Optimization of Extrusion Treatments, Quality Assessments, and Kinetics Degradation of Enzyme Activities during Storage of Rice Bran
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Stabilization of RB
2.3. Simulation of Storage Scenarios
2.4. Experimental Design and Analysis
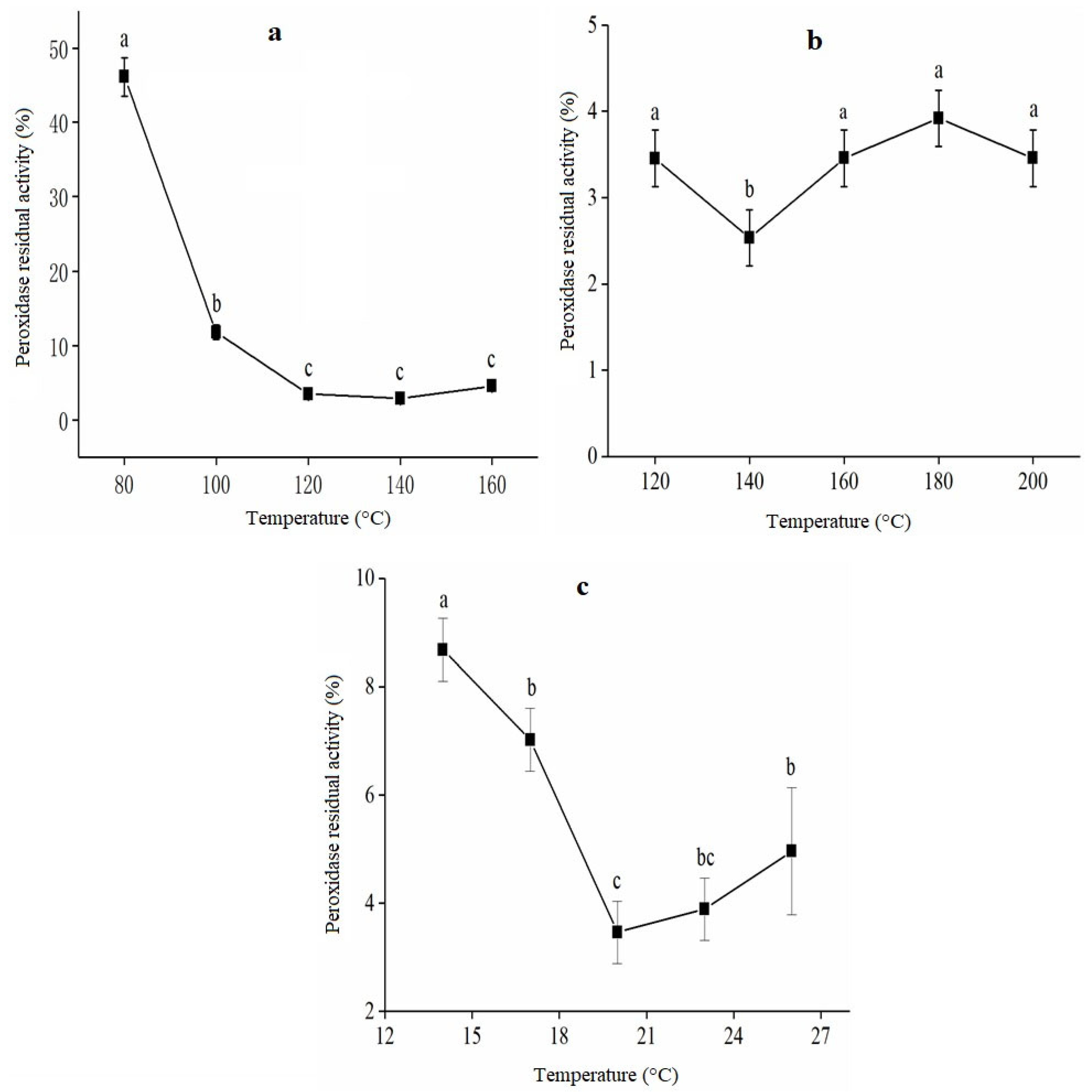
2.4.1. Evaluation of Single-Factor Extrusion Treatment of RB
2.4.2. Effect of Barrel Temperature on RPA
2.4.3. Effect of Screw Speed on RPA
2.4.4. Effect of Moisture Content on RPA
2.5. Response Surface Methodology (RSM) Analysis
2.6. Quality Characteristics of Stabilized RB
2.7. Nutritional Properties of Stabilized RB
2.7.1. Determination of Amino Acid Composition
2.7.2. Determination of Crude Protein Content
2.7.3. Fatty Acid Composition (FAC)
2.8. Protein Electrophoresis Analysis of RB
2.9. Statistical Analysis
3. Results and Discussion
3.1. Single-Factor Results of Extrusion Treatment
3.1.1. RSM of Rice Bran by Extrusion
3.1.2. Optimization
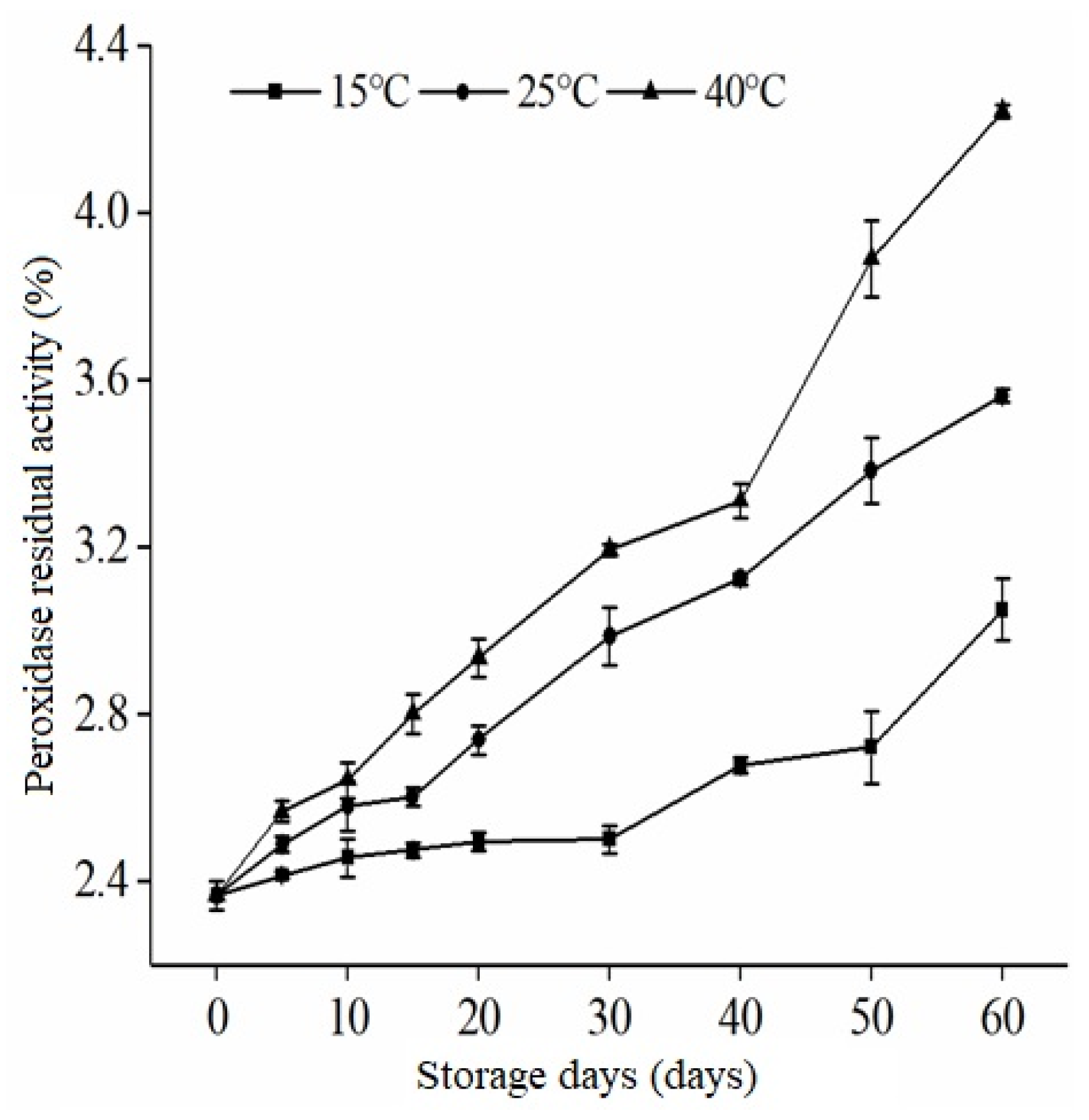
3.2. Peroxidase Activity of Extruded RB during Storage
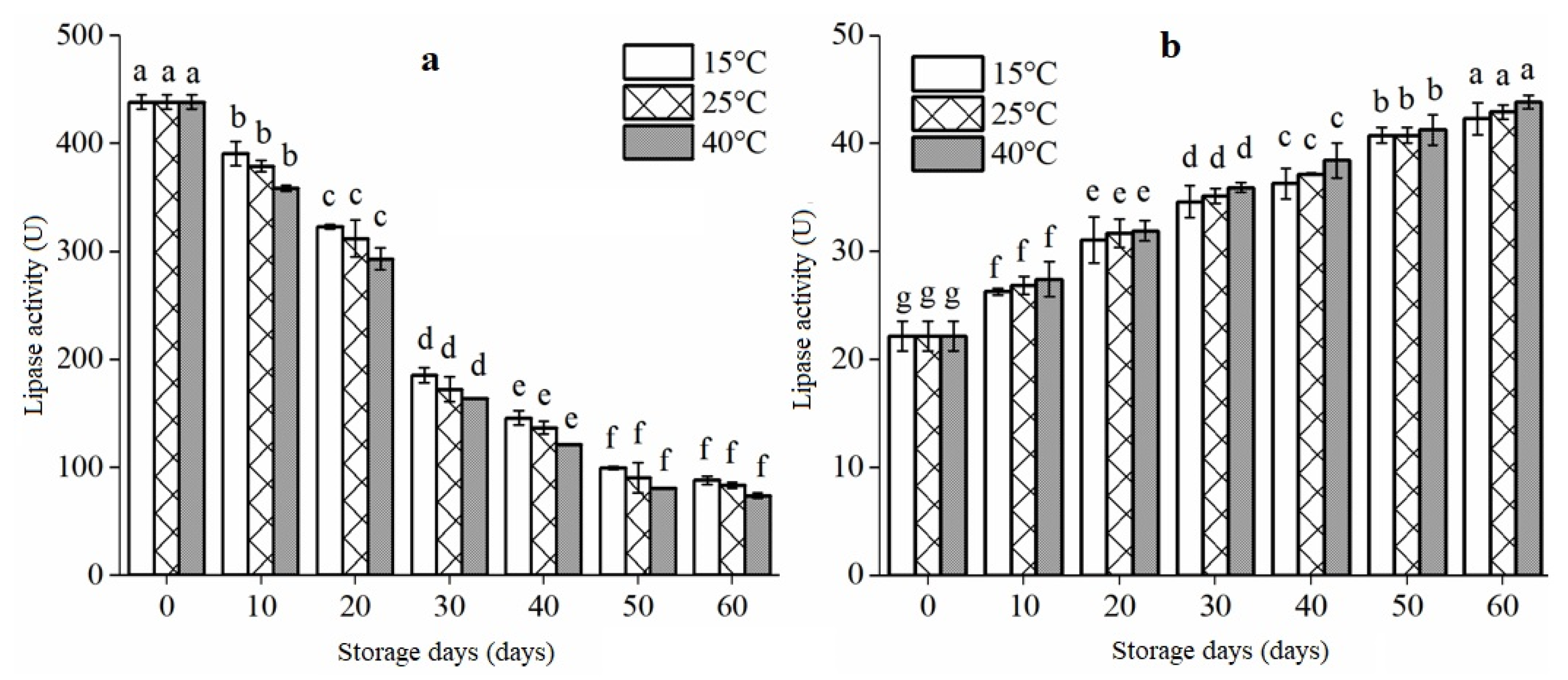
3.3. Lipase Activity of Extruded RB during Storage
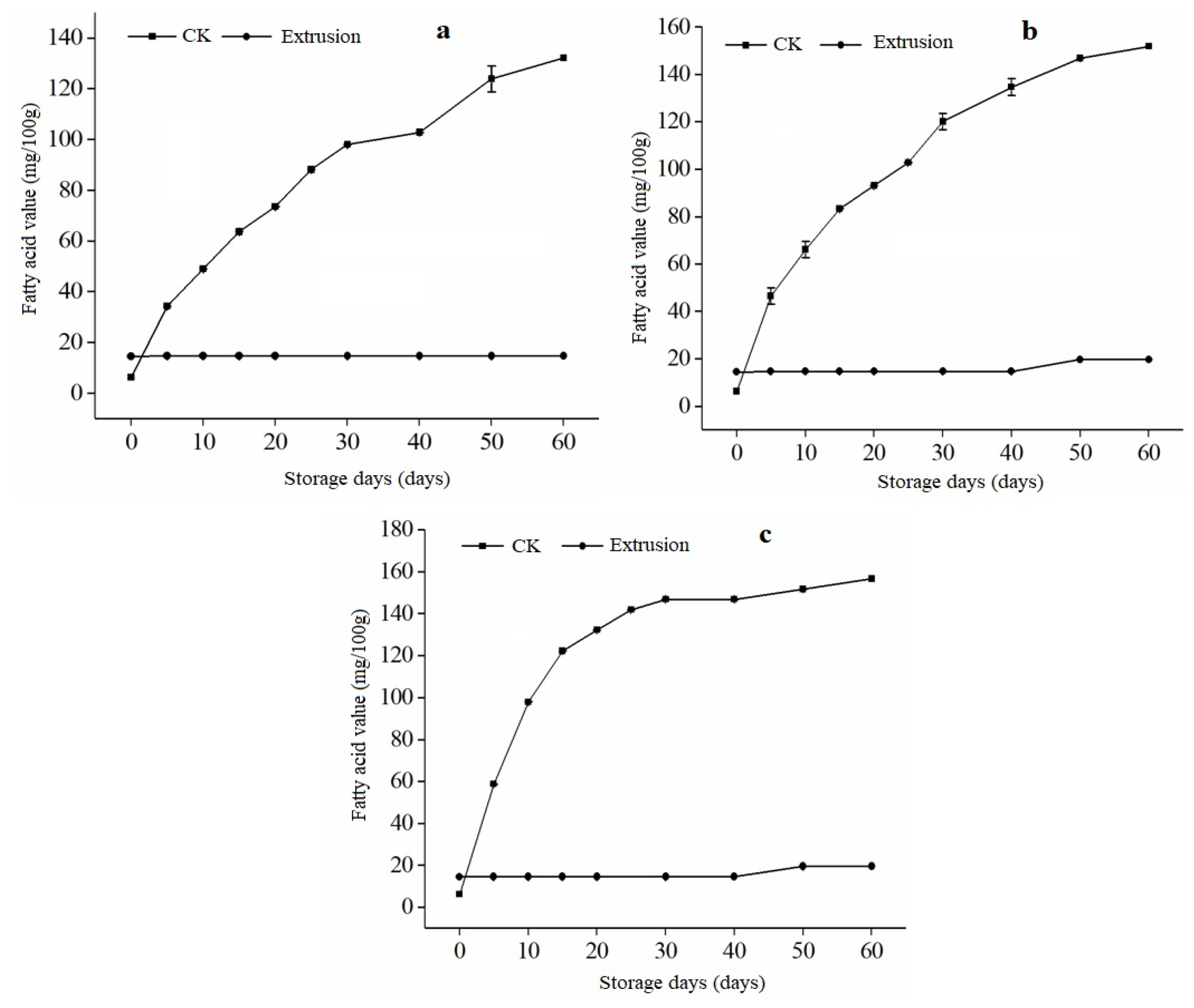
3.4. Free Fatty Acid (FFA) of Extruded RB during Storage
3.5. Peroxide Values of Extrusion RB during Storage
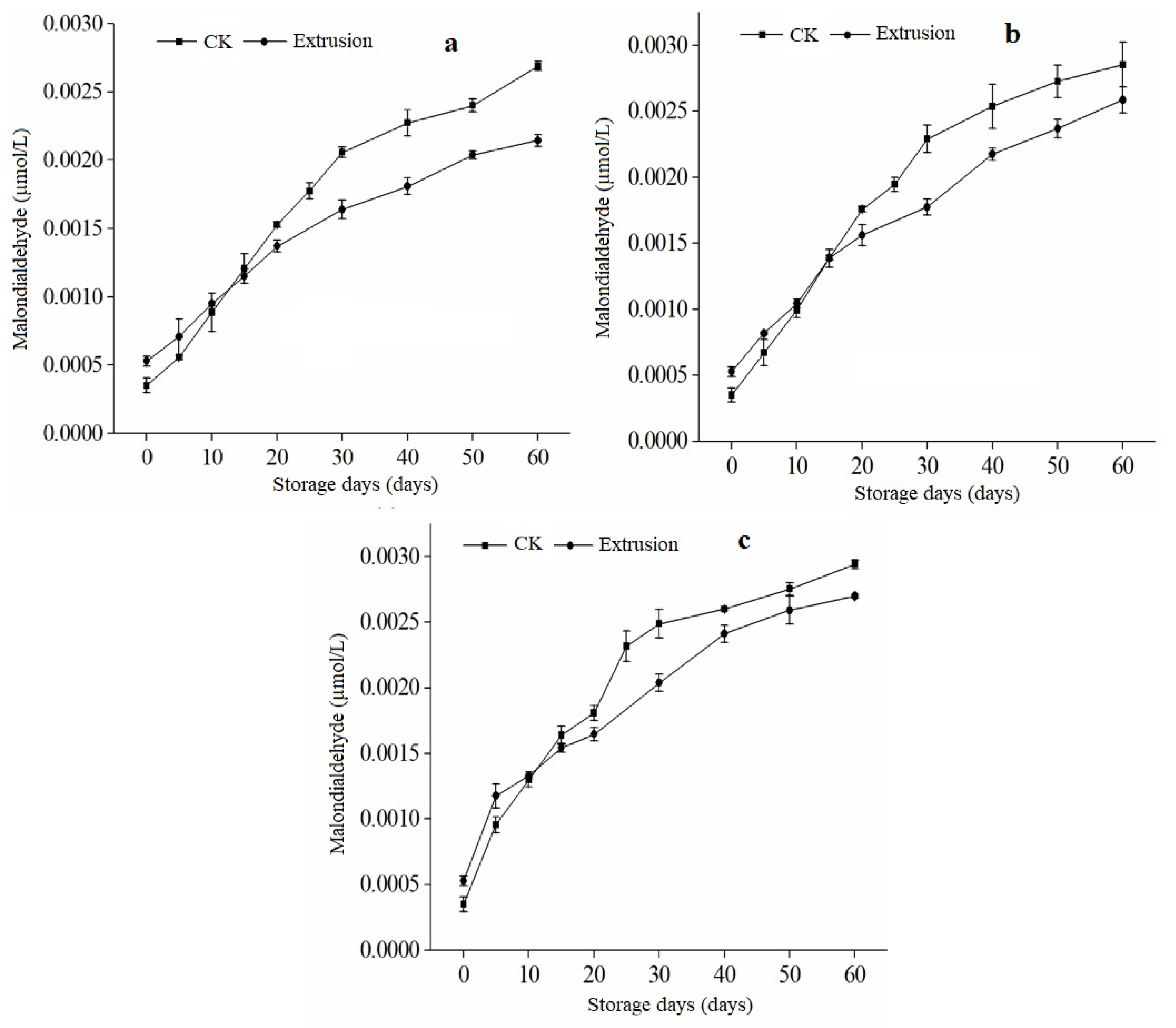
3.6. Malondialdehyde Content of Extruded RB during Storage
3.7. Fatty Acid Composition of Extruded RB during Storage
3.8. Crude Protein Treated by Extruded RB during Storage
3.9. Amino Acid Profile of Extruded RB during Storage
3.10. Protein Electrophoresis Analysis Extruded RB during Storage
3.11. Degradation Kinetics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, R.; Wang, Y.; Zou, Y.; Wang, Z.; Ding, C.; Wu, Y.; Ju, X. Storage Characteristics of Infrared Radiation Stabilized Rice Bran and Its Shelf-Life Evaluation by Prediction Modeling. J. Sci. Food Agric. 2020, 100, 2638–2647. [Google Scholar] [CrossRef]
- Yan, W.; Liu, Q.; Wang, Y.; Tao, T.; Liu, B.; Liu, J.; Ding, C. Inhibition of Lipid and Aroma Deterioration in Rice Bran by Infrared Heating. Food Bioprocess Technol. 2020, 13, 1677–1687. [Google Scholar] [CrossRef]
- Salvador-Reyes, R.; Sampaio, U.M.; de Menezes Alves Moro, T.; de Brito, A.D.C.; Behrens, J.; Campelo, P.H.; Pedrosa Silva Clerici, M.T. Andean Purple Maize to Produce Extruded Breakfast Cereals: Impact on Techno-Functional Properties and Sensory Acceptance. J. Sci. Food Agric. 2023, 103, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Eng, H.Y.; Mohd Rozalli, N.H. Rice Bran and Its Constituents: Introduction and Potential Food Uses. Int. J. Food Sci. Technol. 2022, 57, 4041–4051. [Google Scholar] [CrossRef]
- Andriani, R.; Subroto, T.; Ishmayana, S.; Kurnia, D. Enhancement Methods of Antioxidant Capacity in Rice Bran: A Review. Foods 2022, 11, 2994. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, M.N.; Saikiran, K.C.S.; Venkatachalapathy, N. Stabilization of Rice Bran Milling Fractions Using Microwave Heating and Its Effect on Storage. J. Food Sci. Technol. 2019, 56, 889–895. [Google Scholar] [CrossRef]
- Pokkanta, P.; Yuenyong, J.; Mahatheeranont, S.; Jiamyangyuen, S.; Sookwong, P. Microwave Treatment of Rice Bran and Its Effect on Phytochemical Content and Antioxidant Activity. Sci. Rep. 2022, 12, 7708. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, B.; Pang, H.; Wang, Y.; Fu, H.; Chen, X.; Wang, Y. The Effect of Radio Frequency Heating on the Inactivation and Structure of Horseradish Peroxidase. Food Chem. 2022, 398, 133875. [Google Scholar] [CrossRef]
- Rashid, M.T.; Liu, K.; Han, S.; Jatoi, M.A. The Effects of Thermal Treatment on Lipid Oxidation, Protein Changes, and Storage Stabilization of Rice Bran. Foods 2022, 11, 4001. [Google Scholar] [CrossRef]
