Structure, Merits, Gel Formation, Gel Preparation and Functions of Konjac Glucomannan and Its Application in Aquatic Food Preservation
Abstract
1. Introduction
2. Konjac Glucomannan and Its Structural Features
2.1. Sources
2.2. Structure
2.2.1. Hydrogen Bond Network
2.2.2. Helical Structure
| Object | Research Contents | Result | Method | Reference |
|---|---|---|---|---|
| KGM chain of vacuum | Influence of the degree of polymerization, acetyl group and nonbonding force on the chain morphology | Acetyl is important in maintaining the helical structure of the KGM molecule; the degree of polymerization of KGM significantly affects the shape and stability of its helical structure | Molecular dynamics | [30] |
| Unbranched KGM | KGM helix formation site, helix parameters and hydrogen bond sites | The KGM molecular chain may form a helix on the segments containing acetyl groups | Computer simulation | [35] |
| KGM mono-helix | Local maximum water density near the KGM single-helix | The left-handed single helical conformation is the dominant conformation of KGM in an aqueous environment | Molecular dynamics | [26] |
2.2.3. Topological Structure
2.3. Merits of KGM for Aquatic Food Preservation
2.3.1. Film Forming
2.3.2. Gelation
2.3.3. Water Retention
2.3.4. Thickening
3. Formation Mechanisms of KGM Gels
3.1. Self-Assembly/Conjugate Structure
3.2. Chain Coupling Perforation
3.3. Combination Mechanism of “Polyphenol-Embedded Topological Protection”
4. Preparation of a KGM Gel Carrier Loaded with Active Molecules
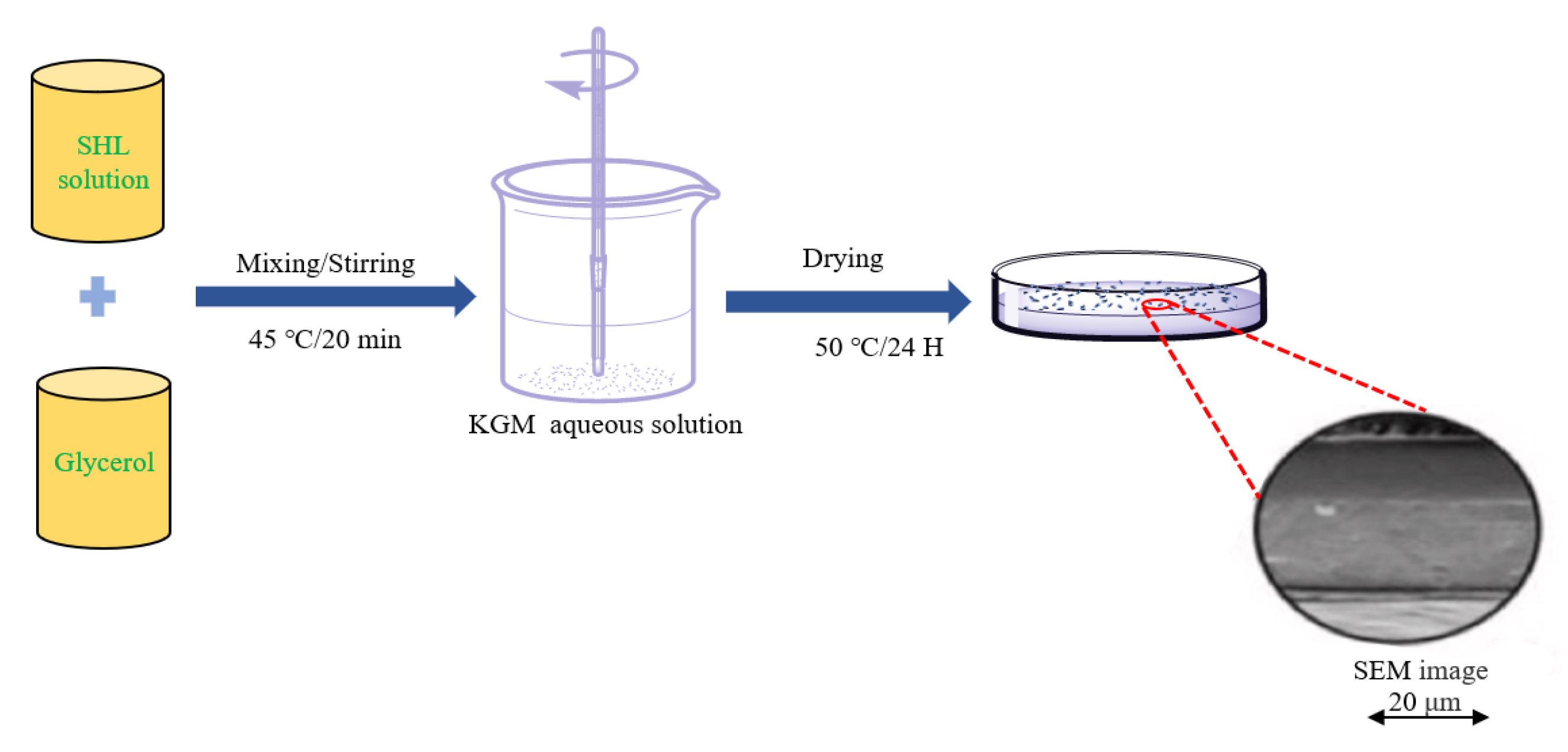
4.1. Casting Method
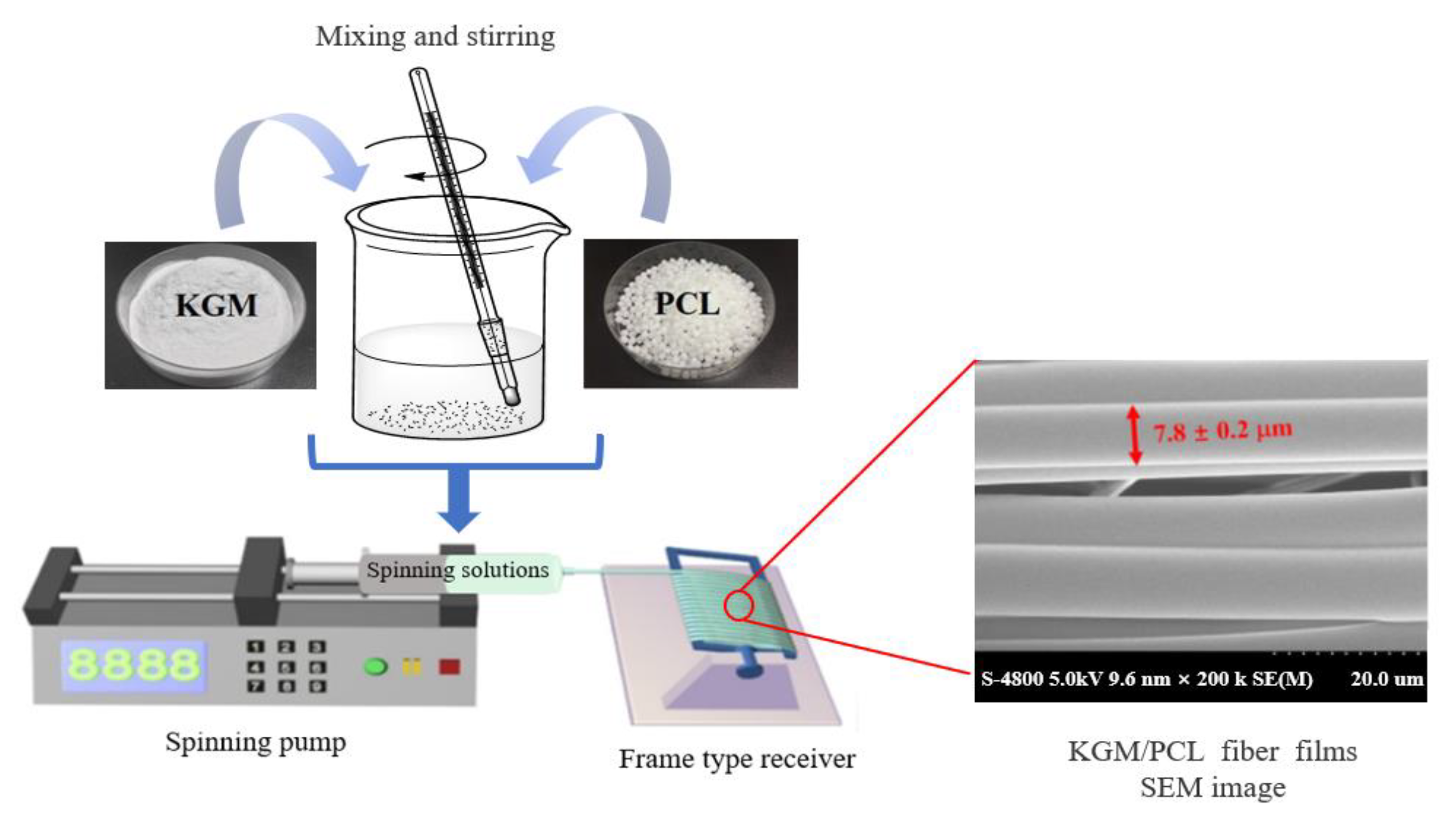
4.2. Microfluidic Spinning Technology (MST)
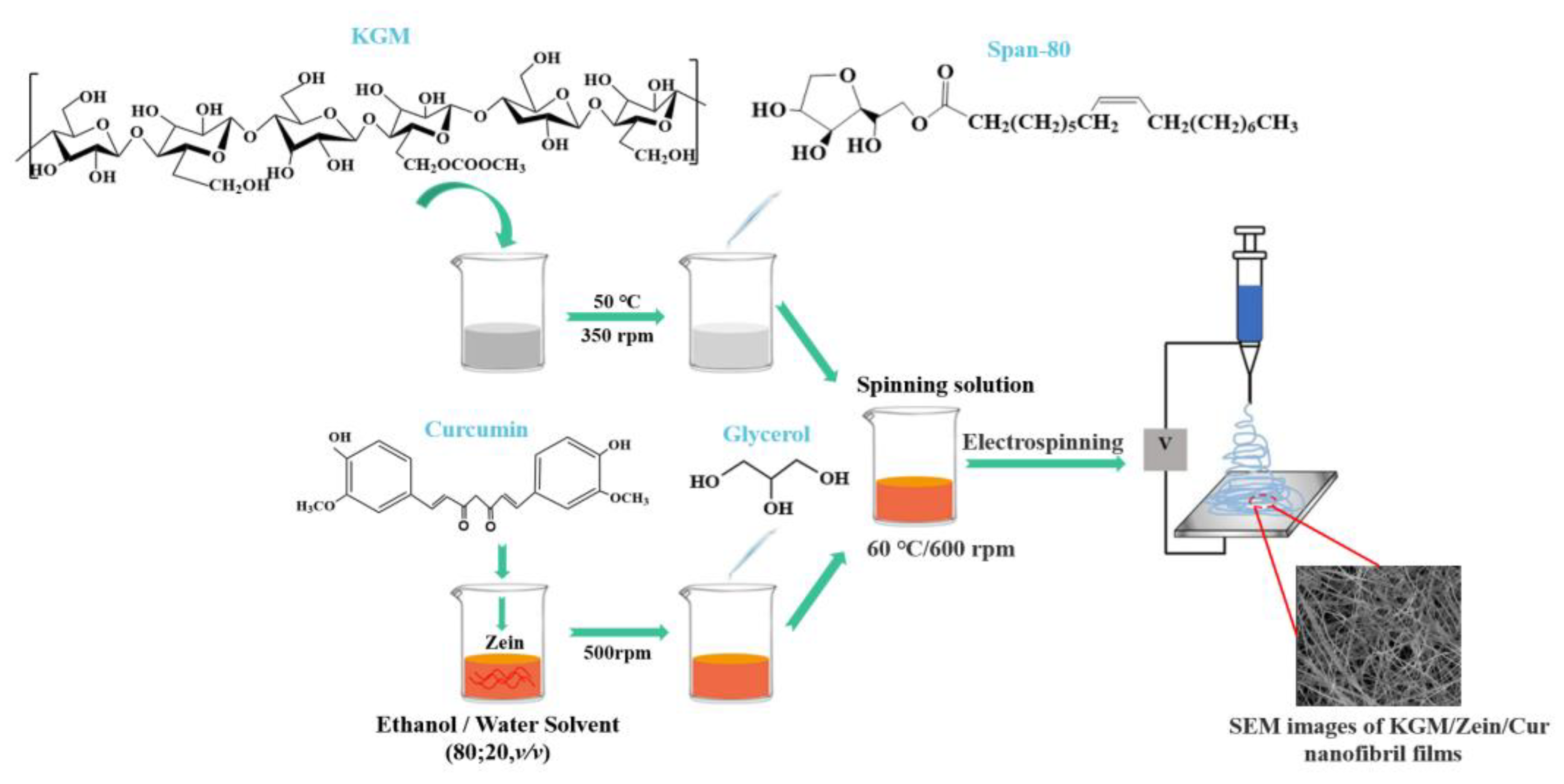
4.3. Electrospinning
4.4. Sol-Gel Conversion Compounding Method
5. Functional Properties of KGM Gel
5.1. Structural Stability
5.2. Oxidation Resistance and Antibacterial Activity
5.3. Sustained Release Capability
5.4. Permeability
5.4.1. Water Vapor Permeability (WVP)
5.4.2. Oxygen Permeability (OP)
5.5. Mechanical Properties
6. Application of KGM Gel in Aquatic Foods
6.1. KGM-Based Sustained Release Gel Loaded with ECGC
6.2. KGM-Based Films Loaded with Anthocyanins
6.3. KGM-Based Composite Coating
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, N.; Zheng, S.; Xie, W.; Cao, G.; Wang, L.; Pang, J. Konjac glucomannan: A review of structure, physicochemical properties, and wound dressing applications. J. Appl. Polym. Sci. 2022, 139, 51780. [Google Scholar] [CrossRef]
- Yang, D.; Yuan, Y.; Wang, L.; Wang, X.; Mu, R.; Pang, J.; Xiao, J.; Zheng, Y. A review on konjac glucomannan gels: Microstructure and application. Int. J. Mol. Sci. 2017, 18, 2250. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhang, S.; Yi, J.; Zhu, Z.; Decker, E.A.; McClements, D.J. The impact of konjac glucomannan on the physical and chemical stability of walnut oil-in-water emulsions coated by whey proteins. J. Sci. Food Agric. 2022, 102, 4003–4011. [Google Scholar] [CrossRef]
- Xingqi, W.; Yong, Z.; Xing, L.; Yang, W.; Jie, H.; Rongfeng, H.; Shuangying, G.; Xiaoqin, C. Cubic and hexagonal liquid crystal gels for ocular delivery with enhanced effect of pilocarpine nitrate on anti-glaucoma treatment. Drug Deliv. 2019, 26, 952–964. [Google Scholar] [CrossRef]
- Song, W.; Wang, Y.; Deng, H. Ion-conducting polymer gels of polyacrylamide embedded with K2CO3. J. Appl. Polym. Sci. 2004, 92, 2076–2081. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Y.; Wang, Y.; Qi, W.; Huang, R.; Su, R.; He, Z. Self-assembled microporous peptide-polysaccharide aerogels for oil-water separation. Langmuir 2018, 34, 10732–10738. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Shen, M.; Wang, Z.; Xie, J. Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. Trends Food Sci. Technol. 2021, 118, 539–557. [Google Scholar] [CrossRef]
