Composition, Microbiota, Mechanisms, and Anti-Obesity Properties of Rice Bran
Abstract
:1. Introduction
2. Phytochemical Composition of Rice Bran
3. Anti-Obesity Properties of Rice Bran
3.1. Results of In Vitro Studies
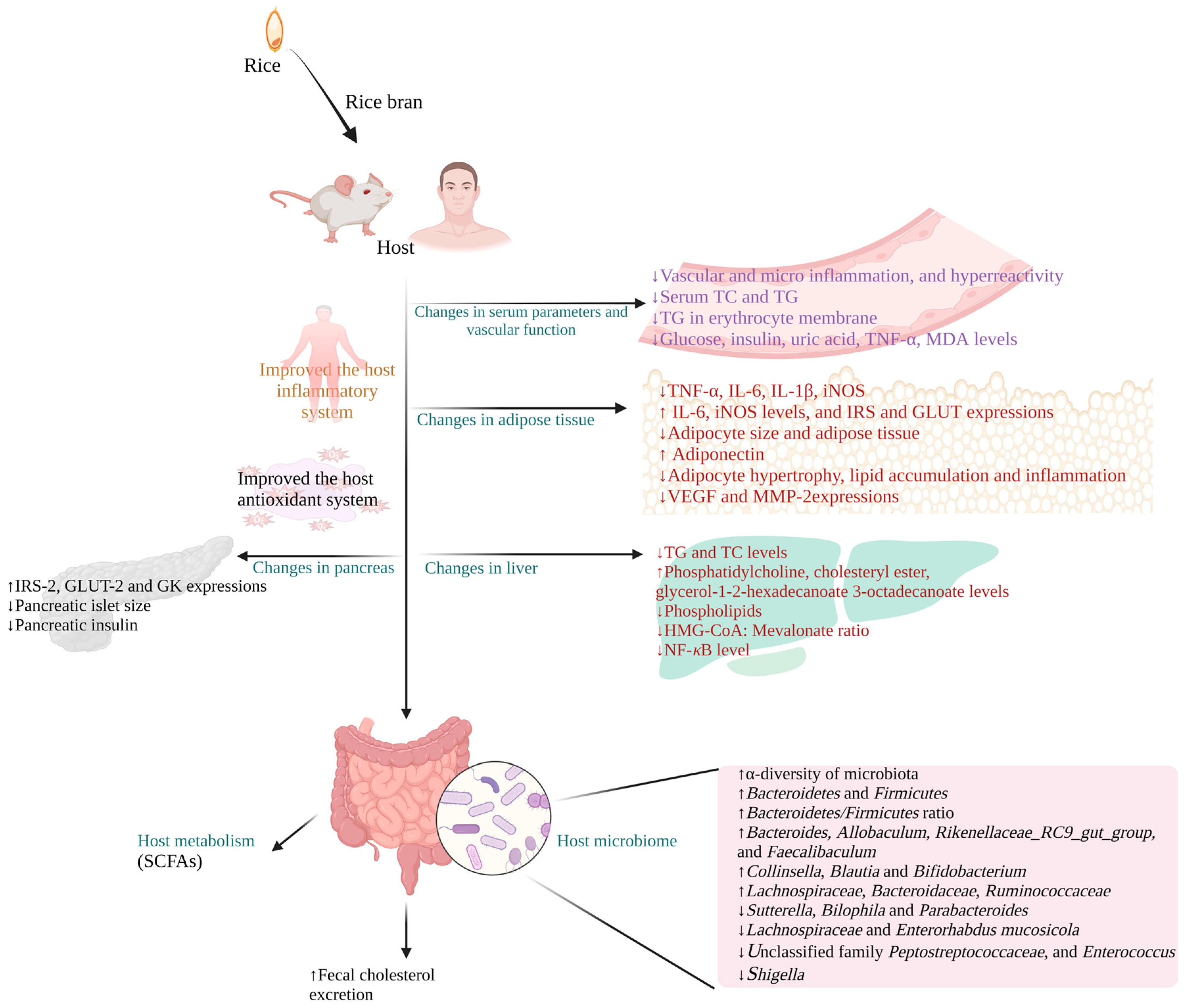
3.2. Results of In Vivo Studies
3.3. Clinical Studies
4. Influence of Rice Bran Supplementation on Host Microbiome
5. Mechanisms Associated with the Anti-Obesity Property of Rice Bran
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.R.; Chauhan, G.S.; Agrawal, K. Physico-Chemical Characteristics of Rice Bran Processed by Dry Heating and Extrusion Cooking. Int. J. Food Prop. 2004, 7, 603–614. [Google Scholar] [CrossRef]
- Spaggiari, M.; Dall’Asta, C.; Galaverna, G.; del Castillo Bilbao, M.D. Rice Bran By-Product: From Valorization Strategies to Nutritional Perspectives. Foods 2021, 10, 85. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Feddern, V.; Kupsk, L.; Cipolatti, E.P.; Furlong, E.B.; Soares, L.A.S. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 2011, 102, 8335–8338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, K.; Yousuf, B.; Singh, A.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food-A review. Bioact. Carbohydr. Diet Fibre 2015, 6, 24–30. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A comprehensive review on anti-diabetic property of rice bran. Asian Pac. J. Trop. Biomed. 2018, 8, 79–84. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Chaiyasut, C.; Sivamaruthi, B.S.; Chalermpong Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Chaiyasut, K.; Kesika, P. The influence of extraction methods on composition and antioxidant properties of rice bran oil. Food Sci. Technol. 2015, 35, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Reis, N.; Castanho, A.; Lageiro, M.; Pereira, C.; Brites, C.M.; Vaz-Velho, M. Rice bran stabilisation and oil extraction using the microwave-assisted method and its effects on GABA and gamma-oryzanol compounds. Foods 2022, 11, 912. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Chongsuwat, R.; Phosat, C.; Butacnum, A. Rice bran oil containing gamma-oryzanol improves lipid profiles and antioxidant status in hyperlipidemic subjects: A randomised double-blind controlled trial. J. Altern. Complement. Med. 2019, 25, 353–358. [Google Scholar] [CrossRef]
- Kozuka, C.; Sunagawa, S.; Ueda, R.; Higa, M.; Tanaka, H.; Shimizu-Okabe, C.; Ishiuchi, S.; Takayama, C.; Matsushita, M.; Tsutsui, M.; et al. γ-Oryzanol protects pancreatic β-cells against endoplasmic reticulum stress in male mice. Endocrinology 2015, 156, 1242–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francisqueti, F.V.; Minatel, I.O.; Ferron, A.J.T.; Bazan, S.G.Z.; Silva, V.D.; Garcia, J.L.; de Campos, D.H.S.; Ferreira, A.L.; Moreto, F.; Cicogna, A.C.; et al. Effect of gamma-oryzanol as therapeutic agent to prevent cardiorenal metabolic syndrome in animals submitted to high sugar-fat diet. Nutrients 2017, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [Green Version]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.; Ma, Q.; Zhang, M.; Zhang, R. Bound Phenolics Ensure the Antihyperglycemic Effect of Rice Bran Dietary Fiber in db/db Mice via Activating the Insulin Signaling Pathway in Skeletal Muscle and Altering Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- Sheflin, A.M.; Borresen, E.C.; Kirkwood, J.S.; Boot, C.M.; Whitney, A.K.; Lu, S.; Brown, R.J.; Broeckling, C.D.; Ryan, E.P.; Weir, T.L. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol. Nutr. Food Res. 2017, 61, 1500905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibayama, J.; Kuda, T.; Shikano, A.; Fukunaga, M.; Takahashi, H.; Kimura, B.; Ishizaki, S. Effects of rice bran and fermented rice bran suspensions on caecal microbiota in dextran sodium sulphate-induced inflammatory bowel disease model mice. Food Biosci. 2018, 25, 8–14. [Google Scholar] [CrossRef]
- Ito, Y.; Nakashima, Y.; Matsuoka, S. Rice bran extract containing acylated steryl glucoside fraction decreases elevated blood LDL cholesterol level in obese Japanese men. J. Med. Investig. 2015, 62, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Haldar, S.; Wong, L.H.; Tay, S.L.; Jacoby, J.J.; He, P.; Osman, F.; Ponnalagu, S.; Jiang, Y.R.; Lian, H.P.R.; Henry, C.J. Two blends of refined rice bran, flaxseed, and sesame seed oils affect the blood lipid profile of Chinese adults with borderline hypercholesterolemia to a similar extent as refined olive oil. J. Nutr. 2020, 150, 3141–3151. [Google Scholar] [CrossRef]