- Rashid, M.T.; Liu, K.; Hen, S.; Jatoi, M.A.; Sarpong, F. Nutritional Composition and Volatile Compounds Stability in Dry-heat and Extruded Stabilized Rice Bran during Storage. Int. J. Food Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Yu, C.W.; Hu, Q.R.; Wang, H.W.; Deng, Z.Y. Comparison of 11 Rice Bran Stabilization Methods by Analyzing Lipase Activities. J. Food Process. Preserv. 2020, 44, e14370. [Google Scholar] [CrossRef]
- Ijaz, U.; Nadeem, H.U.; Shafique, A.; Rasheed, A.; Batool, R.; Azeem, F. Rice Bran Composition and Its Emerging Potential Applications. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, K.; Li, F.; Cao, H.; Duan, Q.; Zhu, M.; Wang, P.; Gao, Y.; Duan, Y. Effect of Extrusion on Physical and Chemical Properties and Storage Stability of Corn Germ. Cereal Chem. 2021, 98, 1135–1145. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, Y.; Zhu, Z.; Wang, Y.; Zeng, Z.; Liu, C. Comparative Study on Physicochemical and Nutritional Properties of Black Rice Influenced by Superheated Steam, Far Infrared Radiation, and Microwave Treatment. Innov. Food Sci. Emerg. Technol. 2023, 84, 103282. [Google Scholar] [CrossRef]
- Allai, F.M.; Azad, Z.R.A.A.; Dar, B.N.; Gul, K.; Jabeen, A. Breakfast Cereals from Whole Grain and Indian Horse Chestnut Flours Obtained through Extrusion: Physical, Mechanical and Functional Characteristics. Appl. Food Res. 2022, 2, 100137. [Google Scholar] [CrossRef]
- Danchai, K.; Kongbangkerd, T.; Ruttarattanamongkol, K. Optimization of rice bran stabilization by infrared-vacuum process and storage stability. J. Food Process Eng. 2021, 44, e13668. [Google Scholar]
- AOAC. AOAC Official Method 996.06, Fat (Total, Saturated, and Unsaturated) in Foods; AOAC: Arlington, TX, USA, 2001. [Google Scholar]
- AOAC. The Association of Official Analytical Chemists International. Off. Methods Anal. 2016, 38, 431. [Google Scholar]
- Liang, K.; Liu, Y.; Liang, S. Analysis of the Characteristics of Foxtail Millet during Storage under Different Light Environments. J. Cereal Sci. 2021, 101, 103302. [Google Scholar] [CrossRef]
- AACC. Soluble, Insoluble, and Total Dietary Fiber in Foods and Food Products, 11th ed.; AACC: Arnold, MD, USA, 2021. [Google Scholar]
- Association of Official Analytical Chemists and Association of Official Agricultural Chemists (US). Off. Methods Anal. Assoc. Off. Anal. Chem. 1921. [CrossRef]
- Irakli, M.; Kleisiaris, F.; Mygdalia, A.; Katsantonis, D. Stabilization of Rice Bran and Its Effect on Bioactive Compounds Content, Antioxidant Activity and Storage Stability during Infrared Radiation Heating. J. Cereal Sci. 2018, 80, 135–142. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, Y.; Chen, F.; Fang, Y. Purification and Identification of Se-Containing Antioxidative Peptides from Enzymatic Hydrolysates of Se-Enriched Brown Rice Protein. Food Chem. 2015, 187, 424–430. [Google Scholar] [CrossRef]
- Kim, J.H.; Tanhehco, E.J.; Ng, P.K.W. Effect of Extrusion Conditions on Resistant Starch Formation from Pastry Wheat Flour. Food Chem. 2006, 99, 718–723. [Google Scholar] [CrossRef]
- Rafe, A.; Sadeghian, A. Stabilization of Tarom and Domesiah Cultivars Rice Bran: Physicochemical, Functional and Nutritional Properties. J. Cereal Sci. 2017, 74, 64–71. [Google Scholar] [CrossRef]
- Dubey, B.N. Comparative Study on the Rice Bran Stabilization Processes: A Review. Res. Dev. Mater. Sci. 2019, 11. [Google Scholar] [CrossRef]
- Ling, B.; Lyng, J.G.; Wang, S. Effects of Hot Air-Assisted Radio Frequency Heating on Enzyme Inactivation, Lipid Stability and Product Quality of Rice Bran. LWT-Food Sci. Technol. 2018, 91, 453–459. [Google Scholar] [CrossRef]
- Basiron, Y.; Shahidi, F. Bailey’s Industrial Oil and Fat Products. Edible Oil Fat Products; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Li, B.; Zhao, L.; Chen, H.; Sun, D.; Deng, B.; Li, J.; Liu, Y.; Wang, F. Inactivation of Lipase and Lipoxygenase of Wheat Germ with Temperature-Controlled Short Wave Infrared Radiation and Its Effect on Storage Stability and Quality of Wheat Germ Oil. PLoS ONE 2016, 11, e0167330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laokuldilok, T.; Rattanathanan, Y. Protease Treatment for the Stabilization of Rice Bran: Effects on Lipase Activity, Antioxidants, and Lipid Stability. Cereal Chem. 2014, 91, 560–565. [Google Scholar] [CrossRef]