- Hong, X.; Ni, Y.; Lin, W.; Mu, R.; Wang, L.; Pang, J.; Wu, C.; Wen, C. Study on the epigallocatechin gallate and konjac glucomannan mosaic topological structure. Chin. J. Struct. Chem. 2017, 36, 1447–1455. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 66–105. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Beigmohammadi, F.; Peighambardoust, S.J. Application of organoclay nanoparticle in low-density polyethylene films for packaging of UF cheese. Packag. Technol. Sci. 2016, 29, 355–363. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J. Nanocomposite films containing organoclay nanoparticles as an antimicrobial (active) packaging for potential food application. J. Food Process. Preserv. 2018, 42, e13488. [Google Scholar] [CrossRef]
- Tafti, A.G.; Peighambardoust, S.H.; Hesari, J.; Bahrami, A.; Bonab, E.S. Physico-chemical and functional properties of spray-dried sourdough in breadmaking. Food Sci. Technol. Int. 2013, 19, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Sapper, M.; Chiralt, A. Starch-based coatings for preservation of fruits and vegetables. Coatings 2018, 8, 152. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Karbowiak, T.; Hervet, H.; Léger, L.; Champion, D.; Debeaufort, F.; Voilley, A. Effect of plasticizers (water and glycerol) on the diffusion of a small molecule in iota-carrageenan biopolymer films for edible coating application. Biomacromolecules 2006, 7, 2011–2019. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, S. Application of chitosan based coating in fruit and vegetable preservation: A review. J. Food Process. Technol. 2013, 4, 227. [Google Scholar]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Lin, L.; Mu, R.; Pang, J. Novel synthesis of mussel inspired and Fe3+ induced pH-sensitive hydrogels: Adhesion, injectable, shapeable, temperature properties, release behavior and rheological characterization. Carbohydr. Polym. 2020, 236, 116045. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Q.; Cao, J.; Zhou, D.; Li, C. Mechanisms of polyphenols on quality control of aquatic products in storage: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–20, ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, X. Research hotspots and development trends of konjac based on bibliometric analysis. Hortscience 2022, 57, 1363–1376. [Google Scholar] [CrossRef]
- Cheng, L.H.; Abd Karim, A.; Seow, C.C. Characterisation of composite films made of konjac glucomannan (KGM), carboxymethyl cellulose (CMC) and lipid. Food Chem. 2008, 107, 411–418. [Google Scholar] [CrossRef]
- Sun, Y.; Guang, Y.; Zhu, Y.; Tian, S. Molecular dynamics simulation of glucomannan solution. Chin. J. Struct. Chem. 2005, 24, 841–845. [Google Scholar] [CrossRef]
- Pang, J.; Sun, Y.; Guan, Y.; Shiping, T. Studies on the effect of structure to property stability of glucomannan. Chin. J. Struct. Chem. 2005, 24, 1061–1065. [Google Scholar] [CrossRef]
- Pang, J.; Sun, Y.; Sun, Y. Studies on single chain structure of konjac glucomannan. Chin. J. Struct. Chem. 2006, 25, 1441–1448. [Google Scholar]
- Ni, Y.; Mu, R.; Tan, X.; Huang, R.; Yuan, Y.; Chen, H.; Pang, J. Stability of the konjac glucomannan topological chain based on quantum spin model. Chin. J. Struct. Chem. 2017, 36, 1043–1048. [Google Scholar] [CrossRef]
- Ran, X.; Lou, X.; Zheng, H.; Gu, Q.; Yang, H. Improving the texture and rheological qualities of a plant-based fishball analogue by using konjac glucomannan to enhance crosslinks with soy protein. Innov. Food Sci. Emerg. Technol. 2022, 75, 102910. [Google Scholar] [CrossRef]
- Fittolani, G.; Seeberger, P.H.; Delbianco, M. Helical polysaccharides. Pept. Sci. 2020, 112, e24124. [Google Scholar] [CrossRef]
