Tisochrysis lutea as a Substrate for Lactic Acid Fermentation: Biochemical Composition, Digestibility, and Functional Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
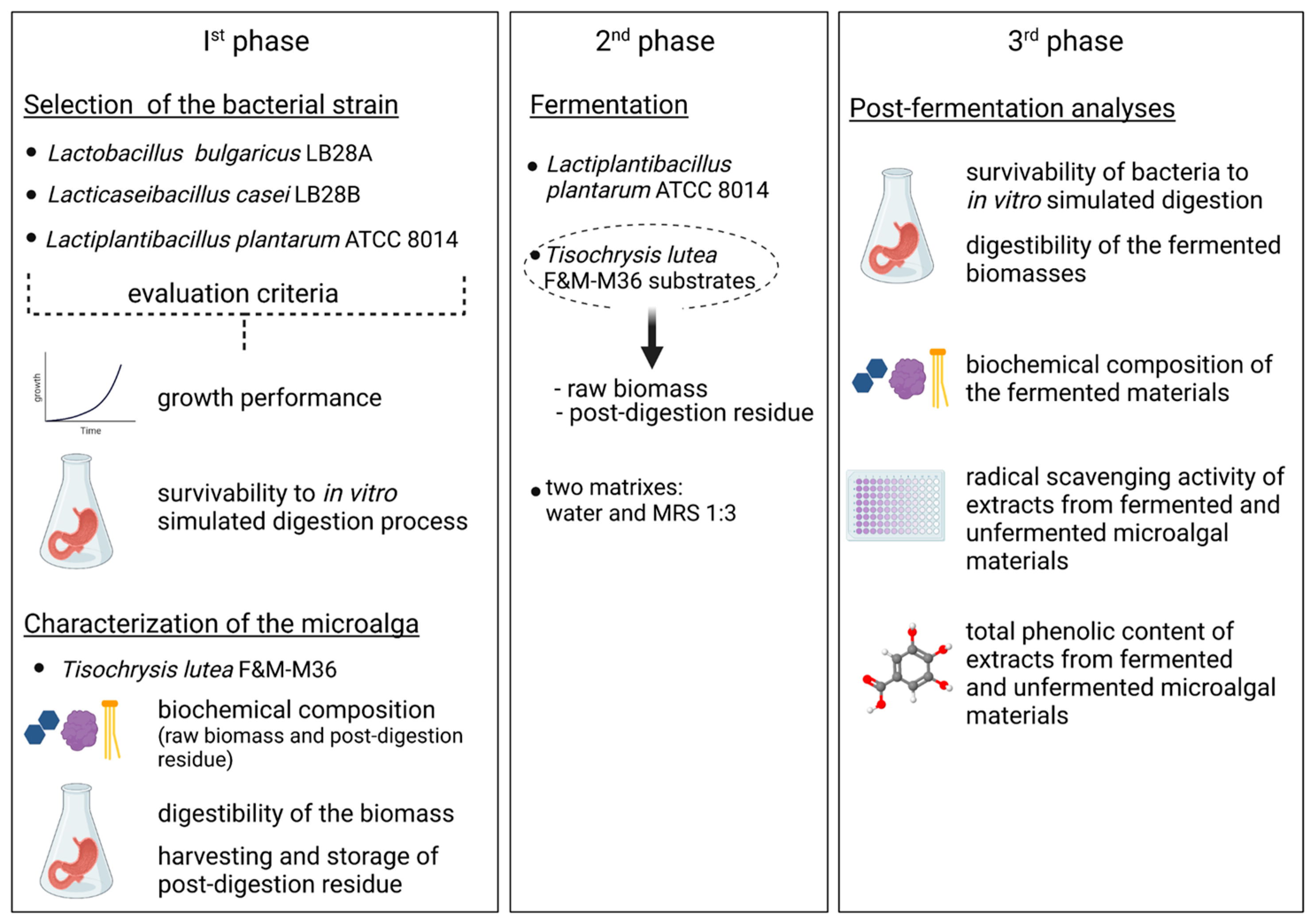
2.2. Experimental Plan
2.3. Characterization of T. lutea F&M-M36 Unfermented and Fermented Materials
2.3.1. Biochemical Composition
2.3.2. In Vitro Digestibility of Microalgal Raw Biomass
2.4. Characterization of Bacterial Strains
2.4.1. Bacterial Growth
2.4.2. Bacterial Survivability to In Vitro Digestion
2.5. Fermentation Trial
2.6. Determination of Extracts Radical Scavenging Activity, Pigment and Total Phenolic Content
2.7. Statistical Analysis
3. Results
3.1. Selection of Bacterial Strain
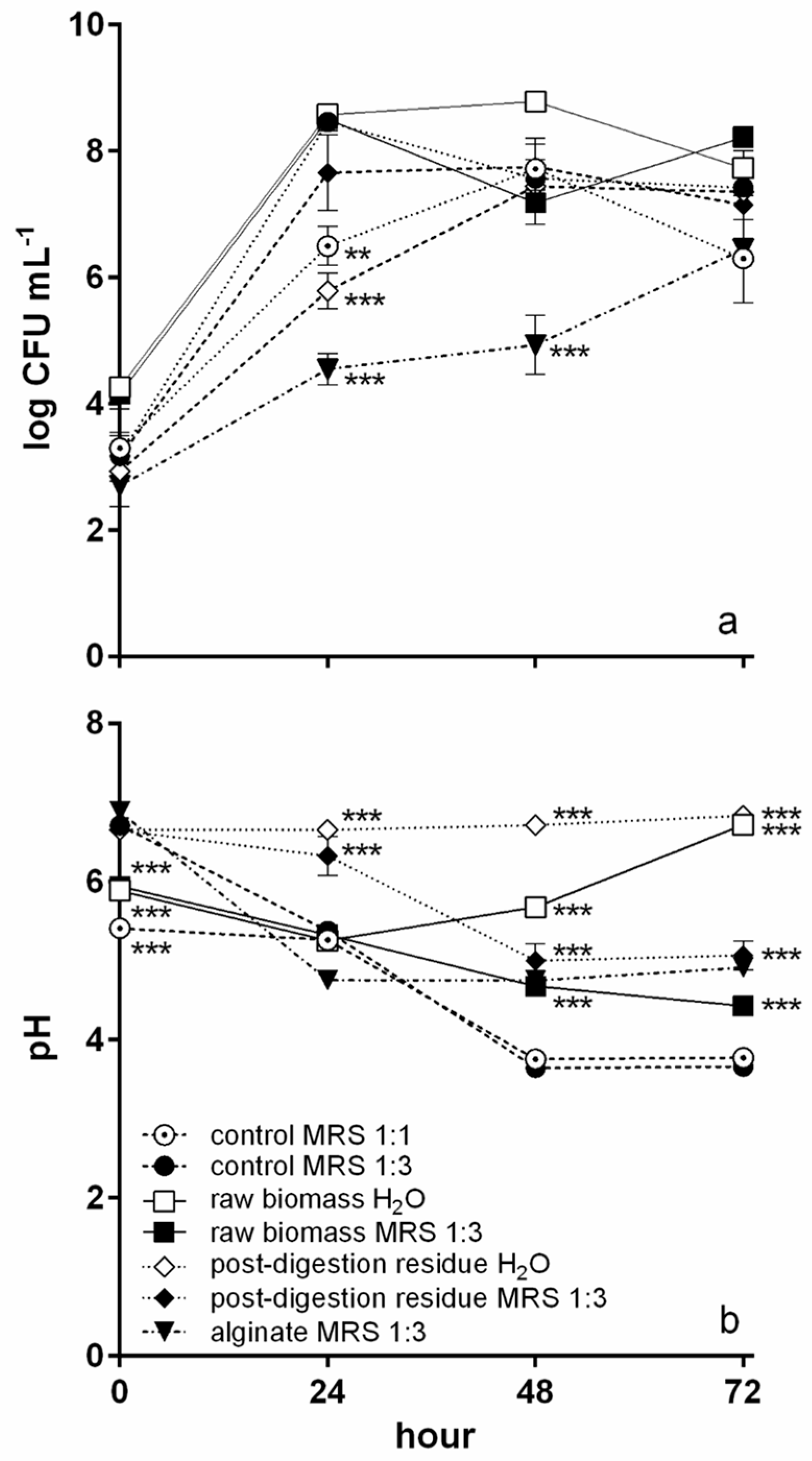
3.2. Fermentation
3.3. Fermented Materials
3.3.1. Biochemical Composition
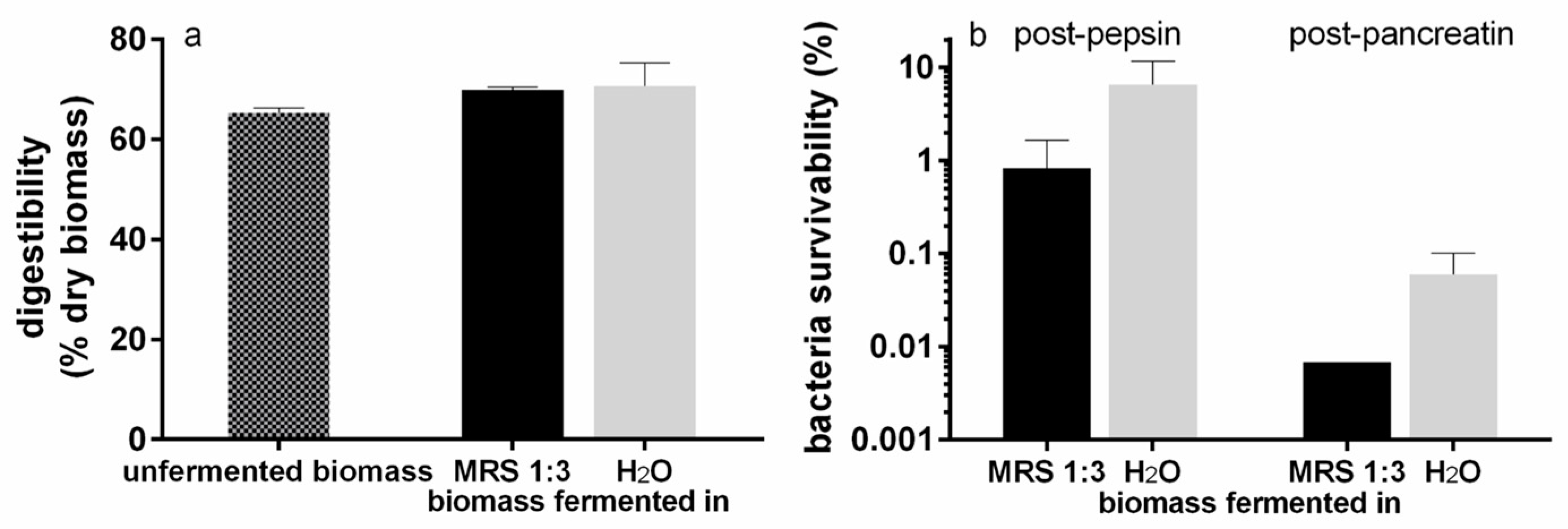
3.3.2. Digestibility of T. lutea F&M-M36 Biomass
3.3.3. Antioxidant Activity of T. lutea F&M-M36 Extracts
3.3.4. Pigment Content of T. lutea F&M-M36 Extracts
3.3.5. Total Phenolic Content of T. lutea F&M-M36 Extracts
4. Discussion
4.1. Probiotic Bacterial Strain Selection
4.2. Fermentation of T. lutea F&M-M36 with L. plantarum ATCC 8014
4.3. Potential Prebiotic Effect of T. lutea F&M-M36
4.4. Characterization of Nutritional and Functional Properties of Unfermented and Fermented T. lutea F&M-M36
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matos, Ậ, P. The impact of microalgae in food science and technology. J. Am. Oil Chem. Soc. 2017, 94, 1333–1350. [Google Scholar] [CrossRef]
- Kaga, Y.; Kuda, T.; Taniguchi, M.; Yamaguchi, Y.; Takenaka, H.; Takahashi, H.; Kimura, B. The effects of fermentation with lactic acid bacteria on the antioxidant and anti-glycation properties of edible cyanobacteria and microalgae. LWT-Food Sci. Technol. 2021, 135, 110029. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Deprá, M.C.; Sartori, R.B.; Dias, R.R.; Zepka, L.Q. Bioactive food compounds from microalgae: An innovative framework on industrial biorefineries. Curr. Opin. Food Sci. 2019, 25, 1–7. [Google Scholar] [CrossRef]
- Lafarga, T.; Rodríguez-Bermúdez, R.; Morillas-España, A.; Villaró, S.; García-Vaquero, M.; Morán, L.; Sánchez-Zurano, A.; González-López, C.V.; Acién-Fernández, F.G. Consumer knowledge and attitudes towards microalgae as food: The case of Spain. Algal Res. 2021, 54, 102174. [Google Scholar] [CrossRef]
