Effect of Physicochemical Characteristics and Storage Atmosphere on Microbiological Stability and Shelf-Life of Minimally Processed European Sea Bass (Dicentrarchus labrax) Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Provision and Processing
2.2. Physicochemical Analysis
2.2.1. Determination of pH and Water Activity (aw)
2.2.2. Determination of Water Phase Salt (WPS)
2.2.3. Determination of Gas Concentrations within the Packages
2.3. Sensory Evaluation
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics
3.2. Gas Changes throughout Storage
3.3. Sensory Analysis and Determination of Shelf-Life
3.4. Microbiological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tacon, A.G.J.; Metian, M. Food Matters: Fish, Income, and Food Supply—A Comparative Analysis. Rev. Fish. Sci. Aquac. 2018, 26, 15–28. [Google Scholar] [CrossRef]
- Speranza, B.; Bevilacqua, A.; Racioppo, A.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Marinated sea bream fillets enriched with Lactiplantibacillus plantarum and Bifidobacterium animalis subsp. lactis: Brine optimization and product design. Foods 2021, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- FAO. World Fisheries and Aquaculture in Review; FAO: Rome, Italy, 2020; Volume 35, ISBN 9789251326923. [Google Scholar]
- Compassion in Foodbusiness European Seabass (Dicentrarchus Labrax) Statistics Summary. 2021. pp. 1–13. Available online: https://www.compassioninfoodbusiness.com/media/7447727/european-seabass-in-numbers.pdf (accessed on 1 February 2023).
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Boziaris, I.S.; Parlapani, F.F. Specific Spoilage Organisms (SSOs) in Fish. Microbiol. Qual. Food Foodborne Spoilers 2017, 61–98. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Boziaris, I.S. The evolution of knowledge on seafood spoilage microbiota from the 20th to the 21st century: Have we finished or just begun? Trends Food Sci. Technol. 2022, 120, 236–247. [Google Scholar] [CrossRef]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Singh, S.; Shalini, R. Effect of Hurdle Technology in Food Preservation: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 641–649. [Google Scholar] [CrossRef]
- Gautam, R.K.; Kakatkar, A.S.; Mishra, P.K.; Kumar, V.; Chatterjee, S. Development of shelf-stable, ready to cook (RTC) intermediate moisture (IM) shrimp and its shelf life extension using hurdle technology. J. Agric. Food Res. 2021, 6, 100199. [Google Scholar] [CrossRef]
- Ashie, I.N.A.; Simpson, B.K.; Smith, J.P. Mechanisms for controlling enzymatic reactions in foods. Crit. Rev. Food Sci. Nutr. 2009, 36, 1–30. [Google Scholar] [CrossRef]
- Gram, L. Microbiological Spoilage of Fish and Seafood Products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9781441908261. [Google Scholar]
- WHO. 2012 Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Kilinc, B.; Cakli, S. The determination of the shelf-life of pasteurized and non-pasteurized sardine (Sardina pilchardus) marinades stored at 4 °C. Int. J. Food Sci. Technol. 2005, 40, 265–271. [Google Scholar] [CrossRef]
- Gökoglu, N.; Cengiz, E.; Yerlikaya, P. Determination of the shelf life of marinated sardine (Sardina pilchardus) stored at 4 °C. Food Control 2004, 15, 1–4. [Google Scholar] [CrossRef]
- Andrighetto, C.; Lombardi, A.; Ferrati, M.; Guidi, A.; Corrain, C.; Arcangeli, G. Lactic acid bacteria biodiversity in Italian marinated seafood salad and their interactions on the growth of Listeria monocytogenes. Food Control 2009, 20, 462–468. [Google Scholar] [CrossRef]
- FDA. Cfsan Fish and Fishery Products Hazards and Controls Guidance; FDA: Silver Spring, MD, USA, 2011. [Google Scholar]
- Peck, M.W. Clostridium botulinum and the safety of refrigerated processed foods of extended durability. Trends Food Sci. Technol. 1997, 8, 186–192. [Google Scholar] [CrossRef]
- Erickson, M.C.; Ma, L.M.; Doyle, M.P. Clostridium botulinum toxin production in relation to spoilage of Atlantic salmon (salmo salar) packaged in films of varying oxygen permeabilities and with different atmospheres. J. Food Prot. 2015, 78, 2006–2018. [Google Scholar] [CrossRef]
- AOAC Official Method 937.09 Salt (Chlorine as Sodium Chloride) in Seafood-Methods of AOAC-International-Food Laws & Regulations-Documents-Global FoodMate. Available online: http://files.foodmate.com/2013/files_2962.html (accessed on 2 February 2023).