- Jian, W.; Yao, M.; Wang, M.; Guan, Y.; Pang, J. Formation mechanism and stability study of konjac glucomannan helical structure. Chin. J. Struct. Chem. 2010, 29, 543–550. [Google Scholar] [CrossRef]
- Paradossi, G.; Chiessi, E.; Barbiroli, A.; Fessas, D. Xanthan and glucomannan mixtures: synergistic interactions and gelation. Biomacromolecules 2002, 3, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tian, J.; Zou, J.; Yuan, X.; Li, J.; Liang, H.; Zhan, F.; Li, B. Partial removal of acetyl groups in konjac glucomannan significantly improved the rheological properties and texture of konjac glucomannan and K-carrageenan blends. Int. J. Biol. Macromol. 2019, 123, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wei, L.; Zhang, W.; Lei, Y.; Kong, Q.; Zhang, B. Production, characterization, and prebiotic activity of oligosaccharides from konjac glucomannan by Bacillus amyloliquefaciens WX-1. J. Funct. Foods 2022, 88, 104872. [Google Scholar] [CrossRef]
- Yui, T.; Ogawa, K.; Sarko, A. Molecular and crystal structure of konjac glucomannan in the mannan II polymorphic form. Carbohydr. Res. 1992, 229, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Wang, M.; Yao, M.; Pang, J. Formation sites and microscopic conformation study on the konjac glucomannan molecular helices. Chin. J. Struct. Chem. 2010, 29, 1084–1090. [Google Scholar]
- Zeng, S.; Long, J.; Sun, J.; Wang, G.; Zhou, L. A review on peach gum polysaccharide: Hydrolysis, structure, properties and applications. Carbohydr. Polym. 2022, 279, 119015. [Google Scholar] [CrossRef]
- Phillips, J.C. Topology of covalent non-crystalline solids I: Short-range order in chalcogenide alloys. J. Non-Cryst. Solids 1979, 34, 153–181. [Google Scholar] [CrossRef]
- Liu, D.; Yin, G.; Le, X.; Chen, T. Supramolecular topological hydrogels: From material design to applications. Polym. Chem. 2022, 13, 1940–1952. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Mao, C.; Song, Z.; Li, X.; Liu, C. Preparation and characterization of konjac glucomannan and gum arabic composite gel. J. Biol. Macromol. 2021, 183, 2121–2130. [Google Scholar] [CrossRef]
- Cheng, H.; Mu, R.; Pang, J.; Tan, X.; Lin, H.; Ma, Z.; Chiang, W. Influence of topology structure on the stability of konjac glucomannan nano gel microfibril. Chin. J. Struct. Chem. 2015, 34, 1939–1941. [Google Scholar] [CrossRef]
- Ni, Y.; Lin, W.; Li, Y.; Wang, L.; Wang, W.; Zhang, X.; Lin, Y.; Wu, X.; Pang, J. The mechanism of konjac glucomannan on the stability of topological chain and tea polyphenols. Food Ferment. Ind. 2017, 43, 78–82. [Google Scholar]
- Ratcliffe, I.; Williams, P.A.; Viebke, C.; Meadows, J. Physicochemical characterization of konjac glucomannan. Biomacromolecules 2005, 6, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.D.; Yang, F.Q. Konjac glucomannan, a promising polysaccharide for OCDDS. Carbohydr. Polym. 2014, 104, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Korkiatithaweechai, S.; Umsarika, P.; Praphairaksit, N.; Muangsin, N. Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar. Drugs 2011, 9, 1649–1663. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, S.; Liang, H.; He, C.; Li, J.; Li, B. The influence of deacetylation degree of konjac glucomannan on rheological and gel properties of konjac glucomannan/κ-carrageenan mixed system. Food Hydrocoll. 2020, 101, 105523. [Google Scholar] [CrossRef]
- Yuan, Y.; Yan, Z.; Mu, R.; Wang, L.; Gong, J.; Hong, X.; Haruna, M.H.; Pang, J. The effects of graphene oxide on the properties and drug delivery of konjac glucomannan hydrogel. J. Appl. Polym. Sci. 2017, 134, 45327. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res. Int. 2012, 46, 270–278. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Z.; Pei, Y.; Li, J.; Li, B. Gelation behaviors of the konjac gum from different origins: A. guripingensis and A. rivirei. Food Hydrocoll. 2021, 111, 106152. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, J.; Bandral, J.D.; Gani, A.; Shams, R. Food hydrocolloids: Functional, nutraceutical and novel applications for delivery of bioactive compounds. Int. J. Biol. Macromol. 2020, 165, 554–567. [Google Scholar] [CrossRef]