- Chini Zittelli, G.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Photobioreactors for mass production of microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley: Oxford, UK, 2013; pp. 225–266. [Google Scholar]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 84. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q. Current status, emerging technologies, and future perspectives of the world microalgal industry. In Book of Abstracts AlgaEurope Conference; European Algae Biomass Association: Florence, Italy, 2019; p. 139. [Google Scholar]
- Niccolai, A.; Bigagli, E.; Biondi, N.; Rodolfi, L.; Cinci, L.; Luceri, C.; Tredici, M.R. In vitro toxicity of microalgal and cyanobacterial strains of interest as food source. J. Appl. Phycol. 2017, 29, 199–209. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 40, 101566. [Google Scholar] [CrossRef]
- Terefe, N.S.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Norici, A.; Mollo, L.; Osimani, A.; Aquilanti, L. Fermentation of microalgal biomass for innovative food production. Microorganisms 2022, 10, 2069. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Shannon, E.; Abu-Ghannam, N.; Biondi, N.; Rodolfi, L.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (spirulina) biomass for probiotic-based products. J. Appl. Phycol. 2019, 31, 1077–1083. [Google Scholar] [CrossRef]
- Niccolai, A.; Bazec, K.; Rodolfi, L.; Biondi, N.; Zlatic, E.; Jamnik, P.; Tredici, M.R. Lactic acid fermentation of Arthrospira platensis (spirulina) in a vegetal soybean drink for developing new functional lactose-free beverages. Front. Microbiol. 2020, 11, 560684. [Google Scholar] [CrossRef]
- Martelli, F.; Alinovi, M.; Bernini, V.; Gatti, M.; Bancalari, E. Arthrospira platensis as natural fermentation booster for milk and soy fermented beverages. Foods 2020, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-state fermentation of Arthrospira platensis to implement new food products: Evaluation of stabilization treatments and bacterial growth on the volatile fraction. Foods 2021, 10, 67. [Google Scholar] [CrossRef]
- Nakashima, A.; Sasaki, K.; Sasaki, D.; Yasuda, K.; Suzuki, K.; Kondo, A. The alga Euglena gracilis stimulates Faecalibacterium in the gut and contributes to increased defecation. Sci. Rep. 2021, 11, 1074. [Google Scholar] [CrossRef]