- Gong, Z.; Yu, G.; Xu, M.; Dou, C. Effect of Heating Treatment of Fresh Rice Bran on Stabilization. Adv. Mater. Res. 2013, 602–604, 1200–1205. [Google Scholar] [CrossRef]
- Sung, J.; Kim, B.K.; Kim, B.S.; Kim, Y. Mass Spectrometry-Based Electric Nose System for Assessing Rice Quality during Storage at Different Temperatures. J. Stored Prod. Res. 2014, 59, 204–208. [Google Scholar] [CrossRef]
- Zhou, Z.; Blanchard, C.; Helliwell, S.; Robards, K. Fatty Acid Composition of Three Rice Varieties Following Storage. J. Cereal Sci. 2003, 37, 327–335. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.; Eitenmiller, R.R. Vitamin E and Oxidative Stability during Storage of Raw and Dry Roasted Peanuts Packaged under Air and Vacuum. J. Food Sci. 2005, 70, C292–C297. [Google Scholar] [CrossRef]
- Guevara-Guerrero, B.; Fernández-Quintero, A.; Montero-Montero, J.C. Free Fatty Acids in Rice Bran during Its Storage after a Treatment by Twin-Screw Extrusion to Prevent Possible Rapid Hydrolytic Rancidity of Lipids1. DYNA 2019, 86, 177–181. [Google Scholar] [CrossRef]
- Amarasinghe, B.M.W.P.K.; Kumarasiri, M.P.M.; Gangodavilage, N.C. Effect of Method of Stabilization on Aqueous Extraction of Rice Bran Oil. Food Bioprod. Process. 2009, 87, 108–114. [Google Scholar] [CrossRef]
- Talcott, S.T.; Duncan, C.E.; Del Pozo-Insfran, D.; Gorbet, D.W. Polyphenolic and Antioxidant Changes during Storage of Normal, Mid, and High Oleic Acid Peanuts. Food Chem. 2005, 89, 77–84. [Google Scholar] [CrossRef]
- Cai, L.; Liu, C.; Ying, T. Changes in Quality of Low-Moisture Conditioned Pine Nut (Pinus Gerardiana) under near Freezing Temperature Storage. CYTA-J. Food 2013, 11, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Qi, P.X.; Onwulata, C.I. Physical Properties, Molecular Structures, and Protein Quality of Texturized Whey Protein Isolate: Effect of Extrusion Temperature. J. Agric. Food Chem. 2011, 59, 4668–4675. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Kar, A.; Mohapatra, D. Stabilization of Rice Bran Using Microwave: Process Optimization and Storage Studies. Food Bioprod. Process. 2016, 99, 204–211. [Google Scholar] [CrossRef]
- Goulas, A.E.; Kontominas, M.G. Combined Effect of Light Salting, Modified Atmosphere Packaging and Oregano Essential Oil on the Shelf-Life of Sea Bream (Sparus Aurata): Biochemical and Sensory Attributes. Food Chem. 2007, 100, 287–296. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, H.S.; Li, Y.Z.; Zhang, W.W.; Xia, K.; Wang, Z.B.; Li, M.; Zhang, Y.G. The Effects of Different Processing Methods on the Estimated Nutritional Value of Rice Bran According to the NRC-2001 Model5or DVE/OEB System6. J. Anim. Feed Sci. 2012, 21, 503–520. [Google Scholar] [CrossRef] [Green Version]
- Mexis, S.F.; Kontominas, M.G. Effect of γ-Irradiation on the Physicochemical and Sensory Properties of Cashew Nuts (Anacardium Occidentale L.). LWT-Food Sci. Technol. 2009, 42, 1501–1507. [Google Scholar] [CrossRef]
- Gong, L.; Huang, L.; Zhang, Y. Effect of Steam Explosion Treatment on Barley Bran Phenolic Compounds and Antioxidant Capacity. J. Agric. Food Chem. 2012, 60, 7177–7184. [Google Scholar] [CrossRef]
- Yilmaz, N.; Tuncel, N.B.; Kocabiyik, H. Infrared Stabilization of Rice Bran and Its Effects on γ-Oryzanol Content, Tocopherols and Fatty Acid Composition. J. Sci. Food Agric. 2014, 94, 1568–1576. [Google Scholar] [CrossRef]
- Ertaş, N. Reutilisation of Rice By-product: Study on the Effect of Rice Bran Addition on Physical, Chemical and Sensory Properties of Erişte. Qual. Assur. Saf. Crops Foods 2014, 6, 249–255. [Google Scholar] [CrossRef]
