Assessment of Nutritional Value and Maillard Reaction in Different Gluten-Free Pasta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Moisture, Protein, Fat and Fibre of Gluten-Free Pasta
2.3. Analysis of Amino Acid
2.4. Determination of Sugars
2.5. Determination of Furosine (FUR)
2.6. Determination of Hydroxymethylfurfural (HMF) and Glucosyl Isomaltol (AGPF)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Pasta Composition
3.2. Amino Acid Analysis and Chemical Score
3.3. Sugar Composition Assessment
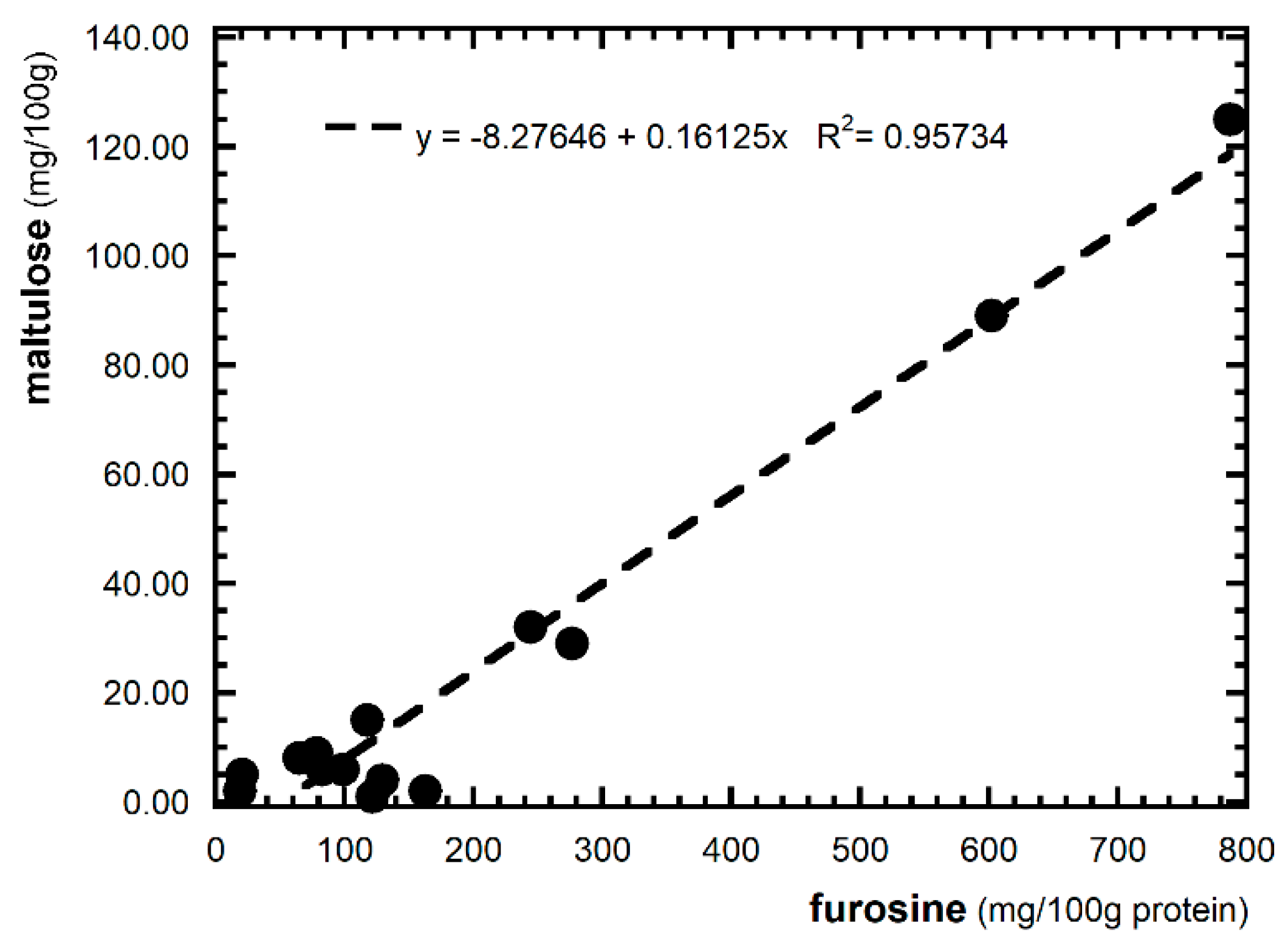
3.4. Assessment of Heat Treatment Incidence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Catassi, C.; Fasano, A. Celiac disease. In Gluten-Free Cereal Products and Beverages; Arendt, E.K., Dal Bello, F., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 1–I. [Google Scholar]
- Balakireva, A.V.; Zamyatnin, A.A. Properties of Gluten Intolerance: Gluten Structure, Evolution, Pathogenicity and Detoxification Capabilities. Nutrients 2016, 8, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, N.; Agostoni, C. Nutritional aspects of gluten-free products. J. Sci. Food Agric. 2015, 95, 2380–2385. [Google Scholar] [CrossRef]
- Jnawali, P.; Kumar, V.; Tanwar, B. Celiac disease: Overview and considerations for development of gluten-free foods. Food Sci. Hum. Wellness 2016, 5, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Marconi, E.; Messia, M.C. Pasta Made from Nontraditional Raw Materials: Technological and Nutritional Aspects. In Durum Wheat, 2nd ed.; Sissons, M., Abecassis, J., Marchylo, B., Carcea, M., Eds.; AACC International Press: Washington, DC, USA, 2012; pp. 201–211. [Google Scholar]
- De Arcangelis, E.; Cuomo, F.; Trivisonno, M.C.; Marconi, E.; Messia, M.C. Gelatinization and pasta making conditions for buckwheat gluten-free pasta. J. Cereal Sci. 2020, 95, 103073. [Google Scholar] [CrossRef]
- FAO/WHO. Dietary protein quality evaluation in human nutrition. FAO Food Nutr. Pap 2013, 92, 1–66. [Google Scholar]
- Decree of the President of the Italian Republic, 9 febbraio 2001, n. 187. Regolamento per la revisione della normativa sulla produzione e commercializzazione di sfarinati e paste alimentari, a norma dell’articolo 50 della legge 22 febbraio 1994. Gazzetta Ufficiale della Repubblica Italiana n. 117. 22 May 2001.