- Campana, R.; Martinelli, V.; Scoglio, S.; Colombo, E.; Benedetti, S.; Baffone, W. Influence of Aphanizomenon flos-aquae and two of its extracts on growth ability and antimicrobial properties of Lactobacillus acidophilus DDS-1. LWT-Food Sci. Technol. 2017, 81, 291–298. [Google Scholar] [CrossRef]
- Bendif, E.M.; Probert, I.; Schoroeder, D.C.; De Vargas, C. On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J. Appl. Phycol. 2013, 25, 1763–1776. [Google Scholar] [CrossRef]
- Muller-Feuga, A. Microalgae for aquaculture: The current global situation and future trends. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Wiley: Oxford, UK, 2013; pp. 615–627. [Google Scholar]
- Cerri, R.; Niccolai, A.; Cardinaletti, G.; Tulli, F.; Mina, F.; Daniso, E.; Bongiorno, T.; Chini Zittelli, G.; Biondi, N.; Tredici, M.R.; et al. Chemical composition and apparent digestibility of a panel of dried microalgae and cyanobacteria biomasses in rainbow trout (Oncorhynchus mykiss). Aquaculture 2021, 544, 737075. [Google Scholar] [CrossRef]
- Delbrut, A.; Albina, P.; Lapierre, T.; Pradelles, R.; Dubreucq, E. Fucoxanthin and polyunsaturated fatty acids co-extraction by a green process. Molecules 2018, 23, 874. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients 2011, 3, 529–554. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2014, 26, 151–161. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Gomes, A.; Campos, A.M.; Falè, P.; Afonso, C.; Bandarra, N.M. Bioprospection of Isochrysis galbana and its potential as a nutraceutical. Food Funct. 2019, 10, 7333–7342. [Google Scholar] [CrossRef]
- Ran, X.; Shen, Y.; Jiang, D.; Wang, C.; Li, X.; Zhang, H.; Pan, Y.; Xie, C.; Xie, T.; Zhang, Y.; et al. Nutrient deprivation coupled with high light exposure for bioactive chrysolaminarin production in the marine microalga Isochrysis zhangjiangensis. Mar. Drugs 2022, 20, 351. [Google Scholar] [CrossRef]
- Nuno, K.; Villarruel-Lopez, A.; Puebla-Perez, A.M.; Romero-Velarde, E.; Puebla-Mora, A.G.; Ascencio, F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J. Funct. Foods 2013, 5, 106–115. [Google Scholar] [CrossRef]
- Bigagli, E.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; D’Ottavio, M.; D’Ambrosio, M.; Lodovici, M.; Tredici, M.R. Preliminary data on the dietary safety, tolerability and effects on lipid metabolism of the marine microalga Tisochrysis lutea. Algal Res. 2018, 34, 244–249. [Google Scholar] [CrossRef]