- Hongu, N.; Kitts, D.D.; Zawistowski, J.; Dossett, C.M.; Kopeć, A.; Pope, B.T.; Buchowski, M.S. Pigmented rice bran and plant sterol combination reduces serum lipids in overweight and obese adults. J. Am. Coll. Nutr. 2014, 33, 231–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edrisi, F.; Salehi, M.; Ahmadi, A.; Fararoei, M.; Rusta, F.; Mahmoodianfard, S. Effects of supplementation with rice husk powder and rice bran on inflammatory factors in overweight and obese adults following an energy-restricted diet: A randomized controlled trial. Eur. J. Nutr. 2018, 57, 833–843. [Google Scholar] [CrossRef]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef]
- Vichapong, J.; Sookserm, M.; Srijesdaruk, V.; Swatsitang, P.; Srijaranai, S. High performance liquid chromatographic analysis of phenolic compounds and their antioxidant activities in rice varieties. LWT-Food Sci. Technol. 2010, 43, 1325–1330. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Juraimi, A.S.; Tayebi-Meigooni, A. Comparative Evaluation of Different Extraction Techniques and Solvents for the Assay of Phytochemicals and Antioxidant Activity of Hashemi Rice Bran. Molecules 2015, 20, 10822–10838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pengkumsri, N.; Chaiyasut, C.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Sivamaruthi, B.S. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci. Technol. 2015, 35, 35–338. [Google Scholar] [CrossRef] [Green Version]
- Zarei, I.; Brown, D.G.; Nealon, N.J.; Ryan, E.P. Rice Bran Metabolome Contains Amino Acids, Vitamins & Cofactors, and Phytochemicals with Medicinal and Nutritional Properties. Rice 2017, 10, 24. [Google Scholar] [PubMed] [Green Version]
- Saunders, R.M. The properties of rice bran as a food stuff. Cereal Foods World 1990, 35, 632–639. [Google Scholar]
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816. [Google Scholar] [CrossRef]
- Zullaikah, S.; Lai, C.C.; Vali, S.R.; Ju, Y.H. A two-step acid-catalyzed process for the production of biodiesel from rice bran oil. Bioresour. Technol. 2005, 96, 1889–1896. [Google Scholar] [CrossRef]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.A.; Salser, W.A.; Parmar, R.; Emeson, E.E. Novel tocotrienols of rice bran inhibit atherosclerotic lesions in C57BL/6 ApoE-deficient mice. J. Nutr. 2001, 131, 2606–2618. [Google Scholar] [CrossRef] [Green Version]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Yamuangmorn, S.; Prom-u-Thai, C. The potential of high-anthocyanin purple rice as a functional ingredient in human health. Antioxidants 2021, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Charoonratana, T.; Songsak, T.; Sakunpak, A.; Pathompak, P.; Charoenchai, L. Using liquid chromatography-mass spectrometry-based metabolomics to discriminate between cold pressed rice bran oils produced from two different cultivars of Oryza sativa L. ssp. indica in Thailand. Chin. J. Chromatogr. 2015, 33, 966–973. [Google Scholar]
- Bramley, P.M.; Elmadfa, I.; Kafatos, A.; Kelly, F.J.; Manios, Y.; Roxborough, H.E.; Schuch, W.; Sheehy, P.J.A.; Wagner, K.H. Vitamin E-A critical review. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Abidi, S.L. Tocol-derived minor constituents in selected plant seed oils. J. Am. Oil Chem. Soc. 2003, 80, 327–333. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Arjin, C.; Satsook, A.; Seel-Audom, M.; Ruksiriwanich, W.; Prom-U-Thai, C.; Sringarm, K. Comparative Analysis of Nutritional Components and Phytochemical Attributes of Selected Thai Rice Bran. Front. Nutr. 2022, 9, 833730. [Google Scholar] [CrossRef]
- Wang, M.; Hettiarachchy, N.S.; Qi, M.; Burks, W.; Siebenmorgen, T. Preparation and functional properties of rice bran protein isolate. J. Agric. Food Chem. 1999, 47, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Fabian, C.; Ju, Y.H. A review on rice bran protein: Its properties and extraction methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 816–827. [Google Scholar] [CrossRef]
- Han, S.W.; Chee, K.M.; Cho, S.J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015, 172, 766–769. [Google Scholar] [CrossRef]
- Lee, S.K.; Jang, I.S.; Kim, M.K.; Park, S.K.; Lee, W.Y.; Youn, K.S.; Bae, D.H. Changes in functional properties of rice bran and sesame meal proteins through chemical modifications. Food Sci. Biotechnol. 2004, 13, 555–560. [Google Scholar]