- Shah, J.C.; Sadhale, Y.; Chilukuri, D.M. Cubic phase gels as drug delivery systems. Adv. Drug Deliv. Rev. 2001, 47, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Water distribution in tofu and application of T2 relaxation measurements in determination of tofu’s water-holding capacity. J. Agric. Food Chem. 2014, 62, 8594–8601. [Google Scholar] [CrossRef] [PubMed]
- Tatirat, O.; Charoenrein, S. Physicochemical properties of konjac glucomannan extracted from konjac flour by a simple centrifugation process. LWT 2011, 44, 2059–2063. [Google Scholar] [CrossRef]
- Wang, S.; Zhan, Y.; Wu, X.; Ye, T.; Li, Y.; Wang, L.; Chen, Y.; Li, B. Dissolution and rheological behavior of deacetylated konjac glucomannan in urea aqueous solution. Carbohydr. Polym. 2014, 101, 499–504. [Google Scholar] [CrossRef]
- Genevro, G.M.; de Moraes, M.A.; Beppu, M.M. Freezing influence on physical properties of glucomannan hydrogels. Int. J. Biol. Macromol. 2019, 128, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, D.; Liu, Y.; Xiong, Y.; Peng, J.; Mahmud, S.; Liu, H. Gold/konjac glucomannan bionanocomposites for catalytic degradation of mono-azo and di-azo dyes. Inorg. Chem. Commun. 2020, 120, 108156. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, X.; Zhang, L.; Chen, J. Preparation and performance of thickened liquids for patients with konjac glucomannan-mediated dysphagia. Molecules 2022, 27, 2194. [Google Scholar] [CrossRef]
- Tian, D.; Xie, H. Graft copolymerization of acrylamide onto konjac glucomannan via inverse emulsion polymerization and its thickening properties. J. Appl. Polym. Sci. 2008, 108, 3122–3127. [Google Scholar] [CrossRef]
- Wei, Y.; Guo, Y.; Li, R.; Ma, A.; Zhang, H. Rheological characterization of polysaccharide thickeners oriented for dysphagia management: Carboxymethylated curdlan, konjac glucomannan and their mixtures compared to xanthan gum. Food Hydrocoll. 2021, 110, 106198. [Google Scholar] [CrossRef]
- Felix Da Silva, D.; Barbosa De Souza Ferreira, S.; Bruschi, M.L.; Britten, M.; Matumoto-Pintro, P.T. Effect of commercial konjac glucomannan and konjac flours on textural, rheological and microstructural properties of low fat processed cheese. Food Hydrocoll. 2016, 60, 308–316. [Google Scholar] [CrossRef]
- Devaraj, R.D.; Reddy, C.K.; Xu, B. Health-promoting effects of konjac glucomannan and its practical applications: A critical review. Int. J. Biol. Macromol. 2019, 126, 273–281. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2003, 92, 1343–1355. [Google Scholar] [CrossRef] [PubMed]
- Rotureau, E.; Chassenieux, C.; Dellacherie, E.; Durand, A. Neutral polymeric surfactants derived from dextran: A study of their aqueous solution behavior. Macromol. Chem. Phys. 2005, 206, 2038–2046. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- McLeish, T. A tangled tale of topological fluids. Phys. Today 2008, 61, 40–45. [Google Scholar] [CrossRef]
- Theodorou, D.N. Hierarchical modelling of polymeric materials. Chem. Eng. Sci. 2007, 62, 5697–5714. [Google Scholar] [CrossRef]
- Pang, J.; Ma, Z.; Shen, B.; Xu, Q.; Sun, Z.; Fu, L.; Fang, W.; Wen, C. Hydrogen bond networks’ QSAR and topological analysis of konjac glucomannan chains. Chin. J. Struct. Chem. 2014, 33, 480–489. [Google Scholar] [CrossRef]
- Zhu, L. Effects of reduced frequency on network configuration and synchronization transition. Chin. Phys. Lett. 2016, 33, 14–17. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Yang, B.; Saeed Bahramy, M.; Nagaosa, N. Topological protection of bound states against the hybridization. Nat. Commun. 2013, 4, 1524. [Google Scholar] [CrossRef]