- Tredici, M.R.; Bassi, N.; Prussi, M.; Biondi, N.; Rodolfi, L.; Chini Zittelli, G.; Sampietro, G. Energy balance of algal biomass production in a 1-ha “Green Wall Panel” plant: How to produce algal biomass in a closed reactor achieving a high Net Energy Ratio. Appl. Energy 2015, 154, 1103–1111. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Prot. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Boisen, S.; Fernández, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Naissinger da Silva, M.; Lago Tagliapietra, B.; do Amaral Flores, V.; Pereira dos Santos Richards, N.S. In vitro test to evaluate survival in the gastrointestinal tract of commercial probiotics. Curr. Res. Food Sci. 2021, 4, 320–325. [Google Scholar] [CrossRef]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Megazyme. L-lactic Acid (L-lactate) Assay Procedure; K-LATE 06/18; Megazyme: Dublin, Ireland, 2018. [Google Scholar]
- Megazyme. Acetic Acid (Rapid, Manual, Simple and End-Point AK/PTA Format) Assay Procedure; K-ACETRM 04/20; Megazyme: Dublin, Ireland, 2020. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Yan, X.; Nagata, T.; Fan, X. Antioxidative activities in some common seaweeds. Plant Food Hum. Nutr. 1998, 52, 253–262. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional foods: Trends and development of the global market. Ital. J. Food Sci. 2016, 28, 338–351. [Google Scholar]
- Petrescu, D.C.; Vermeir, I.; Petrescu-Mag, R.M. Consumer understanding of food quality, healthiness, and environmental impact: A cross-national perspective. Int. J. Environ. Res. Public Health 2020, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Hallak, R.; Onur, I.; Lee, C. Consumer demand for healthy beverages in the hospitality industry: Examining willingness to pay a premium, and barriers to purchase. PLoS ONE 2022, 17, e0267726. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-dairy fermented beverages as potential carriers to ensure probiotics, prebiotics, and bioactive compounds arrival to the gut and their health benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.S.; Chin, Y.L.; Chai, K.F.; Chen, W.N. Fermentation for future food systems: Precision fermentation can complement the scope and applications of traditional fermentation. EMBO Rep. 2021, 22, e52680. [Google Scholar] [CrossRef]
- Luca, L.; Oroian, M. Influence of different prebiotics on viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus encapsulated in alginate microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef]
