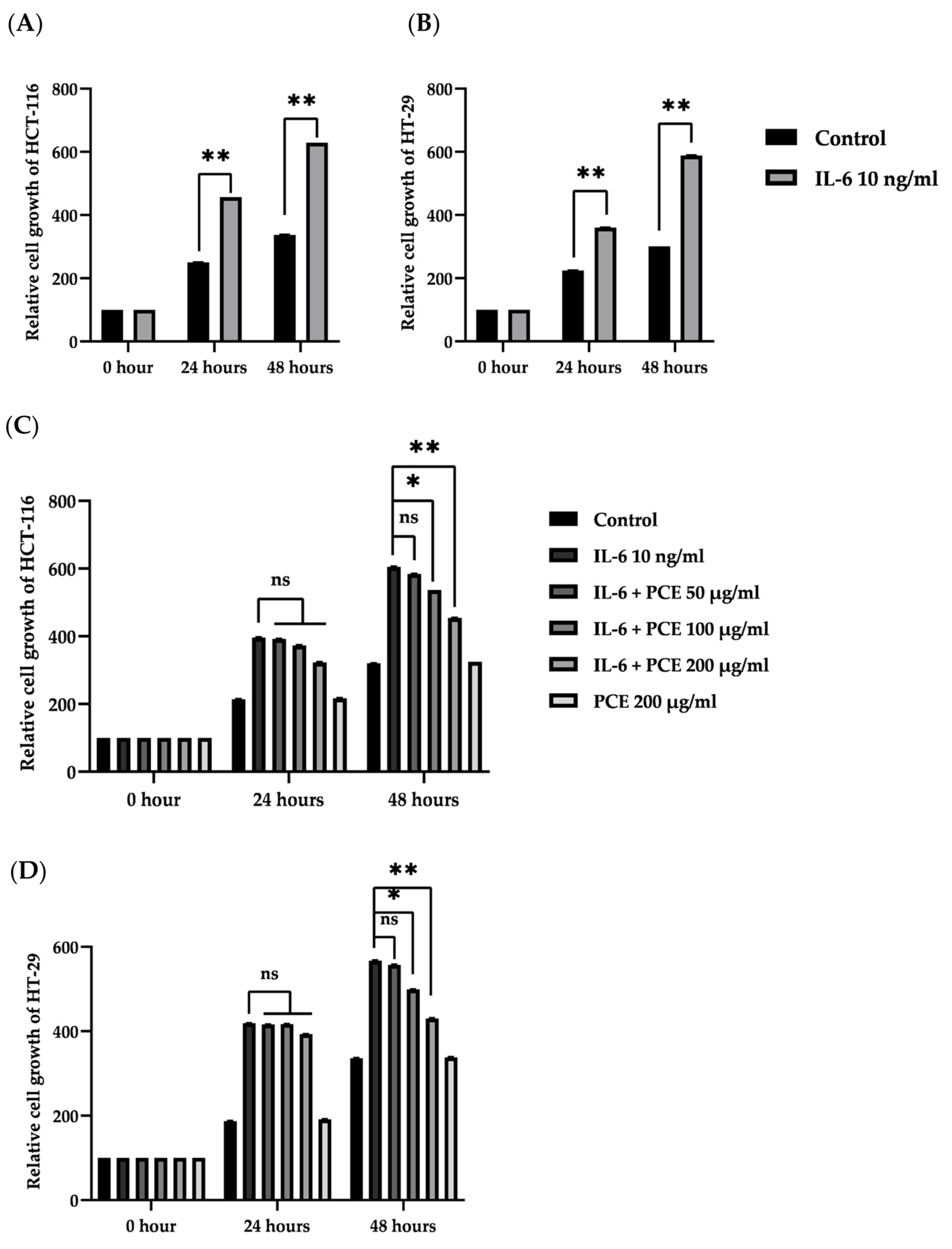
3.6. The Effect of PCE on Cell Proliferation of Colon Cancer Cell Lines
3.7. The Effect of PCE on the Secretion of Pro-Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klampfer, L. Cytokines, Inflammation and Colon Cancer. Curr. Cancer Drug Targets 2011, 11, 451–464. [Google Scholar] [CrossRef]
- Askling, J.; Dickman, P.W.; Ekbom, A.; Karlén, P.; Broström, O.; Lapidus, A.; Löfberg, R. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology 2001, 120, 1356–1362. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Burkholder, B.; Huang, R.Y.; Burgess, R.; Luo, S.; Jones, V.S.; Zhang, W.; Lv, Z.-Q.; Gao, C.-Y.; Wang, B.-L.; Zhang, Y.-M.; et al. Tumor-induced perturbations of cytokines and immune cell networks. BBA Rev. Cancer 2014, 1845, 182–201. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Lai, C.S.; Ho, C.T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Taghinezhad, -S.S.; Mohseni, A.H.; Fu, X. Intervention on gut microbiota may change the strategy for management of colorectal cancer. J. Gastroenterol. Hepatol. 2021, 36, 1508–1517. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Wu, F.; Wang, X. Alterations in the Gut Microbiota and Their Metabolites in Colorectal Cancer: Recent Progress and Future Prospects. Front. Oncol. 2022, 12, 84155. [Google Scholar] [CrossRef]
- Jun, H.-I.; Kim, B.-T.; Song, G.-S.; Kim, Y.-S. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var. acuta) leaves. Food Chem. 2014, 148, 367–372. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules 2008, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.P.; Lin, C.H.; Chen, Y.C.; Kao, S.H. Anti-inflammatory effects of Perilla frutescens leaf extract on lipopolysaccharide-stimulated RAW264.7 cells. Mol. Med. Rep. 2014, 10, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Kuo, C.L.; Wang, J.P.; Cheng, J.S.; Huang, Z.W.; Chen, C.F. Growth inhibitory and apoptosis inducing effect of Perilla frutescens extract on human hepatoma HepG2 cells. J. Ethnopharmacol. 2007, 112, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Khanaree, C.; Pintha, K.; Tantipaiboonwong, P.; Suttajit, M.; Chewonarin, T. The effect of Perilla frutescens leaf on 1, 2-dimethylhydrazine-induced initiation of colon carcinogenesis in rats. J. Food Biochem. 2018, 14, e12493. [Google Scholar] [CrossRef]
- Kwak, Y.; Ju, J. Inhibitory activities of Perilla frutescens britton leaf extract against the growth, migration, and adhesion of human cancer cells. Nutr. Res. Pract. 2015, 9, 11–16. [Google Scholar] [CrossRef]