- Petelski, A.N.; Pamies, S.C.; Benítez, E.I.; Rovaletti, M.M.L.; Sosa, G.L. Molecular insights into protein-polyphenols aggregation: A dynamic and topological description. Chemistryselect 2017, 2, 5608–5615. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Mu, R.; Wang, Y.; Li, Y.; Wu, D.; Wu, C.; Pang, J. Fabrication of novel konjac glucomannan/shellac film with advanced functions for food packaging. Int. J. Biol. Macromol. 2019, 131, 36–42. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Li, M.; Lu, Y.; Du, Y.; Tong, C.; Pang, J.; Wu, C. Preparation and characterization of multifunctional konjac glucomannan/carboxymethyl chitosan biocomposite films incorporated with epigallocatechin gallate. Food Hydrocoll. 2020, 105, 105756. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.; Guo, Y.; Long, J.; Mu, R.; Pang, J. Enhanced functional properties of nanocomposite film incorporated with EGCG-loaded dialdehyde glucomannan/gelatin matrix for food packaging. Food Hydrocoll. 2020, 108, 105863. [Google Scholar] [CrossRef]
- Wu, Z.; Tong, C.; Zhang, J.; Sun, J.; Jiang, H.; Duan, M.; Wen, C.; Wu, C.; Pang, J. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of konjac glucomannan/cellulose nanocrystal bionanocomposite films incorporated with phlorotannin from sargassum. Int. J. Biol. Macromol. 2021, 192, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, Y.; Du, Y.; Wang, L.; Tong, C.; Hu, Y.; Pang, J.; Yan, Z. Preparation and characterization of konjac glucomannan-based bionanocomposite film for active food packaging. Food Hydrocoll. 2019, 89, 682–690. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J.; Wu, C. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Xie, W.; Du, Y.; Yuan, S.; Pang, J. Dihydromyricetin incorporated active films based on konjac glucomannan and gellan gum. Int. J. Biol. Macromol. 2021, 180, 385–391. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, L.; You, P.; Wang, L.; Mu, R.; Pang, J. Preparation of pH-sensitive food packaging film based on konjac glucomannan and hydroxypropyl methyl cellulose incorporated with mulberry extract. Int. J. Biol. Macromol. 2021, 172, 515–523. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, J.; Li, Y.; Ma, J.; Lu, Y.; Pang, J.; Wu, C. Preparation and characterization of citric acid crosslinked konjac glucomannan/surface deacetylated chitin nanofibers bionanocomposite film. Int. J. Biol. Macromol. 2020, 164, 2612–2621. [Google Scholar] [CrossRef]
- Du, Y.; Sun, J.; Wang, L.; Wu, C.; Gong, J.; Lin, L.; Mu, R.; Pang, J. Development of antimicrobial packaging materials by incorporation of gallic acid into Ca2+ crosslinking konjac glucomannan/gellan gum films. Int. J. Biol. Macromol. 2019, 137, 1076–1085. [Google Scholar] [CrossRef]
- Tong, C.; Wu, Z.; Sun, J.; Lin, L.; Wang, L.; Guo, Y.; Huang, Z.; Wu, C.; Pang, J. Effect of carboxylation cellulose nanocrystal and grape peel extracts on the physical, mechanical and antioxidant properties of konjac glucomannan films. Int. J. Biol. Macromol. 2020, 156, 874–884. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, L.; McClements, D.J.; Yang, T.; Zhang, Z.; Ren, F.; Miao, M.; Tian, Y.; Jin, Z. Starch-based biodegradable packaging materials: A review of their preparation, characterization and diverse applications in the food industry. Trends Food Sci. Technol. 2021, 114, 70–82. [Google Scholar] [CrossRef]
- Lin, W.; Ni, Y.; Liu, D.; Yao, Y.; Pang, J. Robust microfluidic construction of konjac glucomannan-based micro-films for active food packaging. Int. J. Biol. Macromol. 2019, 137, 982–991. [Google Scholar] [CrossRef]
