Ultrasound Technology as Inactivation Method for Foodborne Pathogens: A Review
Abstract
:1. Introduction
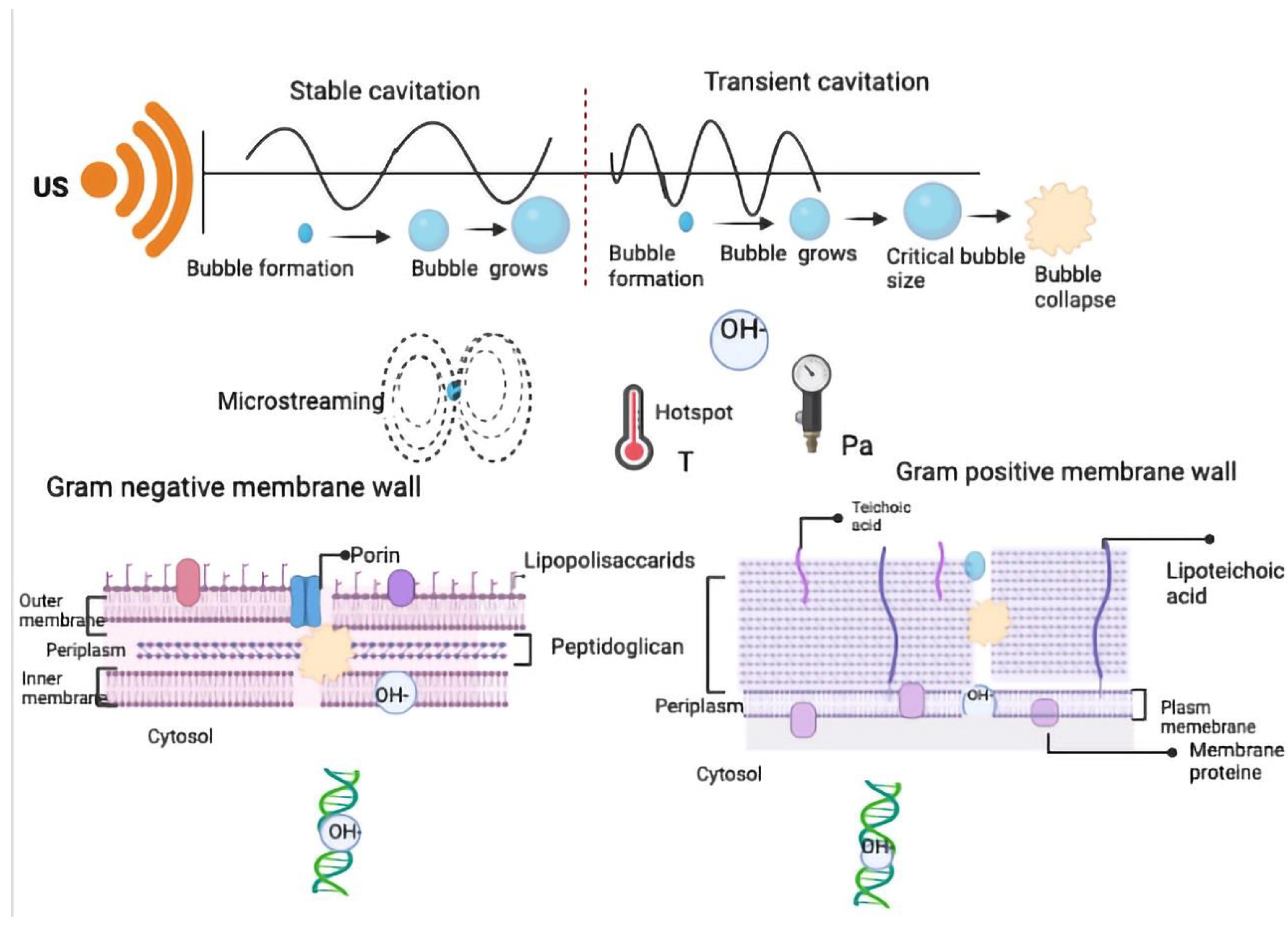
2. Ultrasound: Mechanisms of Action Applied in Food Industry
3. Mechanism of Ultrasound Action against Microorganisms
4. Pathogen Escherichia coli
5. Salmonella spp.
6. Listeria spp.
7. Staphylococcus spp.
8. Campylobacter spp.
9. Vibrio spp.
10. Pseudomonas spp.
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority European. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, Foodborne Illnesses and Germs. Last modified 18 March 2020. 2021. Available online: https://www.cdc.gov/foodsafety/foodborne-germs.html (accessed on 1 October 2022).
- Sango, D.M.; Abela, D.; McElhatton, A.; Valdramidis, V. Assisted ultrasound applications for the production of safe foods. J. Appl. Microbiol. 2014, 116, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Altieri, C.; Sinigaglia, M.; Corbo, M.R. Two Nonthermal Technologies for Food Safety and Quality—Ultrasound and High Pressure Homogenization: Effects on Microorganisms, Advances, and Possibilities: A Review. J. Food Prot. 2019, 82, 2049–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onyeaka, H.; Miri, T.; Hart, A.; Anumudu, C.; Nwabor, O.F. Application of Ultrasound Technology in Food Processing with emphasis on bacterial spores. Food Rev. Int. 2021, 1–26. [Google Scholar] [CrossRef]
- Lee, H.S.; Coates, G.A. Effect of thermal pasteurization on Valencia orange juice color and pigments. LWT Food Sci. Technol. 2003, 36, 153–156. [Google Scholar] [CrossRef]
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of thermal processing on the quality loss of pineapple juice. J. Food Eng. 2005, 66, 259–265. [Google Scholar] [CrossRef]
- Gandy, A.; Schilling, M.; Coggins, P.; White, C.; Yoon, Y.; Kamadia, V. The Effect of Pasteurization Temperature on Consumer Acceptability, Sensory Characteristics, Volatile Compound Composition, and Shelf-Life of Fluid Milk. J. Dairy Sci. 2008, 91, 1769–1777. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, B.K.; O’Donnell, C.P.; Cullen, P.J. Effect of nonthermal processing technologies on the anthocyanin content of fruit juices. Trends Food Sci. Technol. 2009, 20, 137–145. [Google Scholar] [CrossRef]
- Shung, K.K.; Cannata, J.M.; Zhou, Q.F. Piezoelectric materials for high frequency medical imaging applications: A review. J. Electroceramics 2007, 19, 141–147. [Google Scholar] [CrossRef]
- Laborde, J.-L.; Bouyer, C.; Caltagirone, J.-P.; Gérard, A. Acoustic bubble cavitation at low frequencies. Ultrasonics 1998, 36, 589–594. [Google Scholar] [CrossRef]
- Feng, H.; Lee, H. Effect of Power Ultrasound on Food Quality. In Ultrasound Tech for Food and Bioprocessing; Springer: New York, NY, USA, 2011; pp. 154–196. [Google Scholar]
- Chen, F.; Zhang, M.; Yang, C.-H. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef]
- Ojha, K.S.; Tiwari, B.K.; O’Donnell, C.P. Effect of Ultrasound Technology on Food and Nutritional Quality. Adv. Food Nutr. Res. 2018, 84, 207–240. [Google Scholar] [CrossRef] [PubMed]
- Franc, P.; Michel, J.M. Fundamentals of Cavitation; Springer: Dordrecht, The Netherlands, 2004. [Google Scholar] [CrossRef]
- Zupanc, M.; Pandur, Ž.; Perdih, T.S.; Stopar, D.; Petkovšek, M.; Dular, M. Effects of cavitation on different microorganisms: The current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason. Sonochem. 2019, 57, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yang, W.; Hielscher, T. Power Ultrasound. Food Sci. Technol. Int. 2008, 14, 433–436. [Google Scholar] [CrossRef]
- Pokhrel, P.R.; Bermúdez-Aguirre, D.; Martínez-Flores, H.E.; Garnica-Romo, M.G.; Sablani, S.; Tang, J.; Barbosa-Cánovas, G.V. Combined Effect of Ultrasound and Mild Temperatures on the Inactivation of E. coli in Fresh Carrot Juice and Changes on its Physicochemical Characteristics. J. Food Sci. 2017, 82, 2343–2350. [Google Scholar] [CrossRef]
- Chahine, G.L.; Hsiao, C.-T. Modelling cavitation erosion using fluid–material interaction simulations. Interface Focus 2015, 5, 2015–2016. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Charoux, C.M.G.; Ojha, K.S.; O’Donnell, C.P.; Cardoni, A.; Tiwari, B.K. Applications of airborne ultrasonic technology in the food industry. J. Food Eng. 2017, 208, 28–36. [Google Scholar]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent advances on application of ultrasound and pulsed electric field technologies in the extraction of bioactives from agro-industrial by-products. Food Bioprocess Tech. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Magalhães, M.L.; Cartaxo, S.J.; Gallão, M.I.; García-Pérez, J.V.; Cárcel, J.A.; Rodrigues, S.; Fernandes, F.A. Drying intensification combining ultrasound pre-treatment and ultrasound-assisted air drying. J. Food Eng. 2017, 215, 72–77. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhang, M.; Adhikari, B. Ultrasound-assisted freezing of fruits and vegetables: Design, development, and appli-cations. In Global Food Security and Wellness; Barbosa-Canovas, G., Pastore, G., Candoðan, K., Meza, I.M., Da Silva Lannes, C., Lannes, S., Buckle, K., Yada, R., Rosenthal, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 457–487. [Google Scholar]
- Liu, X.; Zhang, C.; Zhang, Z.; Xue, J.; Le, J. The role of ultrasound in hydrogen removal and microstructure refinement by ul-trasonic argon degassing process. Ultrason. Sonochem. 2017, 38, 455–462. [Google Scholar] [CrossRef]