- Dhyani, A.; Chopra, R.; Garg, M. A Review on Nutritional Value, Functional Properties and Pharmacological Application of Perilla (Perilla frutescens, L.). Biomed. Pharmacol. J. 2019, 12, 649–660. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef]
- Zhou, X.-J.; Yan, L.-L.; Yin, P.-P.; Shi, L.-L.; Zhang, J.-H.; Liu, Y.-J.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef]
- Tsai, T.H.; Tsai, P.J.; Ho, S.C. Antioxidant and anti-inflammatory activities of several commonly used spices. J. Food Sci. 2005, 70, 93–97. [Google Scholar] [CrossRef]
- Baskar, R.; Shrisakthi, S.; Sathyapriya, B.; Shyampriya, R.; Nithya, R.; Poongodi, P. Antioxidant potential of peel extracts of banana varieties (Musa sapientum). Food Nutr. Sci. 2011, 2, 1128. [Google Scholar]
- Pintha, K.; Yodkeeree, S.; Pitchakarn, P.; Limtrakul, P. Anti-invasive activity against cancer cells of phytochemicals in red jasmine rice (Oryza sativa L.). Asian Pac. J. Cancer Prev. 2014, 15, 4601–4607. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: Preliminary findings. Cancer Lett. 1987, 37, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P.; Good, C.K. The significance of aberrant crypt foci in understanding the pathogenesis of colon cancer. Toxicol. Lett. 2000, 112, 395–402. [Google Scholar] [CrossRef]
- Robeson, M.S., 2nd; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible sequence taxonomy reference database management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Jang, J.Y.; Ban, Y.-H.; Guo, H.; Shin, K.; Kim, T.-S.; Lee, S.-P.; Choi, J.; An, E.-S.; Seo, D.-W.; et al. Anti-atherosclerotic effects of perilla oil in rabbits fed a high-cholesterol diet. Lab. Anim. Res. J. 2016, 32, 171–179. [Google Scholar] [CrossRef]
- Lee, A.Y.; Choi, J.M.; Lee, M.H.; Lee, J.; Lee, S.; Cho, E.J. Protective effects of perilla oil and alpha linolenic acid on SH-SY5Y neuronal cell death induced by hydrogen peroxide. Nutr. Res. Pract. 2018, 12, 93–100. [Google Scholar] [CrossRef]
- Suttajit, M.; Khanaree, C.; Tantipaiboonwong, P.; Pintha, K. Omega-3, omega-6 fatty acids and nutrients of Nga-mon seeds in Northern Thailand. Naresuan Phayao J. 2015, 8, 80–86. [Google Scholar]
- Wangwongpaibul, S. Effect of Bioactive Rich Fraction from Perilla frutescens Seed Residue on Human Colon Cancer Cell Line. Master’ s Thesis, Chiang Mai University, Chiang Mai, Thailand, June 2020. [Google Scholar]