- Ma, K.; Du, X.; Zhang, Y.; Chen, S. In situ fabrication of halide perovskite nanocrystals embedded in polymer composites via microfluidic spinning microreactors. J. Mater. Chem. C 2017, 5, 9398–9404. [Google Scholar] [CrossRef]
- Ni, Y.; Lin, W.; Mu, R.; Wu, C.; Lin, Z.; Chen, S.; Pang, J. Facile fabrication of novel konjac glucomannan films with antibacterial properties via microfluidic spinning strategy. Carbohydr. Polym. 2019, 208, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ni, Y.; Pang, J. Size effect-inspired fabrication of konjac glucomannan/polycaprolactone fiber films for antibacterial food packaging. Int. J. Biol. Macromol. 2020, 149, 853–860. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, Y.; Zhang, W.; Shi, S.; Zhu, W.; Wang, R.; Zhang, L.; Chen, L.; Sun, J.; Pang, J.; et al. Advanced konjac glucomannan-based films in food packaging: Classification, preparation, formation mechanism and function. LWT 2021, 152, 112338. [Google Scholar] [CrossRef]
- Wang, L.; Mu, R.; Li, Y.; Lin, L.; Lin, Z.; Pang, J. Characterization and antibacterial activity evaluation of curcumin loaded konjac glucomannan and zein nanofibril films. LWT 2019, 113, 108293. [Google Scholar] [CrossRef]
- Norizan, M.N.; Shazleen, S.S.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Zainudin, E.S.; Abdullah, N.; Samsudin, M.S.; Kamarudin, S.H.; Norrrahim, M.N.F. Nanocellulose-based nanocomposites for sustainable applications: A review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef]
- Li, J.; Ma, J.; Chen, S.; He, J.; Huang, Y. Characterization of calcium alginate/ deacetylated konjac glucomannan blend films prepared by Ca2+ crosslinking and deacetylation. Food Hydrocoll. 2018, 82, 363–369. [Google Scholar] [CrossRef]
- Peressini, D.; Bravin, B.; Lapasin, R.; Rizzotti, C.; Sensidoni, A. Starch-methylcellulose based edible films: Rheological properties of film-forming dispersions. J. Food Eng. 2003, 59, 25–32. [Google Scholar] [CrossRef]
- Qiao, D.; Li, H.; Jiang, F.; Zhao, S.; Chen, S.; Zhang, B. Incorporation of κ-carrageenan improves the practical features of agar/konjac glucomannan/κ-carrageenan ternary system. Food Sci. Hum. Wellness 2023, 12, 512–519. [Google Scholar] [CrossRef]
- Wu, K.; Li, X.; Yan, X.; Wan, Y.; Miao, L.; Xiao, M.; Jiang, F.; Chen, S. Impact of curdlan addition on the properties of konjac glucomannan/ethyl cellulose composite films. Starch-Stärke 2022, 74, 2100194. [Google Scholar] [CrossRef]
- Aversa, R.; Modarres, M.H.; Cozzini, S.; Ciancio, R.; Chiusole, A. The first annotated set of scanning electron microscopy images for nanoscience. Sci. Data 2018, 5, 180172. [Google Scholar] [CrossRef] [PubMed]
- Krieg, M.; Fläschner, G.; Alsteens, D.; Gaub, B.M.; Roos, W.H.; Wuite, G.J.; Gaub, H.E.; Gerber, C.; Dufrêne, Y.F.; Müller, D.J. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 2019, 1, 41–57. [Google Scholar] [CrossRef]
- Poolman, J.T.; Anderson, A.S. Escherichia coli and staphylococcus aureus: Leading bacterial pathogens of healthcare associated infections and bacteremia in older-age populations. Expert Rev. Vaccines 2018, 17, 607–618. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Wang, L.; Zhou, N.; Yang, Y.; Pang, J. A pH-intelligent response fish packaging film: Konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int. J. Biol. Macromol. 2022, 204, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, Q.; Qian, H.; Xiao, M.; Corke, H.; Nishinari, K.; Jiang, F. Controllable hydrophilicity-hydrophobicity and related properties of konjac glucomannan and ethyl cellulose composite films. Food Hydrocoll. 2018, 79, 301–309. [Google Scholar] [CrossRef]