- Gram, L.; Trolle, G.; Huss, H.H. Detection of specific spoilage bacteria from fish stored at low (0°C) and high (20°C) temperatures. Int. J. Food Microbiol. 1987, 4, 65–72. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbiological spoilage and investigation of volatile profile during storage of sea bream fillets under various conditions. Int. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Kormas, K.A.; Boziaris, I.S. Microbiological changes, shelf life and identification of initial and spoilage microbiota of sea bream fillets stored under various conditions using 16S rRNA gene analysis. J. Sci. Food Agric. 2015, 95, 2386–2394. [Google Scholar] [CrossRef]
- Syropoulou, F.; Parlapani, F.F.; Anagnostopoulos, D.A.; Stamatiou, A.; Mallouchos, A.; Boziaris, I.S. Spoilage Investigation of Chill Stored Meagre (Argyrosomus regius) Using Modern Microbiological and Analytical Techniques. Foods 2021, 10, 3109. [Google Scholar] [CrossRef]
- Syropoulou, F.; Anagnostopoulos, D.A.; Parlapani, F.F.; Karamani, E.; Stamatiou, A.; Tzokas, K.; Nychas, G.J.E.; Boziaris, I.S. Microbiota Succession of Whole and Filleted European Sea Bass (Dicentrarchus labrax) during Storage under Aerobic and MAP Conditions via 16S rRNA Gene High-Throughput Sequencing Approach. Microorganisms 2022, 10, 1870. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Mallouchos, A.; Angelidou, A.; Syropoulou, F.; Minos, G.; Boziaris, I.S. Volatile Organic Compounds and 16S Metabarcoding in Ice-Stored Red Seabream Pagrus major. Foods 2022, 11, 666. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Syropoulou, F.; Parlapani, F.F.; Tsiartsafis, A.; Exadactylos, A.; Nychas, G.J.E.; Boziaris, I.S. Microbiota profile of filleted gilthead seabream (Sparus aurata) during storage at various conditions by 16S rRNA metabarcoding analysis. Food Res. Int. 2023, 164, 112313. [Google Scholar] [CrossRef] [PubMed]
- Tryfinopoulou, P.; Tsakalidou, E.; Nychas, G.J.E. Characterization of Pseudomonas spp. associated with spoilage of gilt-head sea bream stored under various conditions. Appl. Environ. Microbiol. 2002, 68, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Meziti, A.; Kormas, K.A.; Boziaris, I.S. Indigenous and spoilage microbiota of farmed sea bream stored in ice identified by phenotypic and 16S rRNA gene analysis. Food Microbiol. 2013, 33, 85–89. [Google Scholar] [CrossRef]
- Jääskeläinen, E.; Jakobsen, L.M.A.; Hultman, J.; Eggers, N.; Bertram, H.C.; Björkroth, J. Metabolomics and bacterial diversity of packaged yellowfin tuna (Thunnus albacares) and salmon (Salmo salar) show fish species-specific spoilage development during chilled storage. Int. J. Food Microbiol. 2019, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Parente, E.; Ianniello, R.G.; De Filippis, F.; Ricciardi, A. Dynamics of bacterial communities and interaction networks in thawed fish fillets during chilled storage in air. Int. J. Food Microbiol. 2019, 293, 102–113. [Google Scholar] [CrossRef]
- Leisner, J.J.; Millan, J.C.; Huss, H.H.; Larsen, L.M. Production of histamine and tyramine by lactic acid bacteria isolated from vacuum-packed sugar-salted fish. J. Appl. Bacteriol. 1994, 76, 417–423. [Google Scholar] [CrossRef]