- Daou, C.; Zhang, H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J. Food Sci. Technol. 2014, 51, 3878–3885. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.S.P.; Muralikrishna, G. Non-starch polysaccharide–phenolic acid complexes from native and germinated cereals and millet. Food Chem. 2004, 84, 527–531. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Identification and Quantification of Phenolic Acids and Anthocyanins as Antioxidants in Bran, Embryo and Endosperm of White, Red and Black Rice Kernels (Oryza sativa L.). J. Cereal Sci. 2014, 59, 211–218. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Effect of Extraction Conditions on Phenolic Content, Anthocyanin Content and Antioxidant Activity of Bran Extracts from Thai Rice Cultivars. J. Cereal Sci. 2019, 86, 86–91. [Google Scholar] [CrossRef]
- Aziz, S.; Elfahmi, Y.; Soemardji, A.A.; Sukrasno, S. Anti-Hypercholesterolemic Agent from Indonesian Rice Bran. Int. J. Res. Pharm. Sci. 2019, 10, 2733–2738. [Google Scholar] [CrossRef] [Green Version]
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous Determination of Tocols, γ-Oryzanols, Phytosterols, Squalene, Cholecalciferol and Phylloquinone in Rice Bran and Vegetable Oil Samples. Food Chem. 2019, 271, 630–638. [Google Scholar] [CrossRef]
- Sapwarobol, S.; Saphyakhajorn, W.; Astina, J. Biological Functions and Activities of Rice Bran as a Functional Ingredient: A Review. Nutr. Metab. Insights 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Manosroi, J.; Abe, M.; Manosroi, W.; Manosroi, A. 5αReductase type 1 inhibition of Oryza sativa bran extract prepared by supercritical carbon dioxide fluid. J. Supercrit. Fluids 2011, 59, 61–71. [Google Scholar] [CrossRef]
- Wang, W.; Guo, J.; Zhang, J.; Peng, J.; Liu, T.; Xin, Z. Isolation, identification and antioxidant activity of bound phenolic compounds present in rice bran. Food Chem. 2015, 171, 40–49. [Google Scholar] [CrossRef]
- Wu, F.; Yang, N.; Touré, A.; Jin, Z.; Xu, X. Germinated brown rice and its role in human health. Crit. Rev. Food Sci. Nutr. 2013, 53, 451–463. [Google Scholar] [CrossRef]
- Ahmadifard, N.; Murueta, J.H.; Abedian-Kenari, A.; Motamedzadegan, A.; Jamali, H. Comparison the effect of three commercial enzymes for enzymatic hydrolysis of two substrates (rice bran protein concentrate and soy-been protein) with SDS PAGE. J. Food Sci. Technol. 2016, 53, 1279–1284. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2013, 152, 46–55. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef]
- Ding, C.; Liu, Q.; Li, P.; Pei, Y.; Tao, T.; Wang, Y.; Yan, W.; Yang, G.; Shao, X. Distribution and quantitative analysis of phenolic compounds in fractions of Japonica and Indica rice. Food Chem. 2019, 274, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Liu, L.; Deng, Y.; Ma, Y.; Zhang, Y.; Wei, Z.; Xiao, J.; et al. A Comparison of the Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds from Rice Bran and Its Dietary Fibres. Molecules 2018, 23, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Anthocyanins in Thai rice varieties: Distribution and pharmacological significance. Int. Food Res. J. 2018, 25, 2024–2032. [Google Scholar]
- Maisuthisakul, P.; Changchub, L. Effect of Extraction on Phenolic Antioxidant of Different Thai Rice (Oryza sativa L.) Genotypes. Int. J. Food Prop. 2014, 17, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.P.; Lai, H.M. Bioactive Compounds and Antioxidative Activity of Colored Rice Bran. J. Food Drug Anal. 2016, 24, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, Y.; Dang, P.; Zhao, S.; Lai, D.; Zhou, L. Rice Secondary Metabolites: Structures, Roles, Biosynthesis, and Metabolic Regulation. Molecules 2018, 23, 3098. [Google Scholar] [CrossRef] [Green Version]
- Mohanlal, S.; Maney, S.K.; Santhoshkumar, T.R.; Jayalekshmy, A. Tricin 40 -O-(erythro-β-guaiacylglyceryl) ether and tricin 40 -O-(threo-β-guaiacylglyceryl) ether isolated from Njavara (Oryza sativa L. var. Njavara), induce apoptosis in multiple tumor cells by mitochondrial pathway. J. Nat. Med. 2013, 67, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Abe, D.; Sekiya, K. Sakuranetin induces adipopenesis of 3T3-L1 cells through enhanced expression of PPARγ2. Biochem. Biophys. Res. Commun. 2008, 372, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.-S.; Song, K.-S.; Seong, Y.H.; Bai, K.H. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kinoshita, H.; Okuno, Y. Antimutagenic activity of sakuranetin from Prunus jamasakura. J. Food Sci. 2003, 68, 52–56. [Google Scholar] [CrossRef]