- Torkamani, A.E.; Juliano, P.; Fagan, P.; Jimenez-Flores, R.; Ajlouni, S.; Singh, T.K. Effect of ultrasound-enhanced fat separation on whey powder phospholipid composition and stability. J. Dairy Sci. 2016, 99, 4169–4177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhandari, B.; Zisu, B. Effect of ultrasound treatment on the evolution of solubility of milk protein concentrate powder. In Handbook of Ultrasonics and Sonochemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–19. [Google Scholar]
- Gondrexon, N.; Cheze, L.; Jin, Y.; Legay, M.; Tissot, Q.; Hengl, N.; Baup, S.; Boldo, P.; Pignon, F.; Talansier, E. Intensification of heat and mass transfer by ultrasound: Application to heat exchangers and membrane separation processes. Ultrason. Sonochem. 2015, 25, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; Rey-Salgueiro, L.; Regueiro, J.; Gonzalez-Barreiro, C.; Cancho- Grande, B.; Simal-Gándara, J. Ultrasound-assisted emulsification– microextraction for the determination of phenolic compounds in olive oils. Food Chem. 2014, 150, 128–136. [Google Scholar] [CrossRef]
- Czank, C.; Simmer, K.; Hartmann, P.E. Simultaneous pasteurization and homogenization of human milk by combining heat and ultrasound: Effect on milk quality. J. Dairy Res. 2010, 77, 183–189. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Šimunek, M.; Petrovic, M.; Bedic, H.; Herceg, Z.; Juretic, Z. Aromatic profile and sensory characterization of ultrasound treated cranberry juice and nectar. Ultrason. Sonochem. 2017, 38, 783–793. [Google Scholar] [CrossRef]
- Kentish, S.; Feng, H. Application of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Schneider, Y.; Zahn, S.; Rohm, H. Ultrasonic cutting of foods. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barbosa-Canovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Arnold, G.; Zahn, S.; Legler, A.; Rohm, H. Ultrasonic cutting of foods with inclined moving blades. J. Food Eng. 2011, 103, 394–400. [Google Scholar] [CrossRef]
- Arnold, G.; Leiteritz, L.; Zahn, S.; Rohm, H. Ultrasonic cutting of cheese: Composition affects cutting work reduction and energy demand. Int. Dairy J. 2009, 19, 314–320. [Google Scholar] [CrossRef]
- Shanmugam, A.; Chandrapala, J.; Ashokkumar, M. The effect of ultrasound on the physical and functional properties of skim milk. Innov. Food Sci. Emerg. Technol. 2012, 16, 251–258. [Google Scholar] [CrossRef]
- Flores, D.R.M.; Brasil, C.C.B.; Campagnol, P.C.B.; Jacob-Lopes, E.; Zepka, L.Q.; Wagner, R.; Cichoski, A.J. Application of ultrasound in chicken breast during chilling by immersion promotes a fast and uniform cooling. Food Res. Int. 2018, 109, 59–64. [Google Scholar] [CrossRef]
- Al-Hilphy, A.R.; Al-Temimi, A.B.; Al Rubaiy, H.H.M.; Anand, U.; Delgado-Pando, G.; Lakhssassi, N. Ultrasound applications in poultry meat processing: A systematic review. J. Food Sci. 2020, 85, 1386–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, W.; Zhou, G. Effects of ultrasound-assisted frying on the physiochemical properties and microstructure of fried meatballs. Int. J. Food Sci. Technol. 2019, 54, 2915–2926. [Google Scholar] [CrossRef]
- Dehghannya, J.; Abedpour, L. Influence of a three stage hybrid ultrasound-osmotic-frying process on production of low-fat fried potato strips. J. Sci. Food Agric. 2018, 98, 1485–1491. [Google Scholar] [CrossRef]
- Cadòn-Abant, S.; Arroyo, C.; Álvarez, I.; Brunton, N.; Whyte, P.; Lyng, J.G. An assessment of the application of ultrasound in the processing of ready-to-eat whole brown crab (Cancer pagurus). Ultrason. Sonochem. 2018, 40, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Qui, L.; Zhang, M.; Chitrakar, B.; Bhandari, B. Application of power ultrasound in freezing and thawing Processes: Effect on process efficiency and product quality. Ultrason. Sonochem. 2020, 68, 105230. [Google Scholar] [CrossRef]
- Sun, Q.; Sun, F.; Xia, X.; Xu, H.; Kong, B. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason. Sonochem. 2019, 51, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Zhang, M.; Mujumdar, A.S. Application of airborne ultrasound in the convective drying of fruits and vegetables: A review. Ultrason. Sonochem. 2017, 39, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Başlar, M.; Kılıçlı, M.; Toker, O.S.; Sağdıç, O.; Arici, M. Ultrasonic vacuum drying technique as a novel process for shortening the drying period for beef and chicken meats. Innov. Food Sci. Emerg. Technol. 2014, 26, 182–190. [Google Scholar] [CrossRef]
- Başlar, M.; Kılıçlı, M.; Yalinkilic, B. Dehydration kinetics of salmon and trout fillets using ultrasonic vacuum drying as a novel technique. Ultrason. Sonochem. 2015, 27, 495–502. [Google Scholar] [CrossRef]
- Huang, D.; Men, K.; Li, D.; Wen, T.; Gong, Z.; Sunden, B.; Wu, Z. Application of ultrasound technology in the drying of food products. Ultrason. Sonochem. 2019, 63, 104950. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, X.; Chen, X.; Fang, R.; Zou, Y.; Wang, D.; Xu, W. How ultrasound combined with potassium alginate marination tenderizes old chicken breast meat: Possible mechanisms from tissue to protein. Food Chem. 2020, 328, 127144. [Google Scholar] [CrossRef]
- Yilmaz, B.; Cakmak, H.; Tavman, S. Ultrasonic pretreatment of carrot slices: Effects of sonication source on drying kinetics and product quality. An. Da Acad. Bras. De Ciências 2019, 91, e20180447. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, E.S.; Simal, S.; Femenia, A.; Rosselló, C. Effect of acoustic brining on the transport of sodium chloride and water in Mahon cheese. Eur. Food Res. Technol. 2000, 212, 39–43. [Google Scholar] [CrossRef]