- Brown, W.A.; Skinner, S.A.; Malcontenti-Wilson, C.; Misajon, A.; Dejong, T.; Vogiagis, D.; O’Brien, P.E. Non-steroidal anti-inflammatory drugs with different cyclooxygenase inhibitory profiles that prevent aberrant crypt foci formation but vary in acute gastrotoxicity in a rat model. J. Gastroenterol. Hepatol. 2000, 15, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Suzuki, R.; Yasui, Y.; Miyamoto, S.; Wakabayashi, K.; Tanaka, T. Ursodeoxycholic Acid versus Sulfasalazine in Colitis-Related Colon Carcinogenesis in Mice. Clin. Cancer Res. 2007, 13, 2519–2525. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chou, K.; Fuchs, E.; Havran, W.L.; Boismenu, R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 14338–14343. [Google Scholar] [CrossRef]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef]
- Grab, D.J.; Zedeck, M.S. Organ-specific Effects of the Carcinogen Methylazoxymethanol Related to Metabolism by Nicotinamide Adenine Dinucleotide-dependent Dehydrogenases. Cancer Res. 1997, 37, 4182–4189. [Google Scholar]
- Ma, X.; Aoki, T.; Tsuruyama, T.; Narumiya, S. Definition of Prostaglandin E2-EP2 Signals in the Colon Tumor Microenvironment That Amplify Inflammation and Tumor Growth. Cancer Res. 2015, 75, 2822–2832. [Google Scholar] [CrossRef]
- Rogovskii, V. Modulation of Inflammation-Induced Tolerance in Cancer. Front. Immunol. 2020, 11, 1180. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.K.; Bilsland, A.; Georgakilas, A.G.; Amedei, A.; Amin, A.; Bishayee, A.; Azmi, A.S.; Lokeshwar, B.L.; Grue, B.; Panis, C.; et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin. Cancer Biol. 2015, 35, S151–S184. [Google Scholar] [PubMed]
- Coutinho-Wolino, K.S.; Almeida, P.P.; Mafra, D.; Stockler-Pinto, M.B. Bioactive compounds modulating Toll-like 4 receptor (TLR4)-mediated inflammation: Pathways involved and future perspectives. Nutr. Res. 2022, 107, 96–116. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The Role of Probiotics in Colorectal Cancer Management. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–17. [Google Scholar]
- De Weirdt, R.; Van de Wiele, T. Micromanagement in the gut: Microenvironmental factors govern colon mucosal biofilm structure and functionality. NPJ Biofilms Microbiomes 2015, 1, 15026. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006, 66, 605–612. [Google Scholar] [CrossRef]
- Hochdörfer, T.; Kuhny, M.; Zorn, C.N.; Hendriks, R.W.; Vanhaesebroeck, B.; Bohnacker, T.; Krystal, G.; Huber, M. Activation of the PI3K pathway increases TLR-induced TNF-α and IL-6 but reduces IL-1β production in mast cells. Cell Signal 2011, 23, 866–875. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Śliwiński, T.; Zajdel, R. Anti-Inflammatory Activity of Extracts and Pure Compounds Derived from Plants via Modulation of Signaling Pathways, Especially PI3K/AKT in Macrophages. Int. J. Mol. Sci. 2020, 21, 9605. [Google Scholar] [CrossRef]