- Erbersdobler, H.F.; Somoza, V. Forty years of furosine–Forty years of using Maillard reaction products as indicators of the nutritional quality of foods. Mol. Nutr. Food Res. 2007, 51, 423–430. [Google Scholar] [CrossRef]
- Hellwig, M.; Kühn, L.; Henle, T. Individual Maillard reaction products as indicators of heat treatment of pasta—A survey of commercial products. J. Food Compos. Anal. 2018, 72, 83–92. [Google Scholar] [CrossRef]
- Cavazza, A.; Corradini, C.; Rinaldi, M.; Salvadeo, P.; Borromei, C.; Massini, R. Evaluation of Pasta Thermal Treatment by Determination of Carbohydrates, Furosine, and Color Indices. Food Bioprocess Technol. 2013, 6, 2721–2731. [Google Scholar] [CrossRef]
- de Stefanis, E.; Sgrulletta, D. Effects of high-temperature drying on technological properties of pasta. J. Cereal Sci. 1990, 12, 97–104. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Fast method to determine furosine in breakfast cereals by capillary zone electrophoresis. Eur. Food Res. Technol. 2005, 221, 707–711. [Google Scholar] [CrossRef] [Green Version]
- Morales, V.; Sanz, M.L.; Martín-Álvarez, P.J.; Corzo, N. Combined use of HMF and furosine to assess fresh honey quality. J. Sci. Food Agric. 2009, 89, 1332–1338. [Google Scholar] [CrossRef]
- Giannetti, V.; Boccacci Mariani, M.; Mannino, P.; Testani, E. Furosine and flavour compounds in durum wheat pasta produced under different manufacturing conditions: Multivariate chemometric characterization. LWT Food Sci. Technol. 2014, 56, 15–20. [Google Scholar] [CrossRef]
- Nursten, H.E. The Maillard Reaction: Chemistry, Biochemistry and Implications; Royal Society of Chemistry: London, UK, 2005. [Google Scholar]
- Giannetti, V.; Boccacci Mariani, M.; Colicchia, S. Furosine as marker of quality in dried durum wheat pasta: Impact of heat treatment on food quality and security–A review. Food Control 2021, 125, 108036. [Google Scholar] [CrossRef]
- Resmini, P.; Pellegrino, L.; Battelli, G. Accurate quantification of furosine in milk and dairy products by a direct HPLC method. Ital. J. Food Sci. 1990, 2, 173–183. [Google Scholar]
- Resmini, P.; Pellegrino, L.; Pagani, M.A.; De Noni, I. Formation of 2-acetyl-3-D-glucopyranosylfuran (glucosylisomaltol) from nonenzymatic browning in pasta drying. Ital. J. Food Sci. 1993, 4, 341–353. [Google Scholar]
- García-Baños, J.L.; Corzo, N.; Sanz, M.L.; Olano, A. Maltulose and furosine as indicators of quality of pasta products. Food Chem. 2004, 88, 35–38. [Google Scholar] [CrossRef]