- Hwang, C.-F.; Chen, J.-N.; Huang, Y.-T.; Mao, Z.-Y. Biomass production of Lactobacillus plantarum LP02 isolated from infant feces with potential cholesterol-lowering ability. Afr. J. Biotechnol. 2011, 10, 7010–7020. [Google Scholar]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Zotta, T.; Parente, E.; Ricciardi, A. Aerobic metabolism in the genus Lactobacillus: Impact on stress response and potential applications in the food industry. J. Appl. Microbiol. 2017, 122, 857–869. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. BioMed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.L.; Picariello, G.; Coppola, R.; Tremonte, P.; Spagna Musso, S.; Di Luccia, A. Proteolytic activity of Lactobacillus sakei, Lactobacillus farciminis and Lactobacillus plantarum on sarcoplasmic proteins of pork lean. J. Food Biochem. 2004, 28, 195–212. [Google Scholar] [CrossRef]
- Li, C.; Song, J.; Kwok, L.; Wang, J.; Dong, Y.; Yu, H.; Hou, Q.; Zhang, H.; Chen, Y. Influence of Lactobacillus plantarum on yogurt fermentation properties and subsequent changes during postfermentation storage. J. Dairy Sci. 2017, 100, 2512–2525. [Google Scholar] [CrossRef]
- Ma, C.; Cheng, G.; Liu, Z.; Gong, G.; Chen, Z. Determination of the essential nutrients required for milk fermentation by Lactobacillus plantarum. LWT-Food Sci. Technol. 2016, 65, 884–889. [Google Scholar] [CrossRef]
- Dimitrov Todorov, S.; Gombossy De Melo Franco, B.D. Lactobacillus plantarum: Characterization of the species and application in food production. Food Rev. Int. 2010, 26, 205–229. [Google Scholar] [CrossRef]
- Taniguchi, M.; Kuda, T.; Shibayama, J.; Sasaki, T.; Michihata, T.; Takahashi, H.; Kimura, B. In vitro antioxidant, anti-glycation and immunomodulation activities of fermented blue-green algae Aphanizomenon flos-aquae. Mol. Biol. Rep. 2019, 46, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Beheshtipour, H.; Mortazavian, A.M.; Mohammadi, R.; Sohrabvandi, S.; Khosravi-Darani, K. Supplementation of Spirulina platensis and Chlorella vulgaris algae into probiotic fermented milks. Compr. Rev. Food Sci. Food Saf. 2013, 12, 144–154. [Google Scholar] [CrossRef]
- Yun, Y.-M.; Jung, K.-W.; Kim, D.-H.; Oh, Y.-K.; Shin, H.-S. Microalgal biomass as a feedstock for bio-hydrogen production. Int. J. Hydrogen Energy 2012, 12, 15533–15539. [Google Scholar] [CrossRef]