- Lyhs, U.; Korkeala, H.; Björkroth, J. Identification of lactic acid bacteria from spoiled, vacuum-packaged “gravad” rainbow trout using ribotyping. Int. J. Food Microbiol. 2002, 72, 147–153. [Google Scholar] [CrossRef]
- Silbande, A.; Adenet, S.; Chopin, C.; Cornet, J.; Smith-Ravin, J.; Rochefort, K.; Leroi, F. Effect of vacuum and modified atmosphere packaging on the microbiological, chemical and sensory properties of tropical red drum (Sciaenops ocellatus) fillets stored at 4 °C. Int. J. Food Microbiol. 2018, 266, 31–41. [Google Scholar] [CrossRef]
- Kuuliala, L.; Al Hage, Y.; Ioannidis, A.G.; Sader, M.; Kerckhof, F.M.; Vanderroost, M.; Boon, N.; De Baets, B.; De Meulenaer, B.; Ragaert, P.; et al. Microbiological, chemical and sensory spoilage analysis of raw Atlantic cod (Gadus morhua) stored under modified atmospheres. Food Microbiol. 2018, 70, 232–244. [Google Scholar] [CrossRef]
- Powell, S.M.; Tamplin, M.L. Microbial communities on Australian modified atmosphere packaged Atlantic salmon. Food Microbiol. 2012, 30, 226–232. [Google Scholar] [CrossRef]
- Macé, S.; Cornet, J.; Chevalier, F.; Cardinal, M.; Pilet, M.F.; Dousset, X.; Joffraud, J.J. Characterisation of the spoilage microbiota in raw salmon (Salmo salar) steaks stored under vacuum or modified atmosphere packaging combining conventional methods and PCR-TTGE. Food Microbiol. 2012, 30, 164–172. [Google Scholar] [CrossRef]
- Ponce De Leon, S.; Inoue, N.; Shinano, H. Effect of Acetic and Citric Acids on the Growth and Activity (VB-N) of Pseudomonas sp. and Moraxella sp. Bull. Fac. Fish. Hokkaido Univ. 1993, 44, 80–85. [Google Scholar]