- Lucisano, M.; Cappa, C.; Fongaro, L.; Mariotti, M. Characterisation of gluten-free pasta through conventional and innovative methods: Evaluation of the cooking behaviour. J. Cereal Sci. 2012, 56, 667–675. [Google Scholar] [CrossRef]
- Mariotti, M.; Iametti, S.; Cappa, C.; Rasmussen, P.; Lucisano, M. Characterisation of gluten-free pasta through conventional and innovative methods: Evaluation of the uncooked products. J. Cereal Sci. 2011, 53, 319–327. [Google Scholar] [CrossRef]
- Marti, A.; Caramanico, R.; Bottega, G.; Pagani, M.A. Cooking behavior of rice pasta: Effect of thermal treatments and extrusion conditions. LWT Food Sci. Technol. 2013, 54, 229–235. [Google Scholar] [CrossRef]
- Wang, L.; Duan, W.; Zhou, S.; Qian, H.; Zhang, H.; Qi, X. Effects of extrusion conditions on the extrusion responses and the quality of brown rice pasta. Food Chem. 2016, 204, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Aínsa, A.; Vega, A.; Honrado, A.; Marquina, P.; Roncales, P.; Gracia, J.A.B.; Morales, J.B.C. Gluten-free pasta enriched with fish by-product for special dietary uses: Technological quality and sensory properties. Foods 2021, 10, 3049. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, A.; Giuberti, G.; Cervini, M.; Marti, A. Pasta from yellow lentils: How process affects starch features and pasta quality. Food Chem. 2021, 364, 130387. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, N.; Aslan, M.; Çevik, A. Improvement of Structural and Nutritional Quality of Gluten Free Pasta. J. Culin. Sci. Technol. 2022, 20, 1–19. [Google Scholar] [CrossRef]
- Odabas, E.; Cakmak, H. Partial replacement of starch-based flours with quinoa or yellow lentil flour in the production of gluten-free noodles. J. Food Process. Preserv. 2022, 46, 16776. [Google Scholar] [CrossRef]
- Suo, X.; Dall’Asta, M.; Giuberti, G.; Minucciani, M.; Wang, Z.; Vittadini, E. The effect of chickpea flour and its addition levels on quality and in vitro starch digestibility of corn–rice-based gluten-free pasta. Int. J. Food Sci. Nutr. 2022, 73, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Gasparre, N.; Betoret, E.; Rosell, C.M. Quality Indicators and Heat Damage of Dried and Cooked Gluten Free Spaghetti. Plant Foods Hum. Nutr. 2019, 74, 481–488. [Google Scholar] [CrossRef]