- Grecco, S.S.; Reimao, J.Q.; Tempone, A.G.; Sartorelli, P.; Cunha, R.L.; Romoff, P.; Ferreira, M.J.P.; Favero, O.A.; Lago, J.H.G. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC (Asteraceae). Exp. Parasitol. 2012, 130, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yang, Y.; Yang, X.; Zhu, G.; Lu, X.; Jia, F.; Diao, B.; Yu, S.; Ali, A.; Zhang, H.; et al. Investigation of flavonoid components and their associated antioxidant capacity in different pigmented rice varieties. Food Res. Int. 2022, 161, 111726. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhu, H.; Zhang, Z.; Yang, S.; Li, H. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J. Cereal Sci. 2015, 64, 92–99. [Google Scholar] [CrossRef]
- Eder, R. Pigments. In Food Analysis by HPLC, 2nd ed.; Nollet, L.M.L., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 825–880. [Google Scholar]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Chumpolsri, W.; Wijit, N.; Boontakham, P.; Nimmanpipug, P.; Sookwong, P.; Luangkamin, S.; Wongpornchai, S. Variation of Terpenoid Flavor Odorants in Bran of Some Black and White Rice Varieties Analyzed by GC×GC-MS. J. Food Nutr. Res. 2015, 3, 3–120. [Google Scholar] [CrossRef]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of Different Treatments on the Antioxidant Properties and Phenolic Compounds of Rice Bran and Rice Husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, A.A.; Jayadeep, A. Enzymatic processing of pigmented and non-pigmented rice bran on changes in oryzanol, polyphenols and antioxidant activity. J. Food Sci. Technol. 2015, 52, 6538–6546. [Google Scholar] [CrossRef] [Green Version]
- Ruen-ngam, D.; Thawai, C.; Sukonthamut, S. Pretreatment to increase yield and antioxidant activity of γ-oryzanol in rice bran oil. Scienceasia 2016, 42, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Sawangwan, T.; Porncharoennop, C.; Nimraksa, H. Antioxidant compounds from rice bran fermentation by lactic acid bacteria. AIMS Agric. Food 2021, 6, 578–587. [Google Scholar] [CrossRef]
- Nur, Y.; Dang, L.; Anisah, J.; Shaiful, A.S.; Long, K. Bioactive compounds and antioxidant activity of rice bran fermented with lactic acid bacteria. Malays. J. Microbiol. 2015, 11, 156–162. [Google Scholar]
- Mapoung, S.; Semmarath, W.; Arjsri, P.; Thippraphan, P.; Srisawad, K.; Umsumarng, S.; Phromnoi, K.; Jamjod, S.; Prom-u-thai, C.; Dejkriengkraikul, P. Comparative analysis of bioactive-phytochemical characteristics, antioxidants activities, and anti-inflammatory properties of selected black rice germ and bran (Oryza sativa L.) varieties. Eur. Food Res. Technol. 2022, 249, 451–464. [Google Scholar] [CrossRef]
- Chumchoochart, W.; Sutthanut, K. Anti-obesity potential of glutinous black rice bran extract: Anti-adipogenesis and lipolysis induction in 3T3-L1 adipocyte model. Songklanakarin J. Sci. Technol. 2020, 42, 284–291. [Google Scholar]
- Ketprayoon, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. An in vitro study of lipase inhibitory peptides obtained from de-oiled rice bran. RSC Adv. 2021, 11, 18915–18929. [Google Scholar] [CrossRef]
- Chiou, S.; Lai, J.; Liao, J.; Sung, J.; Lin, S. In vitro inhibition of lipase, a-amylase, a-glucosidase, and angiotensin-converting enzyme by defatted rice bran extracts of red-pericarp rice mutant. Cereal Chem. 2018, 95, 167–176. [Google Scholar] [CrossRef]
- Lim, S.M.; Goh, Y.M.; Kuan, W.B.; Loh, S.P. Effect of germinated brown rice extracts on pancreatic lipase, adipogenesis and lipolysis in 3T3-L1 adipocytes. Lipids Health Dis. 2014, 13, 169. [Google Scholar] [CrossRef] [Green Version]
- Barathikannan, K.; Tyagi, A.; Shan, L.; Kim, N.-H.; Lee, D.-S.; Park, J.-S.; Chelliah, R.; Oh, D.-H. Antiobesity and Antioxidative Effect of Fermented Brown Rice Using In Vitro with In Vivo Caenorhabditis elegans Model. Life 2023, 13, 374. [Google Scholar] [CrossRef]
- Anikisetty, M.; Gopala Krishna, A.G.; Panneerselvam, V.; Kamatham, A.N. Diacylglycerol (DAG) rich rice bran and sunflower oils modulate lipid profile and cardiovascular risk factors in Wistar rats. J. Funct. Foods 2018, 40, 117–127. [Google Scholar] [CrossRef]
- Candiracci, M.; Justo, M.L.; Castaño, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract-supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472. [Google Scholar] [CrossRef]