- Afsana, S.; Molla, M.M.; Nag, P.; Saha, L.K.; Siddiqa, S. MHD natural convection and entropy generation of non-Newtonian ferrofluid in a wavy enclosure. Int. J. Mech. Sci. 2021, 198, 106350. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Sun, J.; Lu, Y.; Tong, C.; Wang, L.; Yan, Z.; Pang, J. Novel konjac glucomannan films with oxidized chitin nanocrystals immobilized red cabbage anthocyanins for intelligent food packaging. Food Hydrocoll. 2020, 98, 105245. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Bhagwat, P.K.; Ozogul, F.; Benjakul, S.; Pillai, S.; Annapure, U.S. Cold plasma for the preservation of aquatic food products: An overview. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4407–4425. [Google Scholar] [CrossRef]
- Rhim, J. Effect of clay contents on mechanical and water vapor barrier properties of agar-based nanocomposite films. Carbohydr. Polym. 2011, 86, 691–699. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef]
- Jia, D.; Fang, Y.; Yao, K. Water vapor barrier and mechanical properties of konjac glucomannan-chitosan-soy protein isolate edible films. Food Bioprod. Process. 2009, 87, 7–10. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.; Sun, J.; Jiang, G. A novel functionalized food packaging film with microwave-modified konjac glucomannan/chitosan/citric acid incorporated with antioxidant of bamboo leaves. LWT 2022, 166, 113780. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Shen, R.; Yang, X. Characterizations of novel konjac glucomannan emulsion films incorporated with high internal phase pickering emulsions. Food Hydrocoll. 2020, 109, 106088. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Meng, X.; Wang, Z.; Wu, S. Preparation and characterization of κ-carrageenan/konjac glucomannan/TiO2 nanocomposite film with efficient anti-fungal activity and its application in strawberry preservation. Food Chem. 2021, 364, 130441. [Google Scholar] [CrossRef]
- Shaikh, M.; Haider, S.; Ali, T.M.; Hasnain, A. Physical, thermal, mechanical and barrier properties of pearl millet starch films as affected by levels of acetylation and hydroxypropylation. Int. J. Biol. Macromol. 2019, 124, 209–219. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed polysaccharide in food contact materials (active packaging, intelligent packaging, edible films, and coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J. Recent progress in konjac glucomannan-based active food packaging films and property enhancement strategies. Food Hydrocoll. 2022, 128, 107572. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.V.; Nugraha, C.; Ng, X.W.; Venkatraman, S. Sustained-release from nanocarriers: A review. J. Control. Release 2014, 193, 122–138. [Google Scholar] [CrossRef]
- Wang, Y.L.; Liang, P.; Wu, J.N.; Zheng, T.; Xie, J.H.; Pang, J. Blackening and blackening control of litopenaeus vannamei during storage at low temperature. CYTA J. Food 2022, 20, 50–59. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J. Anthocyanins: Promising natural products with diverse pharmacological activities. Molecules 2021, 26, 3807. [Google Scholar] [CrossRef] [PubMed]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Zheng, P.; Kang, X.; Chen, M.; Li, Y.; Ge, Y.; Hu, Y.; Pang, J. Preparation of an intelligent film based on chitosan/oxidized chitin nanocrystals incorporating black rice bran anthocyanins for seafood spoilage monitoring. Carbohydr. Polym. 2019, 222, 115006. [Google Scholar] [CrossRef]
- Cao, G.; Bu, N.; Zeng, T.; Sun, R.; Mu, R.; Pang, J.; Wang, L. Development of pH-responsive konjac glucomannan/pullulan films incorporated with acai berry extract to monitor fish freshness. Int. J. Biol. Macromol. 2022, 219, 897–906. [Google Scholar] [CrossRef]
- Yerlikaya, P.; Yatmaz, H.A.; Topuz, O.K. Applications of edible films and coatings in aquatic foods. In Innovative Technologies in Seafood Processing; CRC Press: New York, NY, USA, 2019; pp. 71–91. [Google Scholar]