- International Association for Cereal Science and Technology. Standard Methods of the International Association for Cereal Science and Technology; ICC: Wien, Austria, 1995. [Google Scholar]
- American Association of Cereal Chemists. Approved Methods of AACC, 11th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2010. [Google Scholar]
- Messia, M.C.; Cuomo, F.; Falasca, L.; Trivisonno, M.C.; Arcangelis, E.D.; Marconi, E. Nutritional and technological quality of high protein pasta. Foods 2021, 10, 589. [Google Scholar] [CrossRef]
- Berrios, J.D.J.; Morales, P.; Cámara, M.; Sánchez-Mata, M.C. Carbohydrate composition of raw and extruded pulse flours. Food Res. Int. 2010, 43, 531–536. [Google Scholar] [CrossRef]
- Iqbal, A.; Khalil, I.A.; Ateeq, N.; Sayyar Khan, M. Nutritional quality of important food legumes. Food Chem. 2006, 97, 331–335. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Franczyk, A.J.; Medina, G.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of Processing on the in Vitro and in Vivo Protein Quality of Yellow and Green Split Peas (Pisum sativum). J. Agric. Food Chem. 2017, 65, 7790–7796. [Google Scholar] [CrossRef]
- Larkins, B.A. Chapter 12-Proteins of the Kernel. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 319–336. [Google Scholar]
- Singla, R.K.; Dubey, A.K.; Ameen, S.M.; Montalto, S.; Parisi, S. The Control of Maillard Reaction in Processed Foods. Analytical Testing Methods for the Determination of 5-Hydroxymethylfurfural. In Analytical Methods for the Assessment of Maillard Reactions in Foods; Springer International Publishing: Cham, Switzerland, 2018; pp. 15–26. [Google Scholar]
- Giannetti, V.; Mariani, M.B.; Mannino, P. Furosine as a Pasta Quality Marker: Evaluation by an Innovative and Fast Chromatographic Approach. J. Food Sci. 2013, 78, C994–C999. [Google Scholar] [CrossRef] [PubMed]
- Oral, R.A.; Mortas, M.; Dogan, M.; Sarioglu, K.; Yazici, F. New approaches to determination of HMF. Food Chem. 2014, 143, 367–370. [Google Scholar] [CrossRef] [PubMed]
| Sample | Ingredients |
|---|---|
| Gluten-Free Pasta—100% legume flour | |
| P1 | 100% Green Pea Flour |
| P2 | 100% Red Lentil Flour |
| P3 | 100% Chickpea Flour |
| P4 | 100% Green Pea Flour |
| P5 | 100% Lentil Flour |
| P6 | 100% Green Pea Flour |
| Gluten-Free Pasta—Cereal, pseudocereal and legume flours (100% or mixed) | |