- Justo, M.L.; Candiracci, M.; Dantas, A.P.; de Sotomayor, M.A.; Parrado, J.; Vila, E.; Herrera, M.D.; Rodriguez-Rodriguez, R. Rice bran enzymatic extract restores endothelial function and vascular contractility in obese rats by reducing vascular inflammation and oxidative stress. J. Nutr. Biochem. 2013, 24, 1453–1461. [Google Scholar] [CrossRef]
- Justo, M.L.; Claro, C.; Vila, E.; Herrera, M.D.; Rodriguez-Rodriguez, R. Microvascular disorders in obese Zucker rats are restored by a rice bran diet. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Justo, M.L.; Claro, C.; Zeyda, M.; Stulnig, T.M.; Herrera, M.D.; Rodríguez-Rodríguez, R. Rice bran prevents high-fat diet-induced inflammation and macrophage content in adipose tissue. Eur. J. Nutr. 2016, 55, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Dhara, R.; Dhar, P.; Ghosh, M. Dietary effects of pure and diacylglycerol-rich rice bran oil on growth pattern and lipid profile of rats. J. Oleo Sci. 2012, 61, 369–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuoka, D.; Okahara, F.; Hashizume, K.; Yanagawa, K.; Osaki, N.; Shimotoyodome, A. Triterpene alcohols and sterols from rice bran lower postprandial glucose-dependent insulinotropic polypeptide release and prevent diet-induced obesity in mice. J. Appl. Physiol. 2014, 117, 1337–1348. [Google Scholar] [CrossRef] [Green Version]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of ferulic acid and γ-oryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef] [Green Version]
- Ham, H.; Sung, J.; Lee, L. Effect of rice bran unsaponifiables on high-fat diet-induced obesity in mice. J. Food Biochem. 2015, 39, 673–681. [Google Scholar] [CrossRef]
- Munkong, N.; Hansakul, P.; Yoysungnoen, B.; Wongnoppavich, A.; Sireeratawong, S.; Kaendee, N.; Lerdvuthisopon, N. Vasoprotective effects of rice bran water extract on rats fed with high-fat diet. Asian Pac. J. Trop. Biomed. 2016, 6, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Parklak, W.; Munkong, N.; Somnuk, S.; Somparn, S.; Naowaboot, J.; Yoysungnoen, B.; Lerdvuthisopon, N. Rice bran water extract attenuates pancreatic abnormalities in high-fat diet-induced obese rats. Trop. J. Pharm. Res. 2017, 16, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Munkong, N.; Lonan, P.; Mueangchang, W.; Yadyookai, N.; Kanjoo, V.; Yoysungnoen, B. Red rice bran extract attenuates adipogenesis and inflammation on white adipose tissues in high-fat diet-induced obese mice. Foods 2022, 11, 1865. [Google Scholar] [CrossRef]
- Munkong, N.; Thim-Uam, A.; Pengnet, S.; Hansakul, P.; Somparn, N.; Naowaboot, J.; Tocharus, J.; Tocharus, C. Effects of red rice bran extract on high-fat diet-induced obesity and insulin resistance in mice. Prev. Nutr. Food Sci. 2022, 27, 180–187. [Google Scholar] [CrossRef]
- Park, S.; Chang, H.C.; Lee, J.J. Rice bran fermented with kimchi-derived lactic acid bacteria prevents metabolic complications in mice on a high-fat and -cholesterol diet. Foods 2021, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yu, S.; Kim, W. Rice bran oil attenuates chronic inflammation by inducing m2 macrophage switching in high-fat diet-fed obese mice. Foods 2021, 10, 359. [Google Scholar] [CrossRef]
- Yang, S.C.; Huang, W.C.; Ng, X.E.; Lee, M.C.; Hsu, Y.J.; Huang, C.C.; Wu, H.H.; Yeh, C.L.; Shirakawa, H.; Budijanto, S.; et al. Rice bran reduces weight gain and modulates lipid metabolism in rats with high-energy-diet-induced obesity. Nutrients 2019, 11, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sueratman, A.R.N.E.; Djamiatun, R.K.; Zulfajuniarto, A. Potential of rice bran extract to decrease body weight, triglyceride, and malondialdehyde levels in obese rat. Pak. J. Med. Health Sci. 2019, 13, 1267–1271. [Google Scholar]
- Duansak, N.; Piyabhan, P.; Srisawat, U.; Naowaboot, J.; Lerdvuthisopon, N.; Schmid-Schönbein, G. The effect of rice bran extract on arterial blood pressure, hepatic steatosis, and inflammation in mice fed with a high-fat diet. J. Nutr. Metab. 2020, 2020, 8374287. [Google Scholar] [PubMed]
- Duansak, N.; Schmid-Schönbein, G.W.; Srisawat, U. Anti-obesity effect of rice bran extract on high-fat diet-induced obese mice. Prev. Nutr. Food Sci. 2022, 27, 172–179. [Google Scholar] [CrossRef]
- Laorodphun, P.; Arjinajarn, P.; Thongnak, L.; Promsan, S.; Swe, M.T.; Thitisut, P.; Mahatheeranont, S.; Jaturasitha, S.; Lungkaphin, A. Anthocyanin-rich fraction from black rice, Oryza sativa L. var. indica “Luem Pua,” bran extract attenuates kidney injury induced by high-fat diet involving oxidative stress and apoptosis in obese rats. Phytother. Res. 2021, 35, 5189–5202. [Google Scholar] [CrossRef]