- Turhan, S.; Saricaogliu, T.; Oz, F. The Effect of Ultrasonic Marinating on the Transport of Acetic Acid and Salt in Anchovy Marinades. Food Sci. Technol. Res. 2013, 19, 849–853. [Google Scholar] [CrossRef] [Green Version]
- Alarcon-Rojo, A.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jimenez, M.; Garcia-Galicia, G. Ultrasound, and meat quality: A review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Chang, H.-C.; Wong, R.-X. Textural and biochemical properties of cobia (Rachycentron canadum) sashimi tenderized with the ultrasonic water bath. Food Chem. 2012, 132, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, W.; Zou, M.; Lv, R.; Wang, D.; Hou, F.; Feng, H.; Ma, X.; Zhong, J.; Ding, T.; et al. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Kiani, H.; Sun, D.-W.; Delgado, A.; Zhang, Z. Investigation of the effect of power ultrasound on the nucleation of water during freezing of agar gel samples in tubing vials. Ultrason. Sonochem. 2012, 19, 576–581. [Google Scholar] [CrossRef]
- Musielak, G.; Mierzwa, D.; Kroehnke, J. Food drying enhancement by ultrasound—A review. Trends Food Sci. Technol. 2016, 56, 126–141. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Wiktor, A.; Dadan, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The impact of combination of pulsed electric field and ultrasound treatment on air drying kinetics and quality of carrot tissue. LWT Food Sci. Technol. 2019, 110, 71–79. [Google Scholar] [CrossRef]
- Ozuna, C.; Puig, A.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Influence of high intensity ultrasound application on mass transport, microstructure and textural properties of pork meat (Longissimus dorsi) brined at different NaCl concentrations. J. Food Eng. 2013, 119, 84–93. [Google Scholar] [CrossRef]
- McDonnell, C.K.; Lyng, J.G.; Arimi, J.M.; Allen, P. The acceleration of pork curing by power ultrasound: A pilot-scale pro-duction. Innov. Food Sci. Emerg. Technol. 2014, 26, 191–198. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Burgess, C.M.; Kerry, J.P.; Tiwari, B.K. Ultrasound-Assisted Marination: Role of Frequencies and Treatment Time on the Quality of Sodium-Reduced Poultry Meat. Foods 2019, 8, 473. [Google Scholar] [CrossRef] [Green Version]
- Pedros-Garrido, S.; Condon-Abanto, S.; Beltran, J.; Lyng, J.; Brunton, N.; Bolton, D.; Whyte, P. Assessment of high intensity ultrasound for surface decontamination of salmon (S. salar), mackerel (S. scombrus), cod (G. morhua) and hake (M. mer- luc-cius) fillets, and its impact on fish quality. Innov. Food Sci. Emerg. Technol. 2017, 41, 64–70. [Google Scholar] [CrossRef]
- Khairi, M.T.M.; Ibrahim, S.; Yunus, M.A.M.; Faramarzi, M. Contact and non-contact ultrasonic measurement in the food industry: A review. Meas. Sci. Technol. 2015, 27, 12001. [Google Scholar] [CrossRef]
- Manas, P.; Pagan, R. Microbial inactivation by new technologies of food preservation. J. Appl. Microbiol. 2005, 98, 1387–1399. [Google Scholar] [CrossRef]
- Madigan, M.T.; Bender, K.S.; Buckley, D.H.; Sattley, W.M.; Stahl, D.A. Brock Biology of Microorganisms, 13th ed.; Pearson: Boston, MA, USA, 2012. [Google Scholar]
- Vadillo-Rodríguez, V.; Dutcher, J.R. Viscoelasticity of the bacterial cell envelope. Soft Matter 2011, 7, 4101–4110. [Google Scholar] [CrossRef]
- Wigginton, K.R.; Pecson, B.M.; Sigstam, T.; Bosshard, F.; Kohn, T. Virus inactivation mechanisms: Impact of disinfectants on virus function and structural integrity. Environ. Sci. Tech. 2012, 46, 12069–12078. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.S.M.; Babgi, B.; Alghamdi, Y.; Aksu, M.; Madhavan, J.; Ashokkumar, M. Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrason. Sonochem. 2016, 29, 568–576. [Google Scholar] [CrossRef]
- Labas, M.D.; Zalazar, C.S.; Brandi, R.J.; Cassano, A.E. Reaction kinetics of bacteria disinfection employing hydrogen peroxide. Biochem. Eng. J. 2008, 38, 78–87. [Google Scholar] [CrossRef]
- Rahman, M.; Ninomiya, K.; Ogino, C.; Shimizu, N. Ultrasound-induced membrane lipid peroxidation and cell damage of Escherichia coli in the presence of non- woven TiO2 fabrics. Ultrason. Sonochem. 2010, 17, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Kasper, D.L. Oxidative depolymerization of polysaccharides by reactive oxygen/nitrogen species. Glycobiology 2011, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.K.; Yang, Y.; Gerrity, D.W.; Abbaszadegan, M. The Impact of Capsid Proteins on Virus Removal and Inactivation during Water Treatment Processes. Microbiol. Insights 2015, 8, MBI-S31441. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z. Microbial Inactivation in Foods by Ultrasound. J. Food: Microbiol. Saf. Hyg. 2017, 2, E102. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Zhou, L.; Li, B.; Xu, Z. Effect of ultrasonic field on the enzyme activities and ion balance of potential pathogen Saccharomyces cerevisiae. Microb. Pathog. 2018, 119, 216–220. [Google Scholar] [CrossRef]