- Van Haute, S.; Raes, K.; Devlieghere, F.; Sampers, I. Combined use of cinnamon essential oil and MAP/vacuum packaging to increase the microbial and sensorial shelf life of lean pork and salmon. Food Packag. Shelf Life 2017, 12, 51–58. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Stamatiou, A.P.; Nychas, G.J.E. Microbiological aspects and shelf life of processed seafood products. J. Sci. Food Agric. 2013, 93, 1184–1190. [Google Scholar] [CrossRef]
- Hansen, L.T.; Gill, T.; Røntved, S.D.; Huss, H.H. Importance of autolysis and microbiological activity on quality of cold-smoked salmon. Food Res. Int. 1996, 29, 181–188. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Kendall, P.A.; Sofos, J.N. Effect of Food Processing-Related Stresses on Acid Tolerance of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7514–7516. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Sofos, J.N. Comparative acid stress response of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella Typhimurium after habituation at different pH conditions. Lett. Appl. Microbiol. 2004, 38, 321–326. [Google Scholar] [CrossRef]
- Verdos, G.I.; Makrigiannis, A.; Tsigaras, E.; Boziaris, I.S. Survival of food-borne bacterial pathogens in traditional Mediterranean anchovy products. J. Food Saf. 2019, 39, e12576. [Google Scholar] [CrossRef]
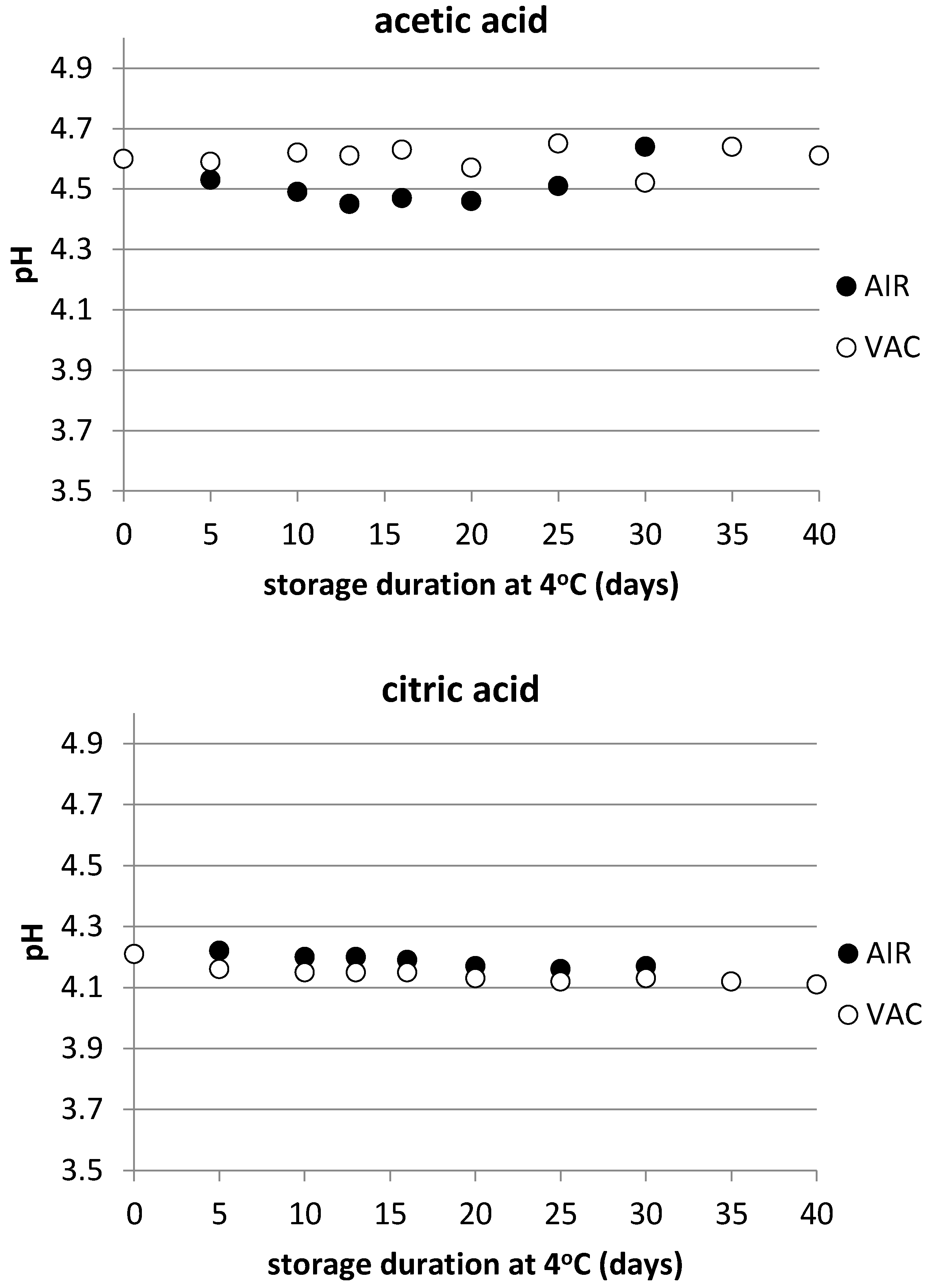
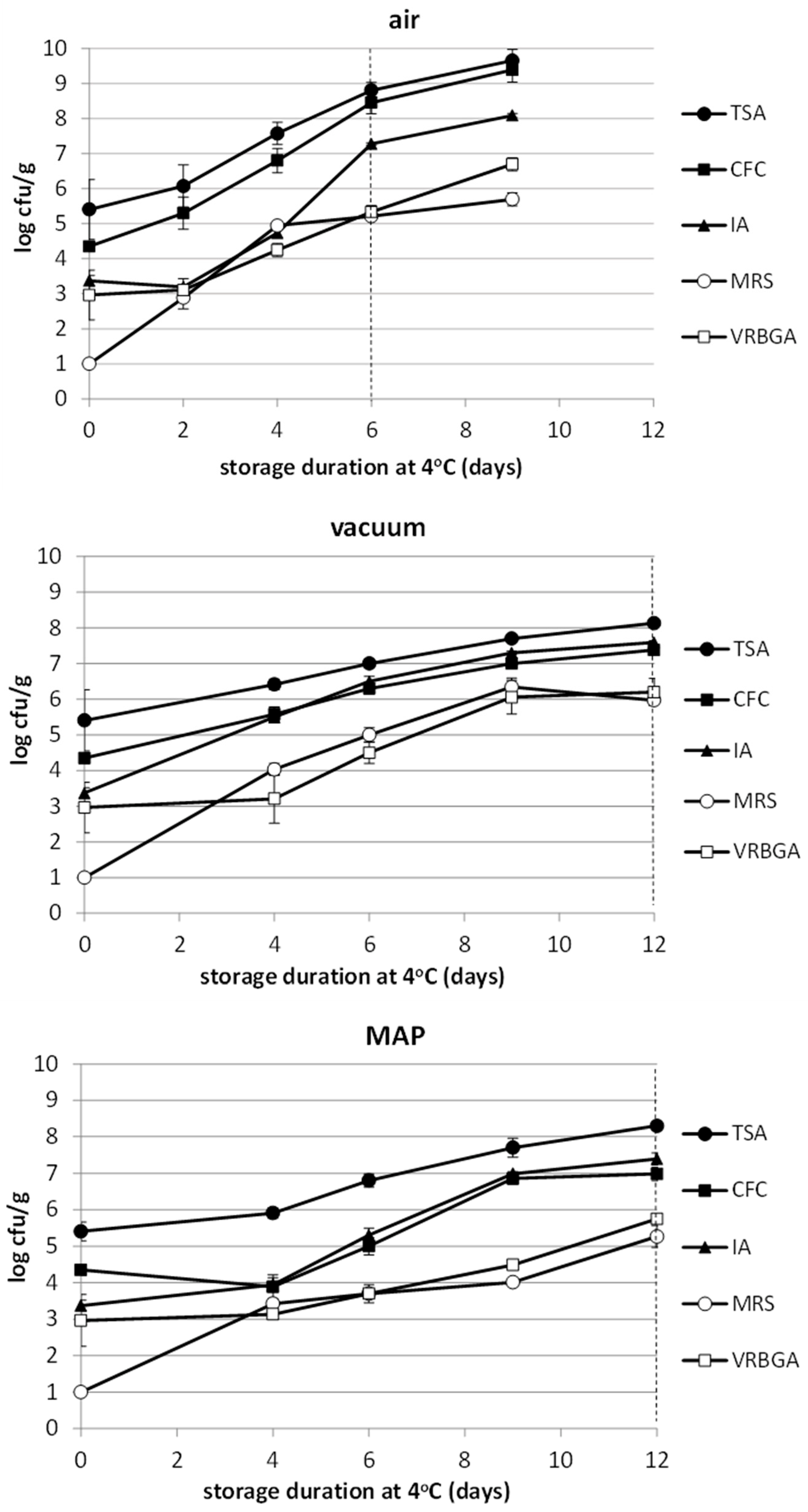
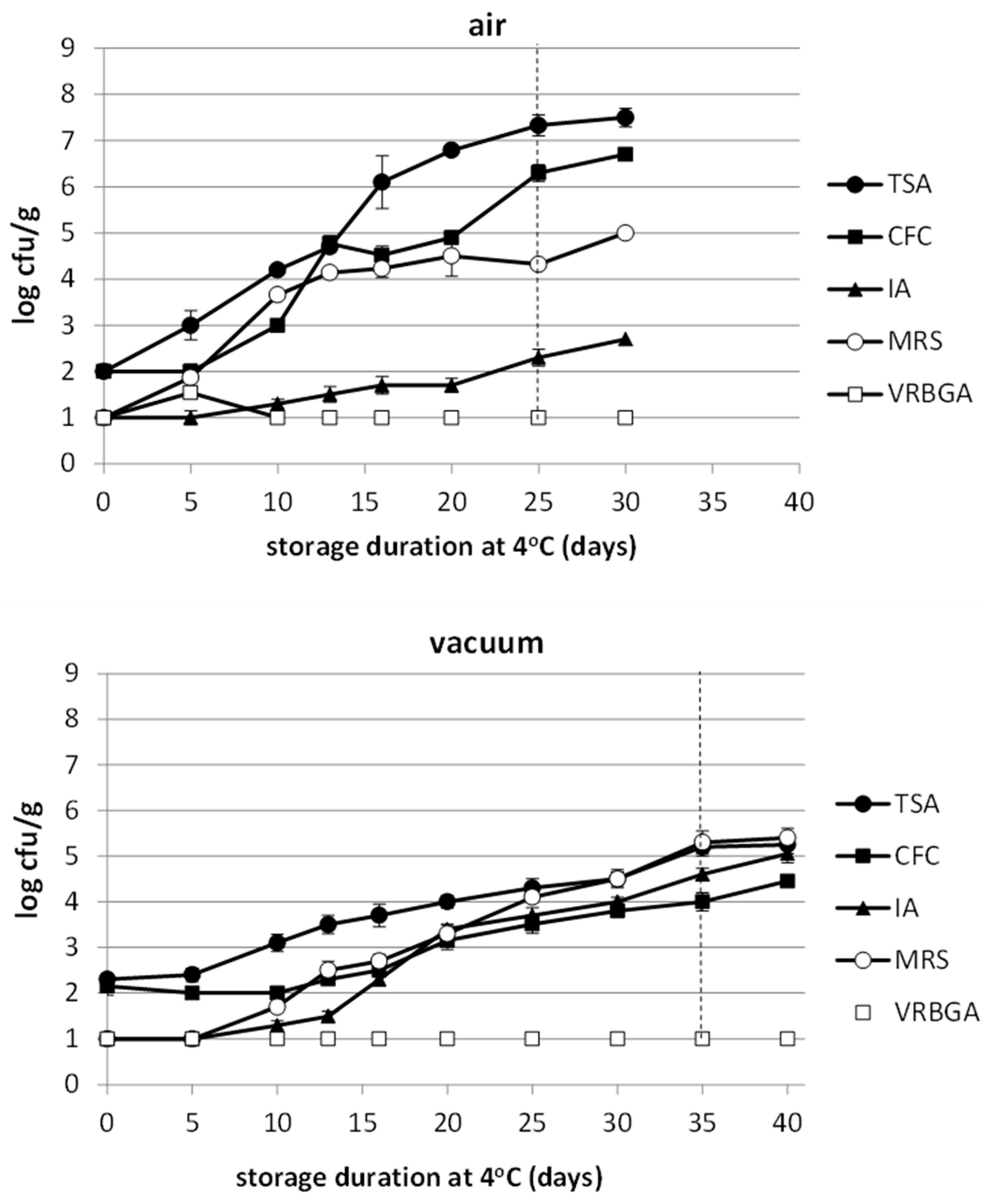
| Product | pH | aw | %Moisture | % Salt | Calculated WPS% |
|---|---|---|---|---|---|
| Brined sea bass fillets | 6.20 ± 0.01 | 0.972 ± 0.002 | 75.54 ± 1.17 | 2.22 ± 0.36 | 2.85 |