- Garcia, J.L.; Vileigas, D.F.; Gregolin, C.S.; Costa, M.R.; Francisqueti-Ferron, F.V.; Ferron, A.J.T.; De Campos, D.H.S.; Moreto, F.; Minatel, I.O.; Bazan, S.G.Z.; et al. Rice (Oryza sativa L.) bran preserves cardiac function by modulating pro-inflammatory cytokines and redox state in the myocardium from obese rats. Eur. J. Nutr. 2022, 61, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Tochitani, S.; Maehara, Y.; Kawase, T.; Tsukahara, T.; Shimizu, R.; Watanabe, T.; Maehara, K.; Asaoka, K.; Matsuzaki, H. Fermented rice bran supplementation ameliorates obesity via gut microbiota and metabolism modification in female mice. J. Clin. Biochem. Nutr. 2022, 70, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, J.; Goto, M.; Kuda, T.; Fukunaga, M.; Takahashi, H.; Kimura, B. Effect of rice bran fermented with Saccharomyces cerevisiae and Lactobacillus plantarum on gut microbiome of mice fed high-sucrose diet. Benef. Microbes. 2019, 10, 811–821. [Google Scholar] [CrossRef]
- Tamura, M.; Hori, S.; Hoshi, C.; Nakagawa, H. Effects of rice bran oil on the intestinal microbiota and metabolism of isoflavones in adult mice. Int. J. Mol. Sci. 2012, 13, 10336–10349. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Zhang, R.; Huang, F.; Dong, L.; Liu, L.; Jia, X.; Chi, J.; Ma, Y.; Deng, M.; Chen, Y.; et al. Hydrolyzed bound phenolics from rice bran alleviate hyperlipidemia and improve gut microbiota dysbiosis in high-fat-diet fed mice. Nutrients 2022, 14, 1277. [Google Scholar] [CrossRef]
- Zou, Y.; Ju, X.; Chen, W.; Yuan, J.; Wang, Z.; Aluko, R.E.; He, R. Rice bran attenuated obesity via alleviating dyslipidemia, browning of white adipocytes and modulating gut microbiota in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2406–2417. [Google Scholar] [CrossRef]
- Chen, T.; Shen, M.; Yu, Q.; Chen, Y.; Wen, H.; Lu, H.; Chen, S.; Xie, J. Purple red rice anthocyanins alleviate intestinal damage in cyclophosphamide-induced mice associated with modulation of intestinal barrier function and gut microbiota. Food Chem. 2022, 397, 133768. [Google Scholar] [CrossRef]
- Ai, X.; Wu, C.; Yin, T.; Zhur, O.; Liu, C.; Yan, X.; Yi, C.; Liu, D.; Xiao, L.; Li, W.; et al. Antidiabetic function of Lactobacillus fermentum MF423-fermented rice bran and its effect on gut microbiota structure in type 2 diabetic mice. Front. Microbiol. 2021, 12, 682290. [Google Scholar] [CrossRef]
- Nealon, N.J.; Parker, K.D.; Lahaie, P.; Ibrahim, H.; Maurya, A.K.; Raina, K.; Ryan, E.P. Bifidobacterium longum-fermented rice bran and rice bran supplementation affects the gut microbiome and metabolome. Benef. Microbes. 2019, 10, 823–839. [Google Scholar] [CrossRef]
- Phannasorn, W.; Pharapirom, A.; Thiennimitr, P.; Guo, H.; Ketnawa, S.; Wongpoomchai, R. Enriched rice berry bran oil exerts chemopreventive properties through anti-inflammation and alteration of gut microbiota in carcinogen-induced liver and colon carcinogenesis in rats. Cancers 2022, 14, 4358. [Google Scholar] [CrossRef]
- Sheflin, A.M.; Borresen, E.C.; Wdowik, M.J.; Rao, S.; Brown, R.J.; Heuberger, A.L.; Broeckling, C.D.; Weir, T.L.; Ryan, E.P. Pilot dietary intervention with heat-stabilized rice bran modulates stool microbiota and metabolites in healthy adults. Nutrients 2015, 7, 1282–1300. [Google Scholar] [CrossRef] [Green Version]
- So, W.K.W.; Chan, J.Y.W.; Law, B.M.H.; Choi, K.C.; Ching, J.Y.L.; Chan, K.L.; Tang, R.S.Y.; Chan, C.W.H.; Wu, J.C.Y.; Tsui, S.K.W. Effects of a rice bran dietary intervention on the composition of the intestinal microbiota of adults with a high risk of colorectal cancer: A pilot randomised-controlled trial. Nutrients 2021, 13, 526. [Google Scholar] [CrossRef]
- Vilander, A.C.; Hess, A.; Abdo, Z.; Ibrahim, H.; Doumbia, L.; Douyon, S.; Koné, K.; Boré, A.; Zambrana, L.E.; Vilchez, S.; et al. A randomized controlled trial of dietary rice bran intake on microbiota diversity, enteric dysfunction, and fecal secretory IgA in Malian and Nicaraguan Infants. J. Nutr. 2022, 152, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Zambrana, L.E.; McKeen, S.; Ibrahim, H.; Zarei, I.; Borresen, E.C.; Doumbia, L.; Boré, A.; Cissoko, A.; Douyon, S.; Koné, K.; et al. Rice bran supplementation modulates growth, microbiota, and metabolome in weaning infants: A clinical trial in Nicaragua and Mali. Sci. Rep. 2019, 9, 13919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tun, S.; Spainhower, C.J.; Cottrill, C.L.; Lakhani, H.V.; Pillai, S.S.; Dilip, A.; Chaudhry, H.; Shapiro, J.I.; Sodhi, K. Therapeutic efficacy of antioxidants in ameliorating obesity phenotype and associated comorbidities. Front. Pharmacol. 2020, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Gu, I.; Lam, W.S.; Marasini, D.; Brownmiller, C.; Savary, B.J.; Lee, J.A.; Carbonero, F.; Lee, S.O. In vitro fecal fermentation patterns of arabinoxylan from rice bran on fecal microbiota from normal-weight and overweight/obese subjects. Nutrients 2021, 13, 2052. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]