- Khan, S.H.; Butt, M.S.; Sharif, M.K.; Sameen, A.; Mumtaz, S.; Sultan, M.T. Functional Properties of Protein Isolates Extracted from Stabilized Rice Bran by Microwave, Dry Heat, and Parboiling. J. Agric. Food Chem. 2011, 59, 2416–2420. [Google Scholar] [CrossRef]
- Oliveira, R.; Oliveira, V.; Aracava, K.K.; Rodrigues, C.E.D.C. Effects of the Extraction Conditions on the Yield and Composition of Rice Bran Oil Extracted with Ethanol—A Response Surface Approach. Food Bioprod. Process. 2012, 90, 22–31. [Google Scholar] [CrossRef]
- Capellini, M.C.; Giacomini, V.; Cuevas, M.S.; Rodrigues, C.E.C. Rice Bran Oil Extraction Using Alcoholic Solvents: Physicochemical Characterization of Oil and Protein Fraction Functionality. Ind. Crops Prod. 2017, 104, 133–143. [Google Scholar] [CrossRef]
- Akinyede, A.I.; Oluwajuyitan, T.D.; Dada, J.B. Influence of Substitution on Amino-Acid Profile, Physicochemical and Sensory Attributes of Breakfast Cereal from Millet, Soycake, Rice Bran and Carrot Pomace Blends. Food Process. Technol. 2020, 8, 19–27. [Google Scholar] [CrossRef]
- Chinma, C.E.; Ilowefah, M.; Shammugasamy, B.; Ramakrishnan, Y.; Muhammad, K. Chemical, Antioxidant, Functional and Thermal Properties of Rice Bran Proteins after Yeast and Natural Fermentations. Int. J. Food Sci. Technol. 2014, 49, 2204–2213. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, E.J.; Lim, S.T.; Han, J.A. Self-Enhancement of GABA in Rice Bran Using Various Stress Treatments. Food Chem. 2015, 172, 657–662. [Google Scholar] [CrossRef]
- Olukosi, O.A.; Adebiyi, A.O. Chemical Composition and Prediction of Amino Acid Content of Maize- and Wheat-Distillers’ Dried Grains with Soluble. Anim. Feed Sci. Technol. 2013, 185, 182–189. [Google Scholar] [CrossRef]
- Yi, J.; Qiu, M.; Liu, N.; Tian, L.; Zhu, X.; Decker, E.A.; McClements, D.J. Inhibition of Lipid and Protein Oxidation in Whey-Protein-Stabilized Emulsions Using a Natural Antioxidant: Black Rice Anthocyanins. J. Agric. Food Chem. 2020, 68, 10149–10156. [Google Scholar] [CrossRef]
- Ling, B.; Ouyang, S.; Wang, S. Effect of Radio Frequency Treatment on Functional, Structural and Thermal Behaviors of Protein Isolates in Rice Bran. Food Chem. 2019, 289, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yamada, C.; Maeda, I.; Techapun, C.; Kuntiya, A.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Takenaka, S.; Shiono, T.; et al. Evaluating of Quality of Rice Bran Protein Concentrate Prepared by a Combination of Isoelectronic Precipitation and Electrolyzed Water Treatment. LWT-Food Sci. Technol. 2019, 99, 262–267. [Google Scholar] [CrossRef]
- Fabian, C.; Ju, Y.H. A Review on Rice Bran Protein: Its Properties and Extraction Methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M.; Normand, M.D.; Corradini, M.G. The Arrhenius Equation Revisited. Crit. Rev. Food Sci. Nutr. 2012, 52, 830–851. [Google Scholar] [CrossRef]
- Kim, A.N.; Kim, O.W.; Kim, H. Degradation Kinetics of Physicochemical and Sensory Properties of Rice during Storage at Different Temperatures. LWT-Food Sci. Technol. 2022, 164, 113688. [Google Scholar] [CrossRef]
- Ball, G.F.M. Vitamins in Foods; Analysis, Bioavailability and Stability; CRC Press: Boca Raton, FL, USA, 2006; p. 7. [Google Scholar]


| Factors | Code | Level | |||
|---|---|---|---|---|---|
| Code | Non-Coding | 1 | 0 | −1 | |
| Barrel temperature (°C) | X1 | A | 140 | 120 | 100 |
| Screw speed (r/min) | X2 | B | 160 | 140 | 120 |
| Moisture (%) | X3 | C | 23 | 20 | 17 |
| Test Number | Factor Level | POD Residual Vitality (%) | ||
|---|---|---|---|---|
| Barrel Temperature (X1) (°C) | Screw Speed (X2) (r/min) | Material Moisture Content (X3) (%) | ||
| 1 | 0 | 0 | 0 | 2.28 |
| 2 | 1 | 0 | 1 | 4.42 |
| 3 | 1 | 1 | 0 | 3.46 |
| 4 | 0 | 0 | 0 | 3.12 |
| 5 | 0 | −1 | 1 | 3.26 |
| 6 | 0 | 0 | 0 | 2.60 |
| 7 | −1 | 1 | 0 | 5.73 |
| 8 | 0 | 0 | 0 | 2.50 |