| P7 | 100% Buckwheat Flour |
| P8 | 75% Corn Flour, 10% Rice Flour, 10% Buckwheat Flour, 5% Quinoa Flour |
| P9 | 75% Rice Flour, 25% Quinoa Flour |
| P10 | 36% Brown Rice Flour, 32.5% Yellow Corn Flour, 20% White Corn Flour, 8% Rice Flour, 3% Potato Starch, Emulsifiers (mono and diglycerides of fatty acids) |
| P11 | 100% Corn Flour |
| P12 | Corn Flour, Rice Flour, 10% Amaranth Flour, 5% Teff Flour, 5% Quinoa Flour |
| P13 | Corn Flour, Rice Flour, 8% Bamboo Fibers |
| P14 | Corn Flour, 30% Pea Flour, Rice Flour |
| P15 | Corn Flour, 30% Red Lentil Flour, Rice Flour |
| Sample | Moisture (%) | Fat | Fibre | Protein | Total Starch |
|---|---|---|---|---|---|
| Gluten-Free Pasta—100% legume flour | |||||
| P1 | 10.8 ± 0.3 abc | 2.02 ± 0.03 de | 9.91 ± 0.16 g | 24.37 ± 0.03 l | 58.2 ± 1.23 c |
| P2 | 10.6 ± 0.2 ab | 1.92 ± 0.15 de | 8.49 ± 0.15 f | 26.60 ± 0.13 n | 54.2 ± 0.98 bc |
| P3 | 11.0 ± 0.1 abc | 6.98 ± 0.12 i | 15.78 ± 0.12 l | 24.17 ± 0.06 il | 46.9 ± 1.03 a |
| P4 | 10.5 ± 0.3 a | 2.63 ± 0.05 g | 8.70 ± 0.15 f | 25.18 ± 0.06 m | 58.9 ± 0.87 c |
| P5 | 10.7 ± 0.2 abc | 1.62 ± 0.11 c | 7.18 ± 0.10 e | 29.53 ± 0.22 o | 56.2 ± 0.25 bc |
| P6 | 11.1 ± 0.1 bc | 6.78 ± 0.12 i | 12.54 ± 0.15 h | 23.97 ± 0.03 h | 52.7 ± 0.77 b |
| Gluten-Free Pasta—Cereal, pseudocereal and legume flours (100% or mixed) | |||||
| P7 | 10.6 ± 0.2 ab | 3.24 ± 0.07 h | 5.06 ± 0.10 d | 9.81 ± 0.02 f | 73.4 ± 2.10 e |
| P8 | 10.8 ± 0.1 abc | 1.82 ± 0.20 cd | 2.83 ± 0.13 b | 9.30 ± 0.08 e | 82.6 ± 2.35 fg |
| P9 | 11.2 ± 0.1 c | 1.21 ± 0.03 b | 1.11 ±0.02 a | 9.40 ± 0.01 e | 84.0 ± 2.11 fg |
| P10 | 10.9 ± 0.1 abc | 2.22 ± 0.10 f | 3.44 ± 0.12 c | 7.69 ± 0.04 c | 81.0 ± 1.98 f |
| P11 | 10.6 ± 0.2 ab | 0.91 ± 0.03 a | 1.01 ± 0.01 a | 5.06 ± 0.04 a | 86.7 ± 2.00 g |
| P12 | 10.5 ± 0.3 a | 3.24 ± 0.02 h | 13.45 ± 0.12 i | 8.80 ± 0.06 d | 64.4 ± 1.54 d |
| P13 | 10.9 ± 0.2 abc | 1.82 ± 0.02 cd | 9.91 ± 0.31 g | 7.38 ± 0.01 b | 72.3 ± 2.14 e |
| P14 | 10.7 ± 0.1 abc | 2.02 ± 0.01 de | 3.74 ± 0.05 c | 10.92 ± 0.01 g | 73.9 ± 2.22 e |
| P15 | 11.1 ± 0.1 bc | 2.12 ± 0.04 ef | 3.34 ± 0.05 c | 12.03 ± 0.01 i | 73.4 ± 2.36 e |
| Mean | 10.8 | 2.70 | 7.10 | 15.6 | 67.9 |
| Min-max | 10.5–11.2 | 0.91–6.98 | 1.11–15.78 | 5.06–29.53 | 46.9–86.7 |
| EAA 1 | Gluten-Free Pasta—100% Legume Flour | Gluten-Free Pasta—Cereal, Pseudocereal and Legume Flours (100% or Mixed) | FAO Amino Acid Scoring Pattern (mg/g Protein) [7] | |||||||||||||