- Sarkinas, A.; Sakalauskiene, K.; Raisutis, R.; Zeime, J.; Salaseviciene, A.; Puidaite, E.; Mockus, E.; Cernauskas, D. Inactivation of some pathogenic bacteria and phytoviruses by ultrasonic treatment. Microb. Pathog. 2018, 123, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Cleach, J.; Watier, D.; Fur, B.L.; Brauge, T.; Duflos, G.; Grard, T.; Lencel, P. Use of ratiometric probes with a spectrofluorometer for bacterial viability measurement. J. Microbiol. Biotech. 2018, 28, 1782–1790. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Ma, L.; Liao, X.; Liu, D.; Lu, X.; Chen, S.; Ye, X.; Ding, T. Ultrasound-Induced Escherichia coli O157:H7 Cell Death Exhibits Physical Disruption and Biochemical Apoptosis. Front. Microbiol. 2018, 9, 2486. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Liu, D.; Ashokkumar, M.; Ye, X.; Jin, T.Z.; Guo, M. Antibacterial mechanism of ultrasound against Escherichia coli: Alterations in membrane microstructures and properties. Ultrason. Sonochem. 2021, 73, 105509. [Google Scholar] [CrossRef]
- Park, S.Y.; Ha, S.-D. Reduction of Escherichia coli and Vibrio parahaemolyticus Counts on Freshly Sliced Shad (Konosirus punctatus) by Combined Treatment of Slightly Acidic Electrolyzed Water and Ultrasound Using Response Surface Methodology. Food Bioprocess Technol. 2015, 8, 1762–1770. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Li, C.; Cui, H. Inactivation mechanism of E. coli O157:H7 under ultrasonic sterilization. Ultrason. Sonochem. 2019, 59, 104751. [Google Scholar] [CrossRef]
- Fitriyanti, M.; Narsimhan, G. Synergistic effect of low power ultrasonication on antimicrobial activity of cecropin P1 against E. coli in food systems. LWT Food Sci. Technol. 2018, 96, 175–181. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Li, Y. Modeling the Thermoultrasound Inactivation of Vibrio parahaemolyticus in Raw Peeled Shrimps. J. Food Prot. 2013, 76, 1712–1718. [Google Scholar] [CrossRef]
- Cruz-Cansino, N.; Reyes-Hernández, I.; Delgado-Olivares, L.; Jaramillo-Bustos, D.P.; Ortega, J.A.A.; Ramírez-Moreno, E. Effect of ultrasound on survival and growth of Escherichia coli in cactus pear juice during storage. Braz. J. Microbiol. 2016, 47, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.; Jiang, Y.; Xing, L.; Zhou, G.; Zhang, W. Inactivation of Escherichia coli O157:H7 and Bacillus cereus by power ultrasound during the curing processing in brining liquid and beef. Food Res. Int. 2017, 102, 717–727. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Hongying, Y.; Aheto, J.H.; Yi, R.; Yu, S.; Tu, H. Nondestructive monitoring, kinetics and antimicrobial properties of ultrasound technology applied for surface decontamina-tion of bacterial foodborne pathogen in pork. Ultrason. Sonochem. 2021, 70, 105344. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Oliveira, M.; Burgess, C.M.; Cropotova, J.; Rustad, T.; Sun, D.-W.; Tiwari, B.K. Combined effects of ultrasound, plasma-activated water, and peracetic acid on decontamination of mackerel fillets. LWT 2021, 150, 111957. [Google Scholar] [CrossRef]
- Wrigley, D.; Llorca, N.G. Decrease of Salmonella typhlmurium in Skim Milk and Egg by Heat and Ultrasonic Wave Treatment. J. Food Prot. 1992, 55, 678–680. [Google Scholar] [CrossRef] [PubMed]
- Techathuvanan, C.; D’Souza, D.H. High Intensity Ultrasound forSalmonellaEnteritidis Inactivation in Culture and Liquid Whole Eggs. J. Food Sci. 2018, 83, 1733–1739. [Google Scholar] [CrossRef]
- Bi, X.; Wang, X.; Chen, Y.; Chen, L.; Xing, Y.; Che, Z. Effects of combination treatments of lysozyme and high power ultrasound on the Salmonella typhimurium inactivation and quality of liquid whole egg. Ultrason. Sonochem. 2020, 60, 104763. [Google Scholar] [CrossRef]
- Kordowska-Wiater, M.; Stasiak, D.M. Effect of ultrasound on survival of gram-negative bacteria on chicken skin surface. Bull. Vet. Inst. Pulawy 2011, 55, 207–210. [Google Scholar]
- Campaniello, D.; Bevilacqua, A.; Speranza, B.; Sinigaglia, M.; Corbo, M.R. Inactivation of Salmonella enterica in a Rice Beverage by Ultrasound: Study of the Parameters Affecting the Antibacterial Effect. Food Bioprocess Technol. 2018, 11, 1139–1148. [Google Scholar] [CrossRef]
- Morbiato, G.; Zambon, A.; Toffoletto, M.; Poloniato, G.; Dall’Acqua, S.; de Bernard, M.; Spilimbergo, S. Supercritical carbon dioxide combined with high power ultrasound as innovate drying process for chicken breast. J. Supercrit. Fluids 2019, 147, 24–32. [Google Scholar] [CrossRef]
- Seo, M.; Jeong, H.; Han, S.; Kang, I.; Ha, S. Impact of ethanol and ultrasound treatment on mesophilic aerobic bacteria, coliforms, and Salmonella Typhimurium on chicken skin. Poult. Sci. 2019, 98, 6954–6963. [Google Scholar] [CrossRef]
- Joo, H.; Mizan, F.R.; Hossain, I.; Lee, D.; Ha, S. Enhanced elimination of Salmonella Typhimurium and Campylobacter jejuni on chicken skin by sequential exposure to ultrasound and peroxyacetic acid. J. Food Saf. 2020, 40, e12803. [Google Scholar] [CrossRef]
- Mortazavi, N.; Aliakbarlu, J. Antibacterial Effects of Ultrasound, Cinnamon Essential Oil, and Their Combination Against Listeria monocytogenes and Salmonella Typhimurium in Milk. J. Food Sci. 2019, 84, 3700–3706. [Google Scholar] [CrossRef] [PubMed]
- Jeyaletchumi, P.; Tunung, R.; Selina, P.M.; Chai, L.C.; Radu, S.; Farinazleen, M.G.; Cheah, Y.K.; Mitsuaki, N.; Yoshitsugu, N.; Kumar, M.P. Assessment of Listeria monocytogenes in salad vegetables through kitchen simulation study. J. Trop. Agric. Food Sci. 2012, 40, 55–62. [Google Scholar]
- Gabriel, A.A. Inactivation of Listeria monocytogenes in milk by multifrequency power ultrasound. J. Food Process. Preserv. 2015, 39, 846–853. [Google Scholar] [CrossRef]
- Gabriel, A.A. Inactivation behaviors of foodborne microorganisms in multi-frequency power ultrasound-treated orange juice. Food Control 2014, 46, 189–196. [Google Scholar] [CrossRef]