| 9 | 1 | 0 | −1 | 5.76 |
| 10 | −1 | 0 | 1 | 3.11 |
| 11 | −1 | −1 | 0 | 4.31 |
| 12 | 1 | −1 | 0 | 3.38 |
| 13 | −1 | 0 | −1 | 7.83 |
| 14 | 0 | 1 | −1 | 6.07 |
| 15 | 0 | −1 | −1 | 7.37 |
| 16 | 0 | 1 | 1 | 6.45 |
| 17 | 0 | 0 | 0 | 2.72 |
| Storage Temp (°C) | Storage Days (d) | Fatty Acid Composition (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myristic Acid | Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | Linolenic Acid | Arachidic Acid | Arachidonic Acid | Saturated Fatty Acid | Unsaturated Fatty Acids | O/L | ||
| Untreated (°C) samples | ||||||||||||
| 15 °C | 0 | 0.17 ± 0.00 a | 16.64 ± 0.02 a | 1.19 ± 0.14 a | 40.81 ± 0.26 a | 38.61± 0.00 b | 0.48 ± 0.04 a | 1.64 ± 0.01 a | 0.45 ± 0.16 a | 19.65 ± 0.14 a | 80.35 ± 0.14 b | 1.06 ± 0.01 a |
| 60 | 0.19± 0.03 aA | 15.13 ± 0.23 bA | 1.19 ± 0.00 aA | 41.31 ± 0.21 aA | 39.55 ± 0.03 aA | 0.43 ± 0.01 aB | 1.66 ± 0.01 aA | 0.54 ± 0.01B | 18.17 ± 0.25 bA | 81.83 ± 0.25 aA | 1.04 ± 0.00 aB | |
| 25 °C | 0 | 0.17 ± 0.00 a | 16.64 ± 0.02 a | 1.19 ± 0.14 a | 40.81 ± 0.26 a | 38.61± 0.00 b | 0.48 ± 0.04a | 1.64 ± 0.01 a | 0.45 ± 0.16 a | 19.65 ± 0.14 a | 80.35 ± 0.14 b | 1.06 ± 0.01 a |
| 60 | 0.20 ± 0.02 aA | 15.03 ± 0.11 bA | 1.21 ± 0.04 aA | 41.70 ± 0.22 aA | 39.15 ± 0.04 aB | 0.49 ± 0.01 aA | 1.66 ± 0.02 aA | 0.56 ± 0.01 aAB | 18.10 ± 0.19 bA | 81.90 ± 0.19 aA | 1.07 ± 0.01 aA | |
| 40 °C | 0 | 0.17 ± 0.00 a | 16.64 ± 0.02 a | 1.19 ± 0.14 a | 40.81 ± 0.26 a | 38.61± 0.00 b | 0.48 ± 0.04 a | 1.64 ± 0.01 a | 0.45 ± 0.16 a | 19.65 ± 0.14 a | 80.35 ± 0.14 b | 1.06 ± 0.01 a |
| 60 | 0.17 ± 0.01 aA | 14.89 ± 0.02 bA | 1.16 ± 0.01 aA | 41.76 ± 0.11 bA | 39.26 ± 0.07 aB | 0.52 ± 0.00 aA | 1.67 ± 0.00 aA | 0.57 ± 0.00 aA | 17.90 ± 0.04 bA | 82.10 ± 0.04 bA | 1.06 ± 0.00 aA | |
| Extrusion (°C) treated samples | ||||||||||||
| 15 °C | 0 | 0.19 ± 0.00 a | 16.51 ± 0.12 a | 1.22 ± 0.01 a | 40.38 ± 0.07 a | 39.04 ± 0.18 a | 0.52 ± 0.02 a | 1.61 ± 0.02 a | 0.53 ± 0.00 a | 19.52 ± 0.12 a | 80.48 ± 0.12 a | 1.03 ± 0.01 a |
| 60 | 0.19 ± 0.03 aA | 16.79 ± 0.19 aA | 1.20 ± 0.11 aA | 40.40 ± 0.46 aA | 38.95 ± 0.49 bA | 0.46 ± 0.04 aAB | 1.57 ± 0.01 aA | 0.44 ± 0.13 aA | 19.75 ± 0.07 aA | 80.25 ± 0.07 aB | 1.04 ± 0.02 aA | |
| 25 °C | 0 | 0.19 ± 0.00 a | 16.51 ± 0.12 a | 1.22 ± 0.01 a | 40.38 ± 0.07 a | 39.04 ± 0.18 a | 0.52 ± 0.02 a | 1.61 ± 0.02 a | 0.53 ± 0.00 b | 19.52 ± 0.12 a | 80.48 ± 0.12 a | 1.03 ± 0.01 a |
| 60 | 0.18 ± 0.01 aA | 16.33 ± 0.23 aA | 1.11 ± 0.10 aA | 40.68 ± 0.47 aA | 39.15 ± 0.32 aA | 0.40 ± 0.02 bB | 1.60 ± 0.01 aA | 0.55 ± 0.00 aA | 19.22 ± 0.12 aB | 80.78 ± 0.12 aA | 1.04 ± 0.02 aA | |
| 40 °C | 0 | 0.19 ± 0.00 a | 16.51 ± 0.12 a | 1.22 ± 0.01 a | 40.38 ± 0.07 a | 39.04 ± 0.18 a | 0.52 ± 0.02 a | 1.61 ± 0.02 a | 0.53 ± 0.00 a | 19.52 ± 0.12 a | 80.48 ± 0.12 a | 1.03 ± 0.01 a |
| 60 | 0.18 ± 0.02 aA | 16.54 ± 0.01 aA | 1.32 ± 0.03 aA | 40.50 ± 0.04 aA | 38.84 ± 0.03 aA | 0.50 ± 0.00 aA | 1..58 ± 0.02 aA | 0.54 ± 0.02 aA | 19.62 ± 0.05 aA | 80.38 ± 0.05 aB | 1.04 ± 0.00 aA | |
| Temperature (°C) | Crude Protein Content (%) | ||||||
|---|---|---|---|---|---|---|---|
| Storage Days (d) | |||||||
| 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| Untreated (°C) samples | |||||||
| 15 °C | 13.37 ± 0.12 a | 13.43 ± 0.03 a | 13.38 ± 0.03 a | 13.28 ± 0.07 a | 13.39 ± 0.07 a | 13.38 ± 0.11 a | 13.39 ± 0.09 a |
| 25 °C | 13.37 ± 0.12 a | 13.47 ± 0.02 a | 13.49 ± 0.06 a | 13.56 ± 0.27 a | 13.42 ± 0.08 a | 13.51 ± 0.24 a | 13.53 ± 0.21 a |
| 40 °C | 13.37 ± 0.12 a | 13.26 ± 0.28 a | 13.32 ± 0.09 a | 13.32 ± 0.03 a | 13.25 ± 0.23 a | 13.34 ± 0.07 a | 13.34 ± 0.17 a |
| Extrusion (°C) treated samples | |||||||
| 15 °C | 13.37 ± 0.12 a | 13.52 ± 0.38 a | 13.48 ± 0.30 a | 13.52 ± 0.19 a | 13.37 ± 0.28 a | 13.54 ± 0.31 a | 14.49 ± 0.03 a |
| 25 °C | 13.55 ± 0.46 a | 13.64 ± 0.20 a | 13.55 ± 0.09 a | 13.61 ± 0.24 a | 13.20 ± 0.21 a | 13.38 ± 0.25 a | 13.49 ± 0.04 a |