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | ||
| His | 2.59 ± 0.01 (a) | 2.64 ± 0.02 (a) | 2.46 ± 0.02 (a) | 2.37 ± 0.12 (a) | 2.35 ± 0.32 (a) | 2.54 ±0.3 (a) | 5.18 ± 0.53 (b) | 2.94 ±0.07 (a) | 2.50 ± 0.06 (a) | 2.56 ± 0.19 (a) | 2.66 ± 0.12 (a) | 2.82 ± 0.08 (a) | 2.54 ±0.17 (a) | 2.64 ±0.23 (a) | 2.70 ± 0.06 (a) | 1.6 |
| Ile | 3.69 ± 0.02 (de) | 2.94 ± 0.06 (ab) | 3.48 ± 0.09 (bcd) | 3.39 ± 0.21 (bcd) | 2.61 ± 0.12 (a) | 3.60 ± 0.22 (cde) | 3.33 ± 0.12 (bcd) | 3.27 ±0.23 (bcd) | 4.11 ± 0.22 (e) | 3.45 ± 0.41 (bcd) | 3.00 ± 0.23 (ab) | 3.06 ± 0.16 (abc) | 3.15 ± 0.12 (abcd) | 3.36 ± 0.08 (bcd) | 3.18 ±0.23 (abcd) | 3 |
| Leu | 7.14 ± 0.03 (bc) | 5.73 ± 0.21 (ab) | 6.77 ± 0.22 (b) | 6.59 ± 0.30 (ab) | 5.12 ± 0.70 (a) | 7.02 ± 0.72 (bc) | 6.04 ± 0.56 (ab) | 10.80 ± 0.67 (ef) | 8.48 ±0.44 (cd) | 10.00 ±0.57 (def) | 11.47 ±0.81 (f) | 9.70 ±0.77 (de) | 10.74 ±0.68 (ef) | 10.07 ±0.22 (def) | 9.76 ± 0.45 (de) | 6.1 |
| Lys | 6.77 ± 0.10 (ef) | 5.28 ± 0.10 (d) | 4.61 ± 0.12 (cd) | 6.19 ± 0.43 (e) | 6.48 ± 0.12 (ef) | 6.62 ±0.44 (ef) | 7.01 ± 0.53 (f) | 3.36 ± 0.23 (ab) | 3.98 ± 0.13 (bc) | 3.07 ± 0.17 (a) | 2.69 ±0.11 (a) | 3.41 ±0.09 (ab) | 2.74 ± 0.16 (a) | 3.98 ±0.08 (bc) | 4.22 ±0.32 (c) | 4.8 |
| Met | 0.87 ± 0.01 (a) | 0.74 ± 0.02 (a) | 0.83 ± 0.04 (a) | 0.80 ± 0.01 (a) | 0.66 ± 0.03 (a) | 0.86 ± 0.11 (a) | 1.56 ± 0.08 (b) | 2.06 ± 0.15 (d) | 2.52 ±0.22 (e) | 2.09 ±0.11 (d) | 1.97 ± 0.04 (cd) | 2.20 ± 0.11 (d) | 2.05 ± 0.10 (d) | 1.75 ± 0.07 (bc) | 1.72 ± 0.09 (bc) | SAA * 2.3 |
| Cys | 1.13 ± 0.02 (b) | 0.77 ± 0.01 (ab) | 1.07 ± 0.07 (b) | 1.04 ± 0.12 (ab) | 0.69 ± 0.01 (a) | 1.11 ± 0.22 (b) | 2.66 ± 0.12 (f) | 1.93 ± 0.15 (de) | 1.63 ± 0.09 (cd) | 1.60 ± 0.17 (cd) | 1.84 ± 0.08 (cde) | 2.05 ± 0.25 (e) | 1.79 ±0.08 (cde) | 1.65 ±0.05 (cd) | 1.56 ± 0.08 (c) | |
| Phe | 4.46 ± 0.03 (cd) | 3.71 ± 0.10 (ab) | 4.22 ± 0.13 (bcd) | 4.08 ± 0.21 (bc) | 3.30 ± 0.23 (a) | 4.36 ± 0.32 (bcd) | 4.66 ±0.33 (cd) | 4.85 ± 0.07 (d) | 5.74 ± 0.12 (e) | 4.84 ±0.41 (d) | 4.58 ±0.27 (cd) | 4.49 ±0.14 (cd) | 4.78 ± 0.09 (d) | 4.77 ±0.23 (d) | 4.62 ± 0.23 (cd) | AAA ** 4.1 |
| Tyr | 2.70 ± 0.05 (bcdef) | 2.30 ± 0.07 (ab) | 2.56 ± 0.09 (abcd) | 2.47 ± 0.13 (abc) | 2.05 ± 0.15 (a) | 2.64 ± 0.11 (bcde) | 4.86 ±0.33 (h) | 3.49 ±0.28 (g) | 3.00 ±0.18 (cdefg) | 3.36 ±0.09 (g) | 3.28 ±0.31 (g) | 3.22 ±0.12 (fg) | 3.15 ± 0.18 (efg) | 3.12 ±0.22 (efg) | 3.04 ± 0.13 (defg) | |