- Choi, S.; Baek, M.; Chung, M.-J.; Lim, S.; Yi, H. Distribution of bacteriocin genes in the lineages of Lactiplantibacillus plantarum. Sci. Rep. 2021, 11, 20063. [Google Scholar] [CrossRef]
- Yao, W.; Yang, L.; Shao, Z.; Xie, L.; Chen, L. Identification of salt tolerance-related genes of Lactobacillus plantarum D31 and T9 strains by genomic analysis. Ann. Microbiol. 2020, 70, 10. [Google Scholar] [CrossRef]
- Vaccalluzzo, A.; Pino, A.; De Angelis, M.; Bautista-Gallego, J.; Romeo, F.V.; Foti, P.; Caggia, C.; Randazzo, C.L. Effects of different stress parameters on growth and on oleuropein-degrading abilities of Lactiplantibacillus plantarum strains selected as tailored starter cultures for naturally table olives. Microorganisms 2020, 8, 1607. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Kermani, Z.J.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; Bernardo de Morais, A.M.M.; Santos Costa de Morais, R.M. Emergent sources of prebiotics: Seaweeds and microalgae. Mar. Drugs 2016, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Barros De Medeiros, V.P.; Leite de Souza, E.; Rodrigues de Albuquerque, T.M.; Da Costa Sassi, C.F.; Dos Santos Lima, M.; Sivieri, K.; Colombo Pimentel, T.; Magnani, M. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Salvetti, E.; Torriani, S.; Felis, G.E. The genus Lactobacillus: A taxonomic update. Probiotic Antimicrob. Proteins 2012, 4, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Okolie, C.L.; Mason, B.; Mohan, A.; Pitts, N.; Udenigwe, C.C. Extraction technology impacts on the structure-function relationship between sodium alginate extracts and their in vitro prebiotic activity. Food Biosci. 2020, 37, 100672. [Google Scholar] [CrossRef]
- Alkhamis, Y.; Qin, J.G. Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. J. Appl. Phycol. 2015, 28, 35–42. [Google Scholar] [CrossRef]
- Da Costa, F.; Petton, B.; Mingant, C.; Bougaran, G.; Rouxel, C.; Quéré, C.; Wikfors, G.H.; Soudant, P.; Robert, R. Influence of one selected Tisochrysis lutea strain rich in lipids on Crassostrea gigas larval development and biochemical composition. Aquac. Nutr. 2016, 22, 813–836. [Google Scholar] [CrossRef]
- Sánchez-Saavedra, M.P.; Maeda-Martínez, A.N.; Acosta-Galindo, S. Effect of different light spectra on the growth and biochemical composition of Tisochrysis lutea. J. Appl. Phycol. 2016, 28, 839–847. [Google Scholar] [CrossRef]