- Franco-Vega, A.; Ramirez-Corona, N.; Lopez-Malo, A.; Palou, E. Estimation of Listeria monocytogenes survival during thermoultrasonic treatments in non-iso- thermal conditions: Effect of ultrasound on temperature and survival profiles. Food Microbiol. 2015, 52, 124–130. [Google Scholar] [CrossRef]
- Dolan, H.L.; Bastarrachea, L.J.; Tikekar, R.V. Inactivation of Listeria innocua by a combined treatment of low-frequency ul-trasound and zinc oxide. Lebensm. Wiss. Technol. 2018, 88, 146–151. [Google Scholar] [CrossRef]
- Bahrami, A.; Baboli, Z.M.; Schimmel, K.; Jafari, S.M.; Williams, L. Efficiency of novel processing technologies for the control of Listeria monocytogenes in food products. Trends Food Sci. Technol. 2020, 96, 61–78. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Cheng, J.-H.; Sun, D.-W. Inactivation of Listeria Monocytogenes at various growth temperatures by ultrasound pretreatment and cold plasma. LWT 2020, 118, 108635. [Google Scholar] [CrossRef]
- Miks-Krajnik, M.; James Feng, L.X.; Bang, W.S.; Yuk, H.-G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolyzed water, ultraviolet light or/and ultrasounds. Food Control 2017, 74, 54–60. [Google Scholar] [CrossRef]
- Pennisi, L.; Di Clerico, D.; Costantini, L.; Festino, A.R.; Vergara, A. Ultrasonic decontamination in smoked salmon experimentally contaminated with Listeria monocytogenes: Preliminary results. Ital. J. Food Saf. 2020, 9, 8398. [Google Scholar] [CrossRef] [Green Version]
- Baumann, A.R.; Martin, S.E.; Feng, H. Power ultrasound treatment of Listeria monocytogenes in apple cider. J. Food Prot. 2005, 68, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsai, S.; Tikekar, R.V. Inactivation of Listeria innocua on blueberries by novel ultrasound washing processes and their impact on quality during storage. Food Control. 2021, 121, 107580. [Google Scholar] [CrossRef]
- Rafeeq, S.; Ovissipour, R. The Effect Ultrasound and Surfactants on Nanobubbles Efficacy against Listeria innocua and Escherichia coli O157:H7, in Cell Suspension and on Fresh Produce Surfaces. Foods 2021, 10, 2154. [Google Scholar] [CrossRef]
- Grace, D.; Fetsch, A. Chapter 1—Staphylococcus aureus—A Foodborne Pathogen: Epidemiology, Detection, Characterization, Prevention, and Control: An Overview. In Staphylococcus aureus; Fetsch, A., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 3–10. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Dai, C.; Sun, L.; Sun, W.; Tang, Y.; Xiong, F.; He, R.; Ma, H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017, 37, 144–149. [Google Scholar] [CrossRef]
- Liao, X.; Li, J.; Suo, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Multiple action sites of ultrasound on Escherichia coli and Staphylococcus aureus. Food Sci. Hum. Wellness 2018, 7, 102–109. [Google Scholar] [CrossRef]
- Mansyur, M.; Yudaningtyas, E.; Prawiro, S.; Widjajanto, E. The Effect of Low Power Ultrasonic Wave Exposure to Suppress Methicillin-Resistant Staphylococcus aureus (MRSA) In Vitro. J. Trop. Life Sci. 2018, 8, 144–150. [Google Scholar] [CrossRef]
- Ahmed, F.; Russell, C. Synergism between ultrasonic waves and hydrogen peroxide in the killing of microorganisms. J. Appl. Bacteriol. 1975, 39, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Sesal, N.C.; Kekeç, Ö. Inactivation of Escherichia coli and Staphylococcus aureus by Ultrasound. J. Ultrasound Med. 2014, 33, 1663–1668. [Google Scholar] [CrossRef]
- Piñon, M.; Alarcon-Rojo, A.; Renteria, A.; Carrillo-López, L.M. Microbiological properties of poultry breast meat treated with high-intensity ultrasound. Ultrasonics 2020, 102, 105680. [Google Scholar] [CrossRef]
- Herceg, Z.; Rezek Jambrak, A.; Lelas, V.; Mededovic Thagard, S. The effect of high intensity ultrasound treatment on the amount of Staphylococcus aureus and Escherichia coli in milk. Food Technol. Biotechnol. 2012, 50, 46–52. [Google Scholar]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef] [Green Version]
- Vetchapitak, T.; Shinki, T.; Sasaki, S.; Taniguchi, T.; Luangtongkum, T.; Misawa, N. Evaluation of chemical treatment combined with vacuum and ultrasonication with a water resonance system for reducing Campylobacter on naturally contaminated chicken carcasses. Food Control. 2020, 112, 107087. [Google Scholar] [CrossRef]
- Moazzami, M.N.M.; Bergenkvist, E.; Fernström, L.-L.; Rydén, J.; Hansson, I. Reducing Campylobacter jejuni,Enterobacteriaceae, Escherichia coli, and Total Aerobic Bacteria on Broiler Carcasses Using Combined Ultrasound and Steam. J. Food Prot. 2021, 84, 572–578. [Google Scholar] [CrossRef]
- Selwet, M. Use of Ultrasounds to Reduce the Count of Campylobacter coli in Water. Pol. J. Microbiol. 2021, 70, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.; Meade, J.; McGill, K.; Walsh, C.; Gibbons, J.; Lyng, J.; Whyte, P. An investigation of high intensity ultrasonication and chemical immersion treatments on Campylobacter jejuni and spoilage bacteria in chicken. Innov. Food Sci. Emerg. Technol. 2018, 45, 298–305. [Google Scholar] [CrossRef]
- Musavian, H.S.; Krebs, N.H.; Nonboe, U.; Corry, J.E.; Purnell, G. Combined steam and ultrasound treatment of broilers at slaughter: A promising intervention to significantly reduce numbers of naturally occurring campylobacters on carcasses. Int. J. Food Microbiol. 2014, 176, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Musavian, H.S.; Butt, T.M.; Ormond, A.; Keeble, D.; Krebs, N.H. Evaluation of Steam-Ultrasound Decontamination on Naturally Contaminated Broilers through the Analysis of Campylobacter, Total Viable Count, and Enterobacteriaceae. J. Food Prot. 2022, 85, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiao, X.; Zhou, X.; Cao, G.; Fang, W.; Gu, R. Isolation and molecular characterization of Vibrio parahaemolyticus from fresh, low temperature preserved, dried, and salted seafood products in two coastal areas of eastern China. Int. J. Food Microbiol. 2008, 125, 279–285. [Google Scholar] [CrossRef]