| Cooked and marinated with acetic acid | 4.60 ± 0.03 | 0.981 ± 0.006 | 77.37 ± 0.77 | 0.94 ± 0.03 | 1.20 |
| Marinated with citric acid | 4.21 ± 0.02 | 0.980 ± 0.003 | 77.54 ± 1.17 | 0.89 ± 0.02 | 1.13 |
| Day | aw | % Moisture | % Salt | Calculated WPS% |
|---|---|---|---|---|
| Air | ||||
| 4 | 0.972 ± 0.002 | 76.60 ± 1.76 | 2.35 ± 1.69 | 2.97 |
| 6 | 0.974 ± 0.003 | 77.96 ± 2.00 | 2.11 ± 0.58 | 2.63 |
| Vacuum | ||||
| 4 | 0.973 ± 0.002 | 76.23 ± 2.32 | 2.53 ± 0.49 | 3.21 |
| 9 | 0.971 ± 0.004 | 77.37 ± 1.97 | 2.67 ± 1.39 | 3.33 |
| 12 | 0.972 ± 0.003 | 75.89 ± 1.93 | 2.44 ± 1.01 | 3.11 |
| MAP | ||||
| 4 | 0.972 ± 0.003 | 74.92 ± 1.66 | 2.49 ± 0.53 | 3.22 |
| 9 | 0.973 ± 0.002 | 77.80 ± 7.76 | 2.28 ± 0.42 | 2.85 |
| 12 | 0.973 ± 0.005 | 76.01 ± 0.19 | 2.13 ± 0.22 | 2.73 |
| Storage Day | MAP (CO2/O2) 1 | Vacuum (CO2/O2) 1 | Storage Day | Vacuum (CO2/O2) 2 |
|---|---|---|---|---|
| 0 | 50.1/9.9 | 0.0/0.1 | 0 | 0.0/0.1 |
| 4 | 23.6/12.1 | 0.4/4.1 | 12 | 0.3/11.6 |
| 9 | 9.4/15.5 | 0.3/10.4 | 25 | 0.3/18.4 |
| 12 | 4.2/18.6 | 0.2/13.7 | 40 | 0.3/20.1 |
| Sample | Shelf-Life (Days) | Shelf-Life Determinants |
|---|---|---|
| Brined sea bass fillets (Air) | 6 | Unpleasant |
| Brined sea bass fillets (Vacuum) Brined sea bass fillets (MAP) | 12 12 | odor and taste |
| Cooked sea bass fillets marinated with acetic acid (Air) | 30 | Softness and |
| Cooked Sea bass fillets marinated with acetic acid (Vacuum) | 40 | bitter taste |
| Sea bass fillets marinated with citric acid (Air) | 25 | Softness and unpleasant |