- Sica, A. Role of tumour-associated macrophages in cancer-related inflammation. Exp. Oncol. 2010, 32, 153–158. [Google Scholar]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Rimando, A.M.; Lee, H.J.; Ji, Y.; Reddy, B.S.; Suh, N. Anti-inflammatory action of pterostilbene is mediated through the p38 mitogen-activated protein kinase pathway in colon cancer cells. Cancer Prev. Res. 2009, 2, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Hodge, D.R.; Hurt, E.M.; Farrar, W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer 2005, 41, 2502–2512. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]


| Group | n | ACF/Rat # | Total Number of ACF # | AC/f # | ||
|---|---|---|---|---|---|---|
| Rectum | Proximal | Distal | ||||
| Negative control | 6 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 |
| DMH + DSS | 6 | 28 ± 10.23 | 201 ± 23.07 | 159 ± 14.63 | 388 ± 90.11 | 3.31 ± 0.41 |
| DMH + DSS + PCE 0.1 g/kg bw | 6 | 19 ± 11.96 * (33.93%) | 115 ± 29.91 ** (42.47%) | 117 ± 7.63 * (26.26%) | 251 ± 56.53 ** (35.20%) | 2.81 ± 0.40 NS (15.12%) |
| DMH + DSS + PCE 1 g/kg bw | 6 | 11 ± 9.29 * (59.82%) | 44 ± 29.45 ** (77.07%) | 75 ± 11.28 ** (53.02%) | 130 ± 31.78 ** (66.46%) | 3.09 ± 0.27 NS (6.69%) |
| PCE 1 g/kg bw | 6 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chantana, W.; Hu, R.; Buddhasiri, S.; Thiennimitr, P.; Tantipaiboonwong, P.; Chewonarin, T. The Extract of Perilla frutescens Seed Residue Attenuated the Progression of Aberrant Crypt Foci in Rat Colon by Reducing Inflammatory Processes and Altered Gut Microbiota. Foods 2023, 12, 988. https://doi.org/10.3390/foods12050988
Chantana W, Hu R, Buddhasiri S, Thiennimitr P, Tantipaiboonwong P, Chewonarin T. The Extract of Perilla frutescens Seed Residue Attenuated the Progression of Aberrant Crypt Foci in Rat Colon by Reducing Inflammatory Processes and Altered Gut Microbiota. Foods. 2023; 12(5):988. https://doi.org/10.3390/foods12050988
Chicago/Turabian StyleChantana, Weerachai, Rentong Hu, Songphon Buddhasiri, Parameth Thiennimitr, Payungsak Tantipaiboonwong, and Teera Chewonarin. 2023. "The Extract of Perilla frutescens Seed Residue Attenuated the Progression of Aberrant Crypt Foci in Rat Colon by Reducing Inflammatory Processes and Altered Gut Microbiota" Foods 12, no. 5: 988. https://doi.org/10.3390/foods12050988
APA StyleChantana, W., Hu, R., Buddhasiri, S., Thiennimitr, P., Tantipaiboonwong, P., & Chewonarin, T. (2023). The Extract of Perilla frutescens Seed Residue Attenuated the Progression of Aberrant Crypt Foci in Rat Colon by Reducing Inflammatory Processes and Altered Gut Microbiota. Foods, 12(5), 988. https://doi.org/10.3390/foods12050988