- Iwahori, J.; Yamamoto, A. Quantitative risk assessment of Vibrio parahaemolyticus in finfish: A model of raw horse mackerel consumption in Japan. Risk Anal. 2010, 30, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Liu, C.C. Vibrio parahaemolyticus: A concern of seafood safety. Food Microbiol. 2007, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Mizan, M.D.F.R.; Ha, S. Inactivation of Cronobacter sakazakii in head lettuce by using a combination of ultrasound and sodium hypochlorite. Food Control 2016, 60, 582–587. [Google Scholar] [CrossRef]
- Borazjani, A.; Andrews, L.S.; Veal, C.D. Novel nonthermal methods to reduce vibrio vulnificus in raw oysters. J. Food Saf. 2003, 23, 179–187. [Google Scholar] [CrossRef]
- Burleson, G.R.B.; Murray, T.M.; Polland, M. Inactivation of Viruses and Bacteria by Ozone, With and Without Sonication. Am. Soc. Microbiol. Appl. Microbiol. 1975, 29, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Fanelli, F.; Caputo, L. Antibiotic Resistant Pseudomonas Spp. Spoilers in Fresh Dairy Products: An Underestimated Risk and the Control Strategies. Foods 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decimo, M.; Cabeza, M.C.; Ordóñez, J.A.; De Noni, I.; Brasca, M. Volatile organic compounds associated with milk spoilage by psychrotrophic bacteria. Int. J. Dairy Technol. 2018, 71, 593–600. [Google Scholar] [CrossRef]
- Villamiel, M.; de Jong, P. Inactivation of Pseudomonas fluorescens and Streptococcus thermophilus in Trypticase® Soy Broth and total bacteria in milk by continuous-flow ultrasonic treatment and conventional heating. J. Food Eng. 2000, 45, 171–179. [Google Scholar] [CrossRef]
- Shin, J.K.; Jung, K.J.; Pyun, Y.R.; Chung, M.S. Application of pulsed electric fields with square wave pulse to milk inoculated with E. coli, P. fluorescens, and B. stearothermophilus. Food Sci. Biotechnol. 2007, 16, 1082–1084. [Google Scholar]
- Mansur, A.R.; Oh, D.-H. Combined Effect of Thermosonication and Slightly Acidic Electrolyzed Water to Reduce Foodborne Pathogens and Spoilage Microorganisms on Fresh-cut Kale. J. Food Sci. 2015, 80, M1277–M1284. [Google Scholar] [CrossRef]
- Su, Y.; Jiang, L.; Chen, D.; Yu, H.; Yang, F.; Guo, Y.; Xie, Y.; Yao, W. In vitro and in silico approaches to investigate antimicrobial and biofilm removal efficacies of combined ultrasonic and mild thermal treatment against Pseudomonas fluorescens. Ultrason. Sonochem. 2022, 83, 105930. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Speranza, B.; Iorio, M.C.; Loi, M.; Sinigaglia, M.; Corbo, M.R. US-Inactivation of foodborne bacteria: Screening in distilled water and combination with citrus extract in skim milk. LWT 2016, 70, 135–141. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skyttä, E.; Helander, I.; Ahvenainen, R. The hurdle concept. In Minimal Processing Technologies in the Food Industries; Woodhead Publishing: Cambridge, UK, 2002; pp. 174–195. [Google Scholar]
- Leistner, L.; Gould, G.W. Hurdle Technologies: Combination Treatments for Food Stability, Safety and Quality; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]

| Application | Conventional Method | Advantages | Ultrasound Principle | Products | Reference |
|---|---|---|---|---|---|
| Cutting | Knife | Small deformation Less cracks and crumbling | Cavitation phenomenon | Fragile and frozen foods Viscoelastic products Heterogenous products | [33] [34,35] [36] |
| Cooking | Stove Fried Water | Homogeneous cooking Less time | Uniform heat transfer | Poultry Beef Vegetables Fruit Crustaceans | [37,38] [39] [40] [39] [41] |
| Freezing | Ice | Less freezing time Homogeneous cooking Less damage to cells | Cavitation Fragmentation of large ice crystals Triggering secondary ice nucleation | Vegetables Meat Fish | [42] [39] [43] |
| Drying | Hot gas streaming Pulverization | Less time Improved heat transfer | Uniform heat transfer | Vegetables Meat Fish Fruit | [44] [45] [46] [47] |
| Pickling/marinating | Brine | Improved organoleptic quality Less time | Uniform heat transfer Microchannel | Meat Vegetables Cheese Fish | [48] [49] [50] [51] |
| Tenderization | Time | Improved meat tenderization | Acoustic cavitation | Meat Fish | [52] [53] |
| Organism | Matrix | Treatment | Parameter | Ultrasound Effects | Reference |
|---|---|---|---|---|---|
| Escherichia coli O157:H7 | Fresh vegetables | Ultrasound at low frequency | P: 100 W, T: 7.0 min, and I: 50 w/cm2 | Inactivation treatment (P: 100 W, T: 4 min, I: 10 w/cm2) | [80] |
| Escherichia coli O157:H7 | Milk Orange juice | Ultrasound at low frequency Ultrasound + antimicrobial peptides | P: 40 W, 160 W, T 30 min, T60 min P: 40 W, 160 W, T 30, T60 | Inactivation of inoculated E. coli Synergic effects | [81] |
| Escherichia coli O157:H7 | Bacterial cell suspension | Ultrasound at low frequency | P: 0.667 and 6.67 W/mL, I: 25.5 and 255 W/cm2, T: 0, 5, 15, 25 min | Different time for low and high intensity | [77] |
| Escherichia coli O157:H7 | Bacterial cell suspension | Ultrasound Ultrasound + nisin | P 20 W, 40 W, 60 W, and 80% by 20 kHz (total P: 950 W) 242.04 W, 484.08 W, 726.12 W, and 968.16 W/cm2; T: 15 min | Inactivation by ultrasound and with nisin | [82] |