| 40 °C | 13.47 ± 0.24 a | 13.36 ± 0.14 a | 13.46 ± 0.04 a | 13.44 ± 0.16 a | 13.51 ± 0.22 a | 13.39 ± 0.20 a | 13.41 ± 0.12 a |
| Amino Acid (mg/g) | Untreated Rice Bran | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 °C | 25 °C | 40 °C | |||||||
| 0 Day | 30 Days | 60 Days | 0 Day | 30 Days | 60 Days | 0 Day | 30 Days | 60 Days | |
| Aspartic acid | 4.22 ± 0.03 b | 4.35 ± 0.04 bB | 4.53 ± 0.07 aA | 4.22 ± 0.03 b | 4.51 ± 0.01 bA | 4.68 ± 0.07 aA | 4.22 ± 0.03 b | 4.58 ± 0.01 aA | 4.71 ± 0.07 aA |
| Threonine | 2.02 ± 0.11 a | 2.02 ± 0.07 aA | 2.11 ± 0.11 aA | 2.02 ± 0.11 a | 2.05 ± 0.04 aA | 2.07 ± 0.11 aA | 2.02 ± 0.11 a | 2.06 ± 0.17 aA | 2.00 ± 0.14 aA |
| Serine | 2.34 ± 0.06 a | 2.45 ± 0.07 aA | 2.53 ± 0.07 aA | 2.34 ± 0.06 a | 2.54 ± 0.07 aA | 2.60 ± 0.04 aA | 2.34 ± 0.06 a | 2.53 ± 0.07 aA | 2.57 ± 0.11 aA |
| Glutamic acid | 10.17 ± 0.03 a | 10.16 ± 0.10 aA | 10.49 ± 0.49 aA | 10.17 ± 0.03 a | 10.56 ± 0.68 aA | 10.7 ±0.68 aA | 10.17 ± 0.03 a | 10.8 ±0.19 aA | 10.96 ± 0.11 aA |
| Glycine | 3.62 ± 0.03 a | 3.67 ± 0.06 aA | 3.64 ± 0.08 aA | 3.62 ± 0.03 a | 3.66 ± 0.01 aA | 3.60 ± 0.09 aA | 3.62 ± 0.03 a | 3.63 ± 0.02 aA | 3.65 ± 0.04 aA |
| Alanine | 4.04 ± 0.20 a | 4.06 ± 0.18 aA | 4.06 ± 0.03 aA | 4.04 ± 0.20 a | 4.09 ± 0.07 aA | 4.02 ± 0.03 aA | 4.04 ± 0.20 a | 4.05 ± 0.16 aA | 4.01 ± 0.17 aA |
| Cystine | 0.22 ± 0.01 a | 0.24 ± 0.01 aA | 0.24 ± 0.04 aA | 0.22 ± 0.01 a | 0.22 ± 0.03 aA | 0.22 ± 0.01 aA | 0.22 ± 0.01 a | 0.26 ± 0.03 aA | 0.23 ± 0.03 aA |
| Valine | 3.22 ± 0.07 b | 3.22 ± 0.04 bB | 3.48 ± 0.10 aA | 3.22 ± 0.07 b | 3.54 ± 0.04 aA | 3.56 ± 0.06 aA | 3.22 ± 0.07 b | 3.48 ± 0.03 aA | 3.56 ± 0.04 aA |
| Methionine | 1.11 ± 0.05 a | 1.08 ± 0.14 aA | 1.06 ± 0.12 aA | 1.11 ± 0.05 a | 1.16 ± 0.08 aA | 1.09 ± 0.11 aA | 1.11 ± 0.05 a | 1.15 ± 0.02 aA | 1.11 ± 0.11 aA |
| Isoleucine | 3.00 ± 0.14 a | 3.04 ± 0.04 aA | 3.04 ± 0.03 aA | 3.00 ± 0.14 a | 3.08 ± 0.05 aA | 3.07 ± 0.07 aA | 3.00 ± 0.14 a | 3.08 ± 0.02 aA | 3.06 ± 0.08 aA |
| Leucine | 6.08 ± 0.14 a | 6.41 ± 0.13 aA | 6.45 ± 0.19 aB | 6.08 ± 0.14 a | 6.53 ± 0.28 aA | 6.64 ± 0.02 aAB | 6.08 ± 0.14 a | 6.68 ± 0.12 aA | 6.94 ± 0.02 aA |
| Tyrosine | 1.73 ± 0.02 a | 1.75 ± 0.09 aA | 1.75 ± 0.08 aA | 1.73 ± 0.02 a | 1.77 ± 0.03 aA | 1.73 ± 0.04 aA | 1.73 ± 0.02 a | 1.74 ± 0.09 aA | 1.75 ± 0.07 aA |
| Phenylalanine | 2.07 ± 0.07 a | 2.07 ± 0.09 aA | 2.07 ± 0.18 aA | 2.07 ± 0.07 a | 2.05 ± 0.10 aA | 2.04 ± 0.04 aA | 2.07 ± 0.07 a | 2.08 ± 0.00 aA | 2.10 ± 0.12 aA |
| Histidine | 2.65 ± 0.02 a | 2.71 ± 0.07 aC | 2.85 ± 0.17 aA | 2.65 ± 0.02 a | 3.09 ± 0.04 aA | 3.11 ± 0.23 aA | 2.65 ± 0.02 a | 2.94 ± 0.01 aB | 3.00 ± 0.09 aA |
| Lysine | 4.31 ± 0.01 a | 4.32 ± 0.13 aA | 4.30 ± 0.12 aA | 4.31 ± 0.01 a | 4.29 ± 0.08 aA | 4.30 ± 0.11 aA | 4.31 ± 0.01 a | 4.33 ± 0.03 aA | 4.30 ± 0.06 aA |
| Arginine | 7.80 ± 0.14 a | 7.84 ± 0.25 aA | 7.80 ± 0.16 aA | 7.80 ± 0.14 a | 7.87 ± 0.30 aA | 7.87 ± 0.06 aA | 7.80 ± 0.14 a | 7.88 ± 0.38 aA | 7.87 ± 0.06 aA |
| Proline | 2.50 ± 0.04 b | 2.51 ± 0.24 bB | 3.25 ± 0.27 aA | 2.50 ± 0.04 b | 3.33 ± 0.15 aA | 3.29 ± 0.19 aA | 2.50 ± 0.04 b | 3.20 ± 0.22 aA | 3.24 ± 0.12 aA |
| TAA | 61.12 ± 0.69 b | 61.90 ± 0.16 a bB | 63.64 ± 0.81 aA | 61.12 ± 0.69 b | 64.36 ± 0.21 aA | 64.61 ± 0.11 aA | 61.12 ± 0.69 b | 64.54 ± 0.83 aA | 65.06 ± 0.50 aA |
| EAA | 21.81 ± 0.22 a | 22.16 ± 0.22 aA | 22.51 ± 0.60 aA | 21.81 ± 0.22 b | 22.70 ± 0.36 aA | 22.78 ± 0.01 aA | 21.81 ± 0.22 b | 22.85 ± 0.02 aA | 23.07 ± 0.34 aA |
| NEAA | 39.31 ± 0.47 b | 39.75 ± 0.37 bA | 41.13 ± 0.21 aB | 39.31 ± 0.47 b | 41.65 ± 0.57 aA | 41.84 ± 0.10 aA | 39.31 ± 0.47 b | 41.69 ± 0.81 aA | 41.99 ± 0.16 aA |
| E/T(%) | 35.68 ± 0.04 a | 35.79 ± 0.44 aA | 35.37 ± 0.50 aA | 35.68 ± 0.04 a | 35.28 ± 0.68 aA | 35.25 ± 0.05 aA | 35.68 ± 0.04 a | 35.41 ± 0.43 aA | 35.46 ± 0.25 aA |
| E/N(%) | 55.47 ± 0.09 a | 55.75 ± 1.07 aA | 54.73 ± 1.19 aA | 55.47 ± 0.09 a | 54.52 ± 1.61 aA | 54.44 ± 0.11 aA | 55.47 ± 0.09 a | 54.83 ± 1.03 aA | 54.94 ± 0.59 aA |