- Wei, X.; Pang, J.; Zhang, C.; Yu, C.; Chen, H.; Xie, B. Structure and properties of moisture-resistant konjac glucomannan films coated with shellac/stearic acid coating. Carbohydr. Polym. 2015, 118, 119–125. [Google Scholar] [CrossRef]
- Pang, J.; Chen, Y.; Chen, Y.; Lin, L.; Chen, S.; Zheng, S. A Modified Atmosphere Preservation Method for Whole Yellow Croaker. CN111802443A, 23 October 2020. (In Chinese). [Google Scholar]
- Xiao, H.; Liao, J.; Chen, Y.; Tong, X.; Sun, X.; Yan, J.; Pang, J. Effects of konjac glucomannan/ε-Polylysine hydrochloride/ferulic acid composite coating on the freshness preservation performance and flavor of refrigerated sea bass fillets. Foods 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]

| Object | Research Contents | Result | Method | Reference |
|---|---|---|---|---|
| KGM single chain in vacuum | The influence of the degree of polymerization and substituents on dynamic conformation | The degree of polymerization affects the chain conformation and stability | Molecular dynamics | [26] |
| KGM segments in solution | The influence of the hydrogen bond change in the KGM chain segment on the structure and energy | A hydrogen bond is the main factor affecting the conformation and properties of KGM molecules | Molecular dynamics | [24] |
| KGM | The effect of pH on the types and quantities of hydrogen bonds in KGM | The gel strength of KGM is increased under alkaline conditions | Molecular dynamics | [25] |
| KGM chain hydrogen network | The stability of the hydrogen network of the KGM chain | Increasing the formation of hydrogen bonds decreases the energy of the acetyl system | Quantum spin model | [27] |
| KGM | Application of KGM in developing plant-based fish balls | KGM promotes the formation of the hydrogen bond and ordered structure | Rheological method | [28] |
| Object | Research Contents | Result | Method | Reference |
|---|---|---|---|---|
| KGM nanogel microfibril | The topological structure of KGM nanogels and nanofibers | Electrospinning improves the intermolecular hydrogen bonding and topological entanglement of KGM molecules | FT-IR, FESEM, DSC | [40] |
| KGM/EGCG nanofibers | Characterizing the microstructure of the nanofibers and discussing the mechanism of formation and the protective effect of KGM/EGCG nanofibers | KGM/EGCG nanofibers exhibit greater antioxidant activity than EGCG solution | Experimental and theoretical analysis | [8] |
| KGM-TP | The microstructure and thermal stability of KGM-TP gel | The KGM topology chain protects TP and has a high degree of release | Direct current | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xu, X.; Wu, Z.; Zhou, H.; Xie, X.; Zhang, Q.; Liu, R.; Pang, J. Structure, Merits, Gel Formation, Gel Preparation and Functions of Konjac Glucomannan and Its Application in Aquatic Food Preservation. Foods 2023, 12, 1215. https://doi.org/10.3390/foods12061215
Sun Y, Xu X, Wu Z, Zhou H, Xie X, Zhang Q, Liu R, Pang J. Structure, Merits, Gel Formation, Gel Preparation and Functions of Konjac Glucomannan and Its Application in Aquatic Food Preservation. Foods. 2023; 12(6):1215. https://doi.org/10.3390/foods12061215
Chicago/Turabian StyleSun, Yilan, Xiaowei Xu, Zhenzhen Wu, Hanlin Zhou, Xiaoyu Xie, Qinhua Zhang, Renyi Liu, and Jie Pang. 2023. "Structure, Merits, Gel Formation, Gel Preparation and Functions of Konjac Glucomannan and Its Application in Aquatic Food Preservation" Foods 12, no. 6: 1215. https://doi.org/10.3390/foods12061215
APA StyleSun, Y., Xu, X., Wu, Z., Zhou, H., Xie, X., Zhang, Q., Liu, R., & Pang, J. (2023). Structure, Merits, Gel Formation, Gel Preparation and Functions of Konjac Glucomannan and Its Application in Aquatic Food Preservation. Foods, 12(6), 1215. https://doi.org/10.3390/foods12061215