- Bonfanti, C.; Cardoso, C.; Afonso, C.; Matos, J.; Garcia, T.; Tanni, S.; Bandarra, N.M. Potential of microalga Isochrysis galbana: Bioactivity and bioaccessibility. Algal Res. 2018, 29, 242–248. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Messina, M.; Bruno, M.; Tulli, F.; Poli, B.M.; Giorgi, G.; Chini Zittelli, G.; Tredici, M.; Tibaldi, E. Effects of graded levels of a blend of Tisochrysis lutea and Tetraselmis suecica dried biomass on growth and muscle tissue composition of European sea bass (Dicentrarchus labrax) fed diets low in fish meal and oil. Aquaculture 2018, 485, 173–182. [Google Scholar] [CrossRef]
- Almutairi, A.W. Improvement of chemical composition of Tisochrysis lutea grown mixotrophically under nitrogen depletion towards biodiesel production. Molecules 2020, 25, 4609. [Google Scholar] [CrossRef]
- Pleissner, D.; Demichelis, F.; Mariano, S.; Fiore, S.; Navarro Gutiérrez, I.M.; Schneider, R.; Venus, J. Direct production of lactic acid based on simultaneous saccharification and fermentation of mixed restaurant food waste. J. Clean. Prod. 2017, 143, 615–623. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Albers, E.; Undeland, I. In vitro bioaccessibility of proteins and lipids of pH-shift processed Nannochloropsis oculata microalga. Food Funct. 2016, 7, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Ueno-Mohri, T.; Okita, T.; Ishibashi, G. Chemical composition, in vitro protein digestibility and in vitro available iron of blue green alga, Nostoc commune. Plant Food Hum. Nutr. 1990, 40, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry of Health. Linee Guida su Probiotici e Prebiotici; Revisione Marzo; Italian Ministry of Health: Rome, Italy, 2018. [Google Scholar]
- Meireles Mafaldo, Í.; Barros de Medeiros, V.P.; Almeida da Costa, W.K.; da Costa Sassi, C.F.; da Costa Lima, M.; Leite de Souza, E.; Barão, C.E.; Colombo Pimentel, T.; Magnani, M. Survival during long-term storage, membrane integrity, and ultrastructural aspects of Lactobacillus acidophilus 05 and Lacticaseibacillus casei 01 freeze-dried with freshwater microalgae biomasses. Food Res. Int. 2022, 159, 111620. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.-M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as potential sources of bioactive compounds for functional foods and pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Jung, W.-K.; Kang, K.-H.; Ryu, B.; Kim, S.-K.; Je, J.-Y.; Heo, S.-J.; Oh, C.; Kang, D.-H.; Park, W.S.; et al. In vitro antioxidant activities of the fermented marine microalga Pavlova lutheri (Haptophyta) with the yeast Hansenula polymorpha. J. Phycol. 2012, 48, 475–482. [Google Scholar] [CrossRef]