| Thr | 3.40 ± 0.01 (cd) | 2.80 ± 0.09 (ab) | 3.23 ± 0.10 (bc) | 3.10 ± 0.22 (bc) | 2.50 ± 0.12 (a) | 3.33 ± 0.28 (bcd) | 3.88 ± 0.41 (d) | 3.55 ±0.22 (cd) | 3.45 ±0.22 (cd) | 3.43 ±0.17 (cd) | 3.40 ±0.12 (cd) | 3.20 ± 0.03 (bc) | 3.30 ±0.11 (bc) | 3.43 ± 0.25 (cd) | 3.30 ±0.02 (bc) | 2.5 |
| Val | 4.16 ± 0.03 (abcd) | 4.32 ± 0.08 (abcde) | 3.96 ± 0.30 (ab) | 3.80 ± 0.14 (a) | 3.84 ± 0.22 (ab) | 4.08 ± 0.31 (abc) | 4.40 ± 0.21 (abcde) | 4.84 ± 0.04 (ef) | 5.88 ± 0.07 (g) | 5.08 ±0.11 (f) | 4.60 ±0.36 (cdef) | 4.44 ±0.09 (bcde) | 4.80 ± 0.33 (ef) | 4.72 ± 0.12 (def) | 4.84 ± 0.12 (ef) | 4 |
| Trp | 0.91 ± 0.01 (e) | 0.80 ± 0.02 (bcde) | 0.86 ± 0.03 (de) | 0.84 ± 0.01 (cde) | 0.71 ± 0.02 (abc) | 0.89 ±0.03 (e) | 1.92 ± 0.08 (g) | 0.82 ±0.05 (bcde) | 1.06 ± 0.10 (f) | 0.86 ±0.09 (de) | 0.61 ± 0.02 (a) | 1.10 ± 0.03 (f) | 0.69 ± 0.02 (ab) | 0.83 ± 0.02 (bcde) | 0.74 ± 0.05 (abcd) | 0.66 |
| CS 2 | 87 | 65 | 82 | 92 | 58 | 85 | 100 | 70 | 83 | 64 | 55 | 71 | 57 | 82 | 88 | |
| Limiting AA | SAA | SAA | SAA | SAA | SAA | SAA | Lys | Lys | Lys | Lys | Lys | Lys | Lys | Lys | ||
| Sample | Galactose | Glucose | Fructose | Maltose | Total Reducing Sugars | Sucrose |
|---|---|---|---|---|---|---|
| Gluten-Free Pasta—100% legume flour | ||||||
| P1 | nd | 12 ± 4 a | 25 ± 12 a | 92 ± 13 bc | 129 | 2753 ± 135 g |
| P2 | 601 ± 22 d | 28 ± 10 a | 63 ± 17 a | 126 ± 02 c | 818 | 2880 ± 222 g |
| P3 | 649 ± 91 d | 61 ± 3 a | 103 ± 45 ab | 315 ± 43 d | 1128 | 3332 ± 114 h |
| P4 | 5 ± 2 a | 232 ± 76 b | 49 ± 5 a | 43 ± 0 ab | 329 | 3195 ± 187 h |
| P5 | 234 ± 54 c | 27 ± 0.0 a | 25 ± 12 a | 120 ± 0 c | 406 | 2240 ± 111 f |
| P6 | 93 ± 18 ab | 24 ± 5 a | 40 ± 8 a | 74 ± 3 abc | 231 | 3798 ± 156 i |
| Gluten-Free Pasta—Cereal, pseudocereal and legume flours (100% or mixed) | ||||||
| P7 | 3 ± 0.0 a | 52 ± 12 a | 36 ± 8 a | 89 ± 4 bc | 180 | 1430 ± 98 e |
| P8 | 11 ± 3 a | 231 ± 32 b | 172 ± 45 bc | 25 ± 3 ab | 439 | 625 ± 14 ab |
| P9 | nd | 318 ± 14 bc | 13 ± 2 a | 40 ± 32 ab | 371 | 637 ± 98 ab |
| P10 | 7 ± 4 a | 252 ± 72 b | 183 ± 24 bcd | 15 ± 4 a | 467 | 762 ± 79 ab |
| P11 | 189 ± 24 bc | 292 ± 67 bc | 293 ± 32 efg | 15 ± 7 a | 789 | 1071 ± 98 cd |
| P12 | nd | 243 ± 54 b | 334 ± 78 fg | 553 ± 78 e | 1130 | 762 ± 45 ab |