| Amino Acid (mg/g) | Extruded Rice Bran | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 °C | 25 °C | 40 °C | |||||||
| 0 Day | 30 Days | 60 Days | 0 Day | 30 Days | 60 Days | 0 Day | 30 Days | 60 Days | |
| Aspartic acid | 1.03 ± 0.03 a | 0.98 ± 0.05 aA | 0.97 ± 0.06 aA | 1.03 ± 0.03 a | 1.00 ± 0.14 aA | 1.02 ± 0.02 aA | 1.03 ± 0.03 a | 1.01 ± 0.14 aA | 1.07 ± 0.07 aA |
| Threonine | 0.74 ± 0.05 a | 0.74 ± 0.04 aA | 0.65 ± 0.05 aA | 0.74 ± 0.05 a | 0.64 ± 0.04 a bA | 0.61 ± 0.01 bA | 0.74 ± 0.05 a | 0.41 ± 0.00 bB | 0.40 ± 0.06 bB |
| Serine | 0.55 ± 0.01 a | 0.49 ± 0.01 bA | 0.50 ± 0.03 aA | 0.55 ± 0.01 a | 0.49 ± 0.04 aA | 0.52 ± 0.05 aA | 0.55 ± 0.01 a | 0.37 ± 0.02 bB | 0.27 ± 0.02cB |
| Glutamic acid | 4.61 ± 0.07 a | 4.35 ± 0.06 bA | 4.45 ± 0.08 a bA | 4.61 ± 0.07 a | 3.99 ± 0.15 bB | 4.09 ± 0.15 bA | 4.61 ± 0.07 a | 4.13 ± 0.0 bAB | 4.14 ± 0.12 bA |
| Glycine | 1.19 ± 0.20 a | 1.14 ± 0.05 aA | 1.16 ± 0.01 aA | 1.19 ± 0.20 a | 1.12 ± 0.06 aA | 1.09 ± 0.01 aA | 1.19 ± 0.20 a | 1.03 ± 0.09 aA | 1.06 ± 0.10 aA |
| Alanine | 1.28 ± 0.14 a | 1.21 ± 0.06 aA | 1.25 ± 0.04 aA | 1.28 ± 0.14 a | 1.18 ± 0.03 aA | 1.18 ± 0.00 aA | 1.28 ± 0.14 a | 1.11 ± 0.12 aA | 1.11 ± 0.20 aA |
| Cystine | 0.11 ± 0.01 a | 0.12 ± 0.01 aA | 0.13 ± 0.02 aA | 0.11 ± 0.01 a | 0.14 ± 0.01 aA | 0.14 ± 0.02 aA | 0.11 ± 0.01 a | 0.12 ± 0.02 aA | 0.12 ± 0.02 aA |
| Valine | 0.63 ± 0.09 a | 0.54 ± 0.05 aA | 0.56 ± 0.02 aA | 0.63 ± 0.09 a | 0.57 ± 0.05 aA | 0.56 ± 0.07 aA | 0.63 ± 0.09 a | 0.39 ± 0.03 bB | 0.40 ± 0.02 bB |
| Methionine | 0.27 ± 0.02 a | 0.25 ± 0.01 aA | 0.25 ± 0.02 aA | 0.27 ± 0.02 a | 0.24 ± 0.00 bA | 0.24 ± 0.01 bA | 0.27 ± 0.02 a | 0.19 ± 0.01 bB | 0.20 ± 0.02 bA |
| Isoleucine | 0.36 ± 0.04 a | 0.33 ± 0.02 aA | 0.35 ± 0.04 aA | 0.36 ± 0.04 a | 0.35 ± 0.09 aA | 0.32 ± 0.07 aA | 0.36 ± 0.04 a | 0.25 ± 0.03 bA | 0.22 ± 0.01 bA |
| Leucine | 1.28 ± 0.15 a | 1.04 ± 0.01 aA | 1.07 ± 0.04 aA | 1.28 ± 0.15 a | 1.07 ± 0.08 aA | 1.02 ± 0.01 aA | 1.28 ± 0.15 a | 0.74 ± 0.00 bB | 0.79 ±0.07 bB |
| Tyrosine | 0.43 ± 0.02 a | 0.40 ± 0.02 aA | 0.41 ± 0.03 aA | 0.43 ± 0.02 a | 0.40 ± 0.03 aA | 0.39 ±0.05 aA | 0.43 ± 0.02 a | 0.36 ± 0.02 bA | 0.35 ± 0.01 bA |
| Phenylalanine | 0.27 ± 0.01 a | 0.27 ± 0.02 aA | 0.27 ± 0.02 aA | 0.27 ± 0.01 a | 0.28 ± 0.01 aA | 0.28 ± 0.02 aA | 0.27 ± 0.01 a | 0.19 ± 0.01 bB | 0.18 ± 0.00 bB |
| Histidine | 1.67 ± 0.01 a | 1.43 ± 0.00 bA | 1.46 ± 0.05 bA | 1.67 ± 0.01 a | 1.30 ± 0.00 bB | 1.34 ± 0.05 bAB | 1.67 ± 0.01 a | 1.28 ± 0.03 bB | 1.29 ± 0.04 bB |
| Lysine | 1.37 ± 0.09 a | 1.21 ± 0.04 aA | 1.23 ± 0.02 aA | 1.37 ± 0.09 a | 1.16 ± 0.02 bA | 1.14 ± 0.01 bA | 1.37 ± 0.09 a | 0.92 ± 0.11 bB | 0.89 ± 0.07 bB |
| Arginine | 3.26 ± 0.05 a | 2.80 ± 0.06 bA | 2.80 ± 0.02 bA | 3.26 ± 0.05 a | 2.55 ± 0.04 bB | 2.53 ± 0.01 bB | 3.26 ± 0.05 a | 2.49 ± 0.05 bB | 2.47 ± 0.07 bB |
| Proline | 0.61 ± 0.03 a | 0.63 ± 0.03 aA | 0.64 ± 0.00 aA | 0.61 ± 0.03 a | 0.61 ± 0.25 aA | 0.59 ± 0.14 aA | 0.61 ± 0.03 a | 0.56 ± 0.07 aA | 0.56 ± 0.07 aA |
| TAA | 19.67 ± 0.76 aB | 17.96 ± 0.05 bA | 17.10 ± 0.38 bA | 19.67 ± 0.76 aB | 18.14 ± 0.05 bB | 17.07 ± 0.21 bB | 19.67 ± 0.76 aB | 15.53 ± 0.20 bC | 15.52 ± 0.42 bC |
| EAA | 4.93 ± 0.34 aA | 4.41 ± 0.10 aA | 4.32 ± 0.21 aA | 4.93 ± 0.34 aA | 4.37 ± 0.14 aA | 4.17 ± 0.15 aA | 4.93 ± 0.34 aA | 3.08 ± 0.15 bB | 3.07± 0.22 bB |
| NEAA | 14.74 ± 0.42 aB | 13.55 ± 0.05 bA | 12.79 ± 0.17 bA | 14.74 ± 0.42 aB | 13.77 ± 1.19 bB | 12.89 ± 0.06 bB | 14.74 ± 0.42 aB | 12.45 ± 0.05 bB | 12.45 ± 0.20 bB |
| E/T(%) | 25.05 ± 0.77 aB | 24.53 ± 0.48 aA | 25.23 ± 0.65 aA | 25.05 ± 0.77 aB | 24.10 ± 0.84 aA | 24.45 ± 0.56 aA | 25.05 ± 0.77 aB | 19.81 ± 0.72 bB | 19.79 ± 0.87 bB |
| E/N(%) | 33.43 ± 1.37 aB | 32.51 ± 0.84 aA | 33.74 ± 1.17 aA | 33.43 ± 1.37 aB | 31.77 ± 1.47 aA | 32.36 ± 0.99 aA | 33.43 ± 1.37 aB | 24.70 ±1.13 bB | 24.67 ± 1.35 bB |