| Cultivar/Strain | Phytochemical Contents | Extraction Methods/Method of Analysis | Ref. |
|---|---|---|---|
| Hashemi RB | TPC: 221.06 ± 10.63 mg/100 g DM TFC: 108.50 ± 10.01 mg/100 g DM Total tocopherol: 38.11 ± 2.04 mg/100 g DM Total tocotrienol 46.54 ± 2.92 mg/100 g DM | Ethanol maceration | [27] |
| TPC: 270.51 ± 11.47 mg/100 g DM TFC: 137.15 ± 12.89 mg/100 g DM Total tocopherol: 36.93 ± 2.26 mg/100 g DM Total tocotrienol: 55.83 ± 1.85 mg/100 g DM | Ethanol–water (50:50) maceration | ||
| TPC: 246.34 ± 12.26 mg/100 g DM TFC: 112.60 ± 13.65 mg/100 g DM Total tocopherol: 37.08 ± 2.21 mg/100 g DM Total tocotrienol: 51.28 ± 2.80 mg/100 g DM | Ethanol ultrasonic | ||
| TPC: 288.40 ± 14.35 mg/100 g DM TFC: 156.20 ± 10.69 mg/100 g DM Total Tocopherol: 37.51 ± 2.05 mg/100 g DM Total Tocotrienol: 56.23 ± 2.37 mg/100 g DM | Ethanol–water (50:50) ultrasonic | ||
| KDML105 | γ-oryzanol: 171.23 ± 0.16 mg/100 g CF α-tocopherol: 6.62 ± 0.01 mg/100 g CF β-tocopherol: 0.38 ± 0.00 mg/100 g CF γ-tocopherol: 7.91 ± 0.00 mg/100 g CF δ-tocopherol: 0.13 ± 0.01 mg/100 g CF Gallic acid: 0.09 ± 0.01 mg/100 g sample 1 Caffeic acid: 0.17 ± 0.00 mg/100 g sample 1 Epigallocatechin gallate: 0.42 ± 0.09 mg/100 g sample 1 p-coumaric acid: 0.36 ± 0.01 mg/100 g sample 1 o-coumaric acid: 0.57 ± 0.04 mg/100 g sample 1 Quercetin: 0.27 ± 0.04 mg/100 g sample 1 Ferulic acid: 0.17 ± 0.01 mg/100 g sample 1 | HPLC-Mass spectrometry | [40] |
| BB3 CMU | γ-oryzanol: 219.90 ± 0.12 mg/100 g CF α-tocopherol: 10.37 ± 0.04 mg/100 g CF β-tocopherol: 0.58 ± 0.00 mg/100 g CF γ-tocopherol: 6.13 ± 0.02 mg/100 g CF δ-tocopherol: 0.18 ± 0.00 mg/100 g CF Gallic acid: 0.14 ± 0.00 mg/100 g sample 1 Caffeic acid: 0.30 ± 0.01 mg/100 g sample 1 Epigallocatechin gallate: 1.34 ± 0.06 mg/100 g sample 1 p-coumaric acid: 1.15 ± 0.07 mg/100 g sample 1 o-coumaric acid: 0.61 ± 0.17 mg/100 g sample 1 Quercetin: 0.26 ± 0.00 mg/100 g sample 1 Ferulic acid: 0.18 ± 0.00 mg/100 g sample 1 | ||
| BB4 CMU | γ-oryzanol: 220.43 ± 0.09 mg/100 g CF α-tocopherol: 15.84 ± 0.03 mg/100 g CF β-tocopherol: 1.16 ± 0.01 mg/100 g CF γ-tocopherol: 6.78 ± 0.04 mg/100 g CF δ-tocopherol: 0.21 ± 0.01 mg/100 g CF Gallic acid: 0.15 ± 0.00 mg/100 g sample 1 Caffeic acid: 0.31 ± 0.00 mg/100 g sample 1 Epigallocatechin gallate: 0.96 ± 0.04 mg/100 g sample 1 p-coumaric acid: 0.87 ± 0.01 mg/100 g sample 1 o-coumaric acid: 0.57 ± 0.01 mg/100 g sample 1 Quercetin: 0.24 ± 0.01 mg/100 g sample 1 Ferulic acid: 0.17 ± 0.01 mg/100 g sample 1 | ||
| RD6 | γ-oryzanol: 207.79 ± 0.03 mg/100 g CF α-tocopherol: 9.22 ± 0.06 mg/100 g CF β-tocopherol: 0.27 ± 0.01 mg/100 g CF γ-tocopherol: 9.19 ± 0.04 mg/100 g CF δ-tocopherol: 0.13 ± 0.00 mg/100 g CF Gallic acid: 0.13 ± 0.00 mg/100 g sample 1 Caffeic acid: 0.19 ± 0.00 mg/100 g sample 1 Epigallocatechin gallate: 1.21 ± 0.02 mg/100 g sample 1 p-coumaric acid: 0.41 ± 0.02 mg/100 g sample 1 o-coumaric acid: 0.51 ± 0.00 mg/100 g sample 1 Quercetin: 0.17 ± 0.00 mg/100 g sample 1 Ferulic acid: 0.21 ± 0.01 mg/100 g sample 1 | ||
| KC CMU107 | γ-oryzanol: 218.76 ± 0.13 mg/100 g CF α-tocopherol: 4.80 ± 0.02 mg/100 g CF β-tocopherol: 0.62 ± 0.00 mg/100 g CF γ-tocopherol: 6.28 ± 0.01 mg/100 g CF δ-tocopherol: 0.11 ± 0.00 mg/100 g CF CY 3-GLU: 40.61 ± 0.39 mg/100 g sample 1 PN 3-GLU: 15.72 ± 0.13 mg/100 g sample 1 Caffeic acid: 0.12 ± 0.00 mg/100 g sample 1 Epigallocatechin gallate: 0.32 ± 0.00 mg/100 g sample 1 p-coumaric acid: 0.22 ± 0.01 mg/100 g sample 1 o-coumaric acid: 0.15 ± 0.00 mg/100 g sample 1 Quercetin: 1.22 ± 0.01 mg/100 g sample 1 Ferulic acid: 0.33 ± 0.00 mg/100 g sample 1 | ||
| BKU5 CMU | γ-oryzanol: 145.16 ± 0.06 mg/100 g CF α-tocopherol: 15.20 ± 0.01 mg/100 g CF β-tocopherol: 0.77 ± 0.00 mg/100 g CF γ-tocopherol: 4.46 ± 0.02 mg/100 g CF δ-tocopherol: 0.18 ± 0.00 mg/100 g CF CY 3-GLU: 331.10 ± 1.64 mg/100 g sample 1 PN 3-GLU: 34.26 ± 0.23 mg/100 g sample 1 Caffeic acid: 0.40 ± 0.02 mg/100 g sample 1 Epigallocatechin gallate: 0.47 ± 0.01 mg/100 g sample 1 p-coumaric acid: 0.46 ± 0.01 mg/100 g sample 1 o-coumaric acid: 0.05 ± 0.03 mg/100 g sample 1 Quercetin: 1.15 ± 0.01 mg/100 g sample 1 Ferulic acid: 0.28 ± 0.00 mg/100 g sample 1 | ||