| P13 | nd | 311 ± 13 bc | 266 ± 23 def | 11 ± 5 a | 588 | 501 ± 25 a |
| P14 | 181 ± 87 bc | 274 ± 45 bc | 212 ± 21 cde | 18 ± 9 a | 685 | 1196 ± 98 de |
| P15 | 153 ± 21 bc | 400 ± 98 c | 365 ± 72 g | 6 ± 2 a | 924 | 888 ± 67 bc |
| Mean | 142 | 184 | 145 | 103 | 574 | 1738 |
| Min-max | 0–649 | 12–400 | 13–365 | 6–553 | 129–1130 | 501–3798 |
| Sample | FUR (mg/100 g Protein) | FUR (mg/100 g) | Maltulose (mg/100 g) | HMF (mg/kg) | AGPF (mg/kg) |
|---|---|---|---|---|---|
| Gluten-free Pasta—100% legume flour | |||||
| P1 | 21.0 ± 0.02 a | 4.6 ± 0.01 b | 5 ± 0 a | 0.099 ± 0.001 | nd |
| P2 | 602.5 ± 2.79 i | 142.8 ± 0.66 g | 89 ± 10 c | 0.236 ± 0.003 | nd |
| P3 | 787.6 ± 14.48 l | 169.3 ± 3.11 h | 125 ± 23 d | 0.147 ± 0.005 | nd |
| P4 | 98.9 ± 1.26 d | 22.1 ± 0.28 d | 6 ± 4 a | nd | nd |
| P5 | 244.6 ± 5.93 g | 64.3 ± 1.56 f | 32 ± 2 b | nd | nd |
| P6 | 163.2 ± 0.61 f | 34.8 ± 0.16 e | 2 ± 0 a | nd | nd |
| Gluten-Free Pasta—Cereal, pseudocereal and legume flours (100% or mixed) | |||||
| P7 | 18.9 ± 0.62 a | 1.6 ± 0.05 a | 2 ± 0 a | 0.106 ± 0.007 | nd |
| P8 | 75.6 ± 0.05 b | 6.3 ± 0.01 b | 8 ± 1 a | 0.220 ± 0.001 | nd |
| P9 | 65.2 ± 3.62 bc | 5.5 ± 0.30 b | 8 ± 5 a | nd | nd |
| P10 | 82.7 ± 0.47 c | 5.6 ± 0.03 b | 6 ± 0 a | nd | nd |
| P11 | 117.9 ± 2.00 l | 5.3 ± 0.09 b | 15 ± 2 ab | nd | nd |
| P12 | 276.7± 9.84 h | 21.6 ± 0.776 d | 29 ± 4 b | nd | nd |
| P13 | 78.3 ±0.78 bc | 5.2 ± 0.05 b | 9 ± 2 a | nd | nd |
| P14 | 121.8 ± 1.55 e | 11.8 ± 0.15 c | 1 ± 0 a | nd | nd |
| P15 | 129.8 ± 7.06 e | 13.9 ± 0.76 b | 4 ± 1 a | nd | nd |
| Mean | 192 | 34.3 | 23 | - | - |
| Min-max | 18.9–787.6 | 1.6–169.3 | 1–125 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Messia, M.C.; Cuomo, F.; Quiquero, M.; Verardo, V.; Marconi, E. Assessment of Nutritional Value and Maillard Reaction in Different Gluten-Free Pasta. Foods 2023, 12, 1221. https://doi.org/10.3390/foods12061221
Messia MC, Cuomo F, Quiquero M, Verardo V, Marconi E. Assessment of Nutritional Value and Maillard Reaction in Different Gluten-Free Pasta. Foods. 2023; 12(6):1221. https://doi.org/10.3390/foods12061221
Chicago/Turabian StyleMessia, Maria Cristina, Francesca Cuomo, Michela Quiquero, Vito Verardo, and Emanuele Marconi. 2023. "Assessment of Nutritional Value and Maillard Reaction in Different Gluten-Free Pasta" Foods 12, no. 6: 1221. https://doi.org/10.3390/foods12061221