| Sea bass fillets marinated with citric acid (Vacuum) | 35 | odor and taste |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anagnostopoulos, D.A.; Parlapani, F.F.; Tsara, E.; Eirinaki, M.G.; Kokioumi, D.; Ampatzidou, E.; Boziaris, I.S. Effect of Physicochemical Characteristics and Storage Atmosphere on Microbiological Stability and Shelf-Life of Minimally Processed European Sea Bass (Dicentrarchus labrax) Fillets. Foods 2023, 12, 1145. https://doi.org/10.3390/foods12061145
Anagnostopoulos DA, Parlapani FF, Tsara E, Eirinaki MG, Kokioumi D, Ampatzidou E, Boziaris IS. Effect of Physicochemical Characteristics and Storage Atmosphere on Microbiological Stability and Shelf-Life of Minimally Processed European Sea Bass (Dicentrarchus labrax) Fillets. Foods. 2023; 12(6):1145. https://doi.org/10.3390/foods12061145
Chicago/Turabian StyleAnagnostopoulos, Dimitrios A., Foteini F. Parlapani, Evangelia Tsara, Maria G. Eirinaki, Despoina Kokioumi, Evdoxia Ampatzidou, and Ioannis S. Boziaris. 2023. "Effect of Physicochemical Characteristics and Storage Atmosphere on Microbiological Stability and Shelf-Life of Minimally Processed European Sea Bass (Dicentrarchus labrax) Fillets" Foods 12, no. 6: 1145. https://doi.org/10.3390/foods12061145
APA StyleAnagnostopoulos, D. A., Parlapani, F. F., Tsara, E., Eirinaki, M. G., Kokioumi, D., Ampatzidou, E., & Boziaris, I. S. (2023). Effect of Physicochemical Characteristics and Storage Atmosphere on Microbiological Stability and Shelf-Life of Minimally Processed European Sea Bass (Dicentrarchus labrax) Fillets. Foods, 12(6), 1145. https://doi.org/10.3390/foods12061145