| Escherichia coli | Cactus pear juice | Ultrasound at high frequency | P: 1500 W 20, 40, 60, and 80% by 20 kHz, t 2 sec 5 min | Inactivation 60%, 80% for 5 min | [83] |
| Escherichia coli (ATCC 11755) | Fresh carrot juice | Ultrasound + temperature | 24 kHz, 120 μm, and 400 W with temperatures of 50, 54, and 58 °C and T: 0 to 10 min | 5 log CFU/mL reduction after 2 min at 54 °C and 58 °C 3.5 log CFU/mL reduction after 10 min at 50 °C | [18] |
| Escherichia coli O157:H7 | Beef | Ultrasound at low frequency | 2.39, 6.23, 11.32, and 20.96 Wcm−2 30, 60, 90, and 120 min | 20.96 W cm−2 for 120 min was the optimal treatment for bacterial reductions | [84] |
| Escherichia coli K12 TEAG 1133 | Pork meat | Ultrasound + NaCl | P: 95 W, T: 1 h | Treatment could assist current sodium reduction strategies, improving processing time and decontamination of brining tanks, increasing the shelf-life | [85] |
| Escherichia coli | Sliced shad (Konosirus punctatus) | Ultrasound Slightly acidic electrolyzed water + ultrasound | Ultrasound 37 kHz, 380 W 0, 50, and 100 min pH range 5.0–6.5, oxidation–reduction potential 650– 1000 mV, available chlorine concentration 10–80 mg/L containing 0, 15, and 30 ppm chlorine and ultrasound 37 kHz, 380 W for 0, 50, and 100 min | Treatment not sufficient 1.04–1.86 log CFU/g Reduction in T | [79] |
| Escherichia coli K12 | Mackerel fillets | Ultrasound Ultrasound + plasma Ultrasound + peracetic acid Ultrasound + plasma-activated water + peracetic acid | 25 kHz, 550 W, 10 min 25 kHz, 550 W, 10 min + 11 L/min 25 kHz, 550 W, 10 min + 200 ppm 25 kHz, 550 W, 10 min + 11 L/min, 10 min + 200 ppm | Inactivation of 0.38 CFU/g Inactivation of 0.2 CFU/g Inactivation of 0.59 CFU/g Inactivation of 0.59 CFU/g | [86] |
| Organism | Matrix | Treatment | Parameter | Ultrasound Effects | Reference |
|---|---|---|---|---|---|
| Salmonella Typhimurium | Liquid whole egg | High-power ultrasound + lysozyme | 35–45 °C and 605–968 W/cm2 for 5–35 min | Ultrasound and ultrasound + Lys caused a reduction of 3.31 and 4.26 log10 cycles | [89] |
| Salmonella Enteritidis | Liquid whole egg | High-power ultrasound | 20 kHz HIU for 0, 1, 5, 10, and 30 min | Significant reduction in cells up to 3.6 log CFU/mL | [88] |
| Salmonella Enterica ATCC 35664 | Rice beverage | Low-power ultrasound | 20 kHz 130 W T 2, 6, 10 min P 40%, 60%, 100% | Confirmation of the strong effect of both power and time, although the correlation with the antibacterial action was not strictly linear | [91] |
| Salmonella spp. | Raw chicken meat | High-power ultrasound + carbon dioxide | 40 kHz/30 min/40 °C | Inactivation of inoculated Salmonella | [92] |
| Salmonella Typhimurium | Chicken skin | Ultrasound + ethanol | Ethanol 70% + ultrasound (37 kHz, 380 W) | Inactivation of inoculated Salmonella, change in Hunter color and skin texture | [93] |
| Salmonella Typhimurium CICC2295 | Pork meat | Ultrasound | 20 kHzf T: 10, 20, 30 min | 1–4.3 and 1–4.6 log CFU/g reduction | [85] |
| Salmonella Typhimurium | Chicken skin | Ultrasound Ultrasound + peroxyacetic acid | 37 kHz, 380 W 5 min 37 kHz, 380 W 5 min + 50–200 ppm | Treatment not sufficient Reductions of 2.21 and 2.08 log CFU/g | [94] |
| Salmonella Typhimurium ATCC 14028 | Low-fat and high-fat milk | Ultrasound Ultrasound + cinnamon essential oil | 24 kHz and 400 W power at 124 μm (100%) wave amplitude 15 min 24 kHz and 400 W power at 124 μm (100%) wave amplitude 15 min + cinnamon | Reduction of 1.6 log cycle Reduction of 2.7 log CFU/mL in low-fat milk and 3.8 log CFU/mL in high-fat milk | [95] |
| Salmonella Enterica Anatum | Chicken skin | Ultrasound Ultrasound + lactic acid aqueous solution | 40 kHz, 2.5 W/cm2 for 3 or 6 min 40 kHz, 2.5 W/cm2 for 3 or 6 min | 0.6 log CFU/cm2 1 log CFU/cm2 1.6 log CFU/cm2 2.7 log CFU/cm2 | [90] |
| Organism | Matrix | Treatment | Parameter | Ultrasound Effects | Reference |
|---|---|---|---|---|---|
| Listeria innocua | Blueberry | Ultrasound + carvacrol + carbonated water | 20 kHz 500 W, 1/3.3 MHz 10 W + solution of carvacrol | After 10 min of treatment with 2 mM carvacrol (CR), carbonated water (CW), 20 kHz ultrasound (20 kHz), or 1 MHz ultrasound (1 MHz) alone, there was a 2.4–2.6 log CFU/g reduction (P < 0.05) in bacteria from blueberry surface from the initial load of 5.2 log CFU/g | [106] |
| Listeria monocytogenes LM ATCC 19114, LM ATCC 15313, LM ATCC 19111, LM ATCC 7644 | Smoked salmon | Ultrasound + temperature | 20 kHz, 100% amplitude, 20 °C, 25 °C, 30 °C, 40 °C, 50 °C, T: 5, 10, 15 min | Inactivation was 2.02, 2.12, and 2.44 log CFU/g at 30 °C for 15 min, at 40 °C for 15 min, and at 50 °C for 5 min | [104] |
| L. monocytogenes ATCC19115 | Bacterial cell suspension | Ultrasound + cold plasma + temperature | 500 W and 40 kHz T 0, 2, 5, 10 min + plasma treatment 2 min | Inactivation by ultrasound and cold plasma, increasing temperature | [102] |
| Listeria innocua | Mackerel fillets | Ultrasound Ultrasound + plasma Ultrasound + peracetic acid Ultrasound + plasma-activated water + peracetic acid | 25 kHz, 550 W, 10 min 25 kHz, 550 W, 10 min + 11 L/min 25 kHz, 550 W, 10 min + 200 ppm 25 kHz, 550 W, 10 min + 11 L/min, 10 min + 200 ppm | Inactivation of 0.33 CFU/g Inactivation of 0.20 CFU/g Inactivation of 0.72 CFU/g Inactivation of 0.65 CFU/g | [86] |