| OD600 | Neubauer Chamber | |
|---|---|---|
| Bacterial Strain | Growth Rate (h−1) | Growth Rate (h−1) |
| L. plantarum ATCC 8014 | 0.33 | 0.48 |
| L. bulgaricus LB28A | 0.24 | 0.33 |
| L. casei LB28B | 0.17 | 0.21 |
| Protein | Carbohydrate | Lipid | Ash | TDF | Total Carotenoids | Fucoxanthin | |
|---|---|---|---|---|---|---|---|
| % (Dry Weight) | mg g–1 (Dry Weight) | ||||||
| Raw biomass | |||||||
| unfermented | 42.0 ± 2.5 | 12.6 ± 0.7 a | 29.3 ± 0.3 | 11.9 | 9.3 | 20.8 ± 0.3 a | 5.86 ± 0.20 a |
| fermented in H2O | 48.6 ± 4.0 | 7.2 ± 0.5 b | 32.6 ± 5.7 | 9.1 | nd | 12.3 ± 1.5 b | 2.00 ± 0.16 b |
| fermented in MRS 1:3 | 48.9 ± 1.1 | 7.9 ± 0.4 b | 33.4 ± 4.4 | 6.2 | nd | 13.3 ± 0.3 b | 1.88 ± 0.03 b |
| Post-digestion residue | |||||||
| unfermented | 45.9 ± 4.1 | 10.2 ± 0.4 a | 30.6 ± 0.3 | 10.7 | 7.4 ± 1.3 | 11.3 ± 1.1 | 1.26 ± 0.01 |
| fermented in H2O | 43.8 ± 1.0 | 8.8 ± 0.8 b | 33.4 ± 3.4 | 5.8 | nd | 11.2 ± 0.4 | 1.19 ± 0.00 |
| fermented in MRS 1:3 | 39.8 ± 1.6 | 8.3 ± 0.1 b | 32.9 ± 6.6 | 5.7 | nd | 11.4 ± 0.0 | 0.85 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnini, C.; Sampietro, G.; Santini, G.; Biondi, N.; Rodolfi, L. Tisochrysis lutea as a Substrate for Lactic Acid Fermentation: Biochemical Composition, Digestibility, and Functional Properties. Foods 2023, 12, 1128. https://doi.org/10.3390/foods12061128
Pagnini C, Sampietro G, Santini G, Biondi N, Rodolfi L. Tisochrysis lutea as a Substrate for Lactic Acid Fermentation: Biochemical Composition, Digestibility, and Functional Properties. Foods. 2023; 12(6):1128. https://doi.org/10.3390/foods12061128
Chicago/Turabian StylePagnini, Caterina, Giacomo Sampietro, Gaia Santini, Natascia Biondi, and Liliana Rodolfi. 2023. "Tisochrysis lutea as a Substrate for Lactic Acid Fermentation: Biochemical Composition, Digestibility, and Functional Properties" Foods 12, no. 6: 1128. https://doi.org/10.3390/foods12061128
APA StylePagnini, C., Sampietro, G., Santini, G., Biondi, N., & Rodolfi, L. (2023). Tisochrysis lutea as a Substrate for Lactic Acid Fermentation: Biochemical Composition, Digestibility, and Functional Properties. Foods, 12(6), 1128. https://doi.org/10.3390/foods12061128