| Enzymatic Activity | Temp (°C) | Zero-Order | First-Order | Second-Order | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k | R2 | RMSE | X2 | k | R2 | RMSE | X2 | k | R2 | RMSE | X2 | ||
| PVuntreated | 15 °C | 0.057 | 0.841 | 0.974 | 0.950 | 0.020 | 0.986 | 0.056 | 0.00323 | −0.004 | 0.985 | 0.012 | 1.48 × 10−4 |
| 25 °C | 0.059 | 0.836 | 1.026 | 1.053 | 0.02 | 0.995 | 0.030 | 9.49 × 10−4 | −0.003 | 0.969 | 0.016 | 2.78 × 10−4 | |
| 40 °C | 0.065 | 0.843 | 1.091 | 1.191 | 0.023 | 0.973 | 0.090 | 0.008 | −0.004 | 0.893 | 0.033 | 0.001 | |
| LPS untreated | 15 °C | 3.514 | 0.215 | 261.216 | 68,233.82 | −6.466 | 0.947 | 36.160 | 1307.565 | 1.88 × 10−4 | 0.975 | 0.001 | 1.17 × 10−6 |
| 25 °C | 3.313 | 0.201 | 257.083 | 66,092.11 | −6.495 | 0.941 | 38.465 | 1479.565 | 2.01 × 10−4 | 0.975 | 0.001 | 1.31 × 10−6 | |
| 40 °C | 3.408 | 0.209 | 257.874 | 66,499.10 | −6.470 | 0.964 | 29.254 | 855.832 | 2.14 × 10−4 | 0.952 | 0.001 | 2.94 × 10−6 | |
| FFA untreated | 15 °C | 2.541 | 0.967 | 18.263 | 333.556 | 1.988 | 0.935 | 12.360 | 152.793 | −0.00174 | 0.443 | 0.046 | 0.002 |
| 25 °C | 3.073 | 0.952 | 26.776 | 716.993 | 2.286 | 0.890 | 18.957 | 359.384 | −1.72 × 10−3 | 0.421 | 0.047 | 0.002 | |
| 40 °C | 3.394 | 0.889 | 46.520 | 2164.192 | 2.047 | 0.675 | 3.36 × 101 | 1128.244 | −1.68 × 10−3 | 3.93 × 10−1 | 0.049 | 0.002 | |
| POD | 15 °C | 0.063 | 0.758 | 1.386 | 1.922 | 0.009 | 0.868 | 0.089 | 0.008 | 0.008 | 0.623 | 0.256 | 0.065 |
| 25 °C | 0.074 | 0.807 | 1.413 | 1.999 | 0.019 | 0.996 | 0.027 | 7.36 × 10−4 | 0.007 | 0.561 | 0.248 | 0.061 | |
| 40 °C | 0.083 | 0.846 | 1.388 | 1.927 | 0.030 | 0.975 | 0.113 | 0.012 | 0.006 | 0.511 | 0.247 | 0.061 | |
| PV | 15 °C | 0.060 | 0.696 | 1.392 | 1.939 | 0.006 | 0.947 | 0.036 | 0.001 | −0.001 | 0.965 | 0.004 | 2.12 × 10−6 |
| 25 °C | 0.062 | 0.704 | 1.409 | 1.988 | 0.007 | 0.994 | 0.013 | 1.94 × 10−4 | −0.001 | 0.987 | 0.003 | 9.93 × 10−6 | |
| 40 °C | 0.066 | 0.731 | 1.408 | 1.983 | 0.011 | 0.974 | 0.045 | 0.002 | −0.001 | 0.954 | 0.008 | 7.59 × 10−5 | |
| LPS | 15 °C | 0.873 | 0.830 | 14.010 | 196.301 | 0.334 | 0.983 | 1.038 | 1.077 | −3.37 × 10−4 | 0.920 | 0.002 | 5.54 × 10−5 |
| 25 °C | 0.884 | 0.831 | 14.145 | 200.096 | 0.341 | 0.980 | 1.154 | 1.333 | −3.42 × 10−4 | 0.898 | 0.002 | 7.41 × 10−6 | |
| 40 °C | 0.903 | 0.834 | 14.290 | 204.227 | 0.354 | 0.978 | 1.254 | 1.573 | −3.46 × 10−4 | 0.888 | 0.002 | 8.39 × 10−6 | |
| FFA | 15 °C | 0.338 | 0.694 | 8.757 | 76.689 | 0.001 | 0.341 | 0.052 | 0.002 | −7.53 × 10−4 | 0.341 | 2.47 × 10−4 | 6.12 × 10−8 |
| 25 °C | 0.398 | 0.783 | 8.150 | 66.436 | 0.089 | 0.639 | 1.582 | 2.503 | −3.12 × 10−4 | 0.644 | 0.005 | 3.00 × 10−5 | |
| 40 °C | 0.397 | 0.783 | 8.151 | 66.4470 | 0.088 | 0.640 | 1.578 | 2.490 | −3.12 × 10−4 | 0.645 | 0.005 | 2.98 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, M.T.; Liu, K.; Han, S.; Jatoi, M.A.; Sarpong, F. Optimization of Extrusion Treatments, Quality Assessments, and Kinetics Degradation of Enzyme Activities during Storage of Rice Bran. Foods 2023, 12, 1236. https://doi.org/10.3390/foods12061236
Rashid MT, Liu K, Han S, Jatoi MA, Sarpong F. Optimization of Extrusion Treatments, Quality Assessments, and Kinetics Degradation of Enzyme Activities during Storage of Rice Bran. Foods. 2023; 12(6):1236. https://doi.org/10.3390/foods12061236
Chicago/Turabian StyleRashid, Muhammad Tayyab, Kunlun Liu, Simeng Han, Mushtaq Ahmed Jatoi, and Frederick Sarpong. 2023. "Optimization of Extrusion Treatments, Quality Assessments, and Kinetics Degradation of Enzyme Activities during Storage of Rice Bran" Foods 12, no. 6: 1236. https://doi.org/10.3390/foods12061236
APA StyleRashid, M. T., Liu, K., Han, S., Jatoi, M. A., & Sarpong, F. (2023). Optimization of Extrusion Treatments, Quality Assessments, and Kinetics Degradation of Enzyme Activities during Storage of Rice Bran. Foods, 12(6), 1236. https://doi.org/10.3390/foods12061236