| Listeria monocytogenes ATCC 19115 | Low-fat and high-fat milk | Ultrasound Ultrasound + cinnamon essential oil | 24 kHz and 400 W power at 124 μm (100%) wave amplitude 15 min 24 kHz and 400 W power at 124 μm (100%) wave amplitude 15 min + cinnamon | Reduction of 2.5 and 3 log cycles Reduction of 4.3 and 4.5 log cycles | [95] |
| Listeria innocua | Spinach leaves | Ultrasound Ultrasound + nanobubble | Did not significantly reduce bacteria More than 6 log CFU/mL reduction after 15 min | [107] | |
| Listeria monocytogenes | Salmon filets | Ultrasound | 200 W, 45 kHz | Reduction of 0.6 log CFU/mL | [103] |
| Organism | Matrix | Treatment | Parameter | Ultrasound Effects | Reference |
|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | Broth colony | Ultrasound | 30 kHz 100 W from 5 to 3° min | Not sufficient | [112] |
| Methicillin-resistant Staphylococcus aureus | Broth colony | Ultrasound | 20 kHz 2, 3, 4, 5, or 6 watts for 2 min | Lethal power = 8.432 watts | [113] |
| Staphylococcus aureus ATCC 25923 | Broth colony | Ultrasound | 198 W, 252 W/cm2, 20 kHz | Bacterial damage | [110] |
| Staphylococcus aureus | Chicken breast | Ultrasound | Ultrasonic bath 9.6 W/cm2 /40 kHz/0, 30, and 50 min/5 °C | S. aureus increased | [114] |
| Staphylococcus aureus | Milk | Ultrasound + temperature | 20 kHz, 600 W, 120 lm, 12 min + 60 C | 0.94 log CFU ml1 | [115] |
| Organism | Matrix | Treatment | Parameter | Ultrasound Effects | Reference |
|---|---|---|---|---|---|
| Campylobacter jejuni | Chicken carcasses | Ultrasound + vacuum | 1200 W/130 Hz/15 min + 0.1% cetylpyridinium chloride or 0.01% sodium hypochlorite and a vacuum of −0.02 MPa | From 0.94 to 1.19 log10 MPN (most probable number)/10 gr | [117] |
| Campylobacter jejuni | Chicken carcasses | Ultrasound + steam | 30 to 40 kHz and steam at 84 to 85 °C | 0.5–0.8 log CFU/g | [118] |
| Campylobacter jejuni | Chicken skin | Ultrasound Ultrasound + peroxyacetic acid | 37 kHz, 380 W 5 min 37 kHz, 380 W 5 min + 50–200 ppm | Not sufficient at 0.25 log CFU/g Reduction of 2.08 log CFU/g | [94] |
| Campylobacter coli ATCC 33559 | Water | Ultrasound | 37 kHz and 80 kH + 5 min | Frequency of 80 kHz reduction from 6.86 log CFU/mL to 3.08 log CFU/mL, 37 kHz reduction 6.75 log CFU/mL to 4.04 log CFU/mL | [119] |
| Campylobacter jejuni | Raw chicken | Ultrasound + temperature | 4, 25, and 54 °C, 40 kHz, ultrasound power of 120 W, 1, 2 or 3 min | Reduction | [120] |
| Campylobacter jejuni | Chicken carcass | Ultrasound + temperature | 90–94 °C and + t 30–40 kHz | Reduction of 0.7 log10 CFU | [121] |
| Organism | Matrix | Treatment | Parameter | Ultrasound Effects | Reference |
|---|---|---|---|---|---|
| Vibrio paraemoliticus KCTC 2471 | Sliced shad (Konosirus punctatus) | Ultrasound Slightly acidic electrolyzed water + ultrasound | Ultrasound 37 kHz, 380 W 0, 50, and 100 min pH range 5.0–6.5, oxidation–reduction potential 650– 1000 mV, available chlorine concentration 10–80 mg/L containing 0, 15, and 30 ppm chlorine and ultrasound 0.37 kHz, 380 W, 0, 50, and 100 min | Treatment not sufficient 1.02–1.42 log CFU/g reduction | [126] |
| Vibrio paraemoliticus ATCC 33847 | Raw peeled shrimp | Ultrasound Ultrasound + temperature | 0, 96, 150, and 204 W 0, 96, 150, and 204 W, 47, 50, and 53 °C | Limited reduction of 0.59, 0.60, and 0.68 log CFU/g 47 °C reductions of 1.76, 2.63, and 4.01 log CFU/g 96, 150, and 204 W, respectively, for 8 min | [82] |
| Vibrio vulnificus | Oysters (Crossostrea virginica) | Ultrasound | 100 W, 500 W/cm for 30 min | Treatment not sufficient | [127] |
| Vibrio cholerae | Broth solution | Ultrasound Ultrasound + ozone | 40 kHz, 150 W 10 min | Treatment not sufficient Treatment not sufficient | [128] |
| Organism | Matrix | Treatment | Parameter | Ultrasound Effects | Reference |
|---|---|---|---|---|---|
| Pseudomonas fluorescens | Mackerel fillets | Ultrasound Ultrasound + plasma Ultrasound + peracetic acid Ultrasound + plasma-activated water + peracetic acid | 25 kHz, 550 W, 10 min 25 kHz, 550 W, 10 min + 11 L/min 25 kHz, 550 W, 10 min + 200 ppm 25 kHz, 550 W, 10 min + 11 L/min, 10 min + 200 ppm | Inactivation of 0.50 CFU/g Inactivation of 0.13 CFU/g Inactivation of 0.46 CFU/g Inactivation of 0.30 CFU/g | [86] |
| Pseudomonas fluorescens | Chicken skin | Ultrasound Ultrasound + lactic acid aqueous solution | 40 kHz, 2.5 W/cm2 for 3 or 6 min 40 kHz, 2.5 W/cm2 for 3 or 6 min | 0.5 log CFU/cm2 1 log CFU/cm2 3 log CFU/cm2 4.1 log CFU/cm2 | [94] |
| Pseudomonas fluorescens | Milk | Ultrasound + temperature | 20 kHz, 150 W + 62 °C | 3.1 CFU/mL | [131] |
| Pseudomonas fluorescens | Raw milk | Ultrasound + temperature | 60 kV/cm, 200 μs, 40 °C (Tin) | 5 CFU/mL | [132] |
| Pseudomonas putida | Milk | Ultrasound | 20 kHz, 100 W | Bacteriostatic effect | [133] |
| Pseudomonas fluorescens | Fresh-cut kale | Ultrasound + temperature | 100 W/L at 25, 40, or 50 °C | Reduced by 3 log CFU/mL | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauteri, C.; Ferri, G.; Piccinini, A.; Pennisi, L.; Vergara, A. Ultrasound Technology as Inactivation Method for Foodborne Pathogens: A Review. Foods 2023, 12, 1212. https://doi.org/10.3390/foods12061212
Lauteri C, Ferri G, Piccinini A, Pennisi L, Vergara A. Ultrasound Technology as Inactivation Method for Foodborne Pathogens: A Review. Foods. 2023; 12(6):1212. https://doi.org/10.3390/foods12061212
Chicago/Turabian StyleLauteri, Carlotta, Gianluigi Ferri, Andrea Piccinini, Luca Pennisi, and Alberto Vergara. 2023. "Ultrasound Technology as Inactivation Method for Foodborne Pathogens: A Review" Foods 12, no. 6: 1212. https://doi.org/10.3390/foods12061212