| K4 CMU | γ-oryzanol: 228.96 ± 0.02 mg/100 g CF α-tocopherol: 6.39 ± 2.77 mg/100 g CF β-tocopherol: 0.47 ± 0.95 mg/100 g CF γ-tocopherol: 8.51 ± 2.92 mg/100 g CF δ-tocopherol: 0.36 ± 0.25 mg/100 g CF Epigallocatechin gallate: 0.68 ± 0.02 mg/100 g sample 1 p-coumaric acid: 0.44 ± 0.01 mg/100 g sample 1 o-coumaric acid: 0.25 ± 0.02 mg/100 g sample 1 Quercetin: 1.26 ± 0.02 mg/100 g sample 1 Ferulic acid: 0.20 ± 0.01 mg/100 g s sample 1 | ||
| KDK | γ-oryzanol: 222.34 ± 0.25 mg/100 g CF α-tocopherol: 5.65 ± 0.04 mg/100 g CF β-tocopherol: 0.97 ± 0.00 mg/100 g CF γ-tocopherol: 6.81 ± 0.02 mg/100 g CF δ-tocopherol: 0.22 ± 0.00 mg/100 g CF CY 3-GLU: 41.86 ± 0.12 mg/100 g sample 1 PN 3-GLU: 23.75 ± 0.68 mg/100 g sample 1 Caffeic acid: 0.09 ± 0.00 mg/100 g sample 1 Epigallocatechin gallate: 0.28 ± 0.01 mg/100 g sample 1 p-coumaric acid: 0.15 ± 0.00 mg/100 g sample 1 o-coumaric acid: 0.18 ± 0.04 mg/100 g sample 1 Quercetin: 0.81 ± 0.00 mg/100 g sample 1 Ferulic acid: 0.13 ± 0.00 mg/100 g sample 1 | ||
| KAK1 CMU | γ-oryzanol: 175.74 ± 0.25 mg/100 g CF α-tocopherol: 17.56 ± 0.00 mg/100 g CF β-tocopherol: 0.87 ± 0.01 mg/100 g CF γ-tocopherol: 4.44 ± 0.02 mg/100 g CF δ-tocopherol: 0.17 ± 0.00 mg/100 g CF CY 3-GLU: 525.72 ± 1.72 mg/100 g sample 1 PN 3-GLU: 46.01 ± 0.51 mg/100 g sample 1 Caffeic acid: 0.15 ± 0.00 mg/100 g sample 1 Epigallocatechin gallate: 0.50 ± 0.00 mg/100 sample 1 p-coumaric acid: 0.33 ± 0.01 mg/100 g sample 1 o-coumaric acid: 0.33 ± 0.02 mg/100 g sample 1 Quercetin: 1.21 ± 0.01 mg/100 g sample 1 Ferulic acid: 0.23 ± 0.01 mg/100 g sample 1 | ||
| Sang5 CMU | γ-oryzanol: 111.36 ± 0.22 mg/100 g CF α-tocopherol: 4.77 ± 0.06 mg/100 g CF β-tocopherol: 0.64 ± 0.00 mg/100 g CF γ-tocopherol: 6.02 ± 0.04 mg/100 g CF δ-tocopherol: 0.18 ± 0.00 mg/100 g CF CY 3-GLU: 166.40 ± 0.57 mg/100 g sample 1 PN 3-GLU: 13.09 ± 0.01 mg/100 g sample 1 Caffeic acid: 0.16 ± 0.01 mg/100 g sample 1 Epigallocatechin gallate: 0.42 ± 0.03 mg/100 g sample 1 p-coumaric acid: 0.23 ± 0.04 mg/100 g sample 1 o-coumaric acid: 0.55 ± 0.03 mg/100 g sample 1 Quercetin: 1.27 ± 0.01 mg/100 g sample 1 Ferulic acid: 0.22 ± 0.00 mg/100 g sample 1 | ||
| PES1 CMU | γ-oryzanol: 139.58 ± 0.04 mg/100 g CF α-tocopherol: 17.02 ± 0.03 mg/100 g CF β-tocopherol: 0.72 ± 0.00 mg/100 g CF γ-tocopherol: 4.35 ± 0.03 mg/100 g CF δ-tocopherol: 0.25 ± 0.00 mg/100 g CF CY 3-GLU: 650.55 ± 1.65 mg/100 g sample 1 PN 3-GLU: 67.54 ± 0.32 mg/100 g sample 1 Caffeic acid: 0.26 ± 0.00 mg/100 g sample 1 Epigallocatechin gallate: 0.39 ± 0.00 mg/100 g sample 1 p-coumaric acid: 0.26 ± 0.00 mg/100 g sample 1 o-coumaric acid: 0.39 ± 0.01 mg/100 g sample 1 Quercetin: 1.44 ± 0.00 mg/100 g sample 1 Ferulic acid: 0.24 ± 0.01 mg/100 g sample 1 | ||
| Taibalang black waxy rice | Outer RB: Total ACN: 6.29 ± 0.08 mg CY 3-GLU Eq/g DM CY 3-GLU: 2.44 ± 0.27 mg/g DM PN 3-GLU: 0.53 ± 0.04 mg/g DM CY 3-RUT: 0.46 ± 0.04 mg/g DM Vitamin E total: 85.49 ± 3.24 µg/g DM γ-oryzanol: 3.95 ± 0.32 mg/g DM Inner RB: Total ACN: 3.46 ± 0.11 mg CY 3-GLU Eq/g DM CY 3-GLU: 1.43 ± 0.19 mg/g DM PN 3-GLU: 0.34 ± 0.05 mg/g DM CY 3-RUT: 0.25 ± 0.04 mg/g DM | 80% ethanol extraction and HPLC analysis | [63] |
| Black rice western Taiwan | Outer RB: Total ACN: 6.70 ± 0.06 mg CY 3-GLU Eq/g DM CY 3-GLU: 3.07 ± 0.14 mg/g DM PN 3-GLU: 1.32 ± 0.03 mg/g DM CY 3-RUT: 0.42 ± 0.05 mg/g DM Vitamin E total: 129.97 ± 1.23 µg/g DM γ-oryzanol: 4.85 ± 0.11 mg/g DM Inner RB: Total ACN: 4.92 ± 0.30 mg CY 3-GLU Eq/g DM CY 3-GLU: 2.06 ± 0.18 mg/g DM PN 3-GLU: 0.89 ± 0.07 mg/g DM CY 3-RUT: 0.28 ± 0.05 mg/g DM | ||
| Black rice Thailand | Outer RB: Total ACN: 11.27 ± 0.38 mg CY 3-GLU Eq/g DM CY 3-GLU: 10.63 ± 0.66 mg/g DM PN 3-GLU: 0.81 ± 0.06 mg/g DM CY 3-RUT: 0.52 ± 0.12 mg/g DM Vitamin E total: 137.28 ± 9.75 µg/g DM γ-oryzanol: 7.72 ± 0.39 mg/g DM Inner RB: Total ACN: 6.85 ± 0.36 mg CY 3-GLU Eq/g DM CY 3-GLU: 6.62 ± 0.39 mg/g DM PN 3-GLU: 0.40 ± 0.05 mg/g DM CY 3-RUT: 0.36 ± 0.11 mg/g DM | ||
| Taibalang red waxy rice | Outer RB: Pro-ACN: 19.13 ± 0.41 mg CE/g DM Total ACN: 0.31 ± 0.01 mg CY 3-GLU Eq/g DM CY 3-GLU: 0.05 ± 0.01 mg/g DM Vitamin E total: 99.68 ± 9.14 µg/g DM γ-oryzanol: 3.62 ± 0.16 mg/g DM Inner RB: Pro-ACN: 3.41 ± 0.08 mg CE/g DM Total ACN: 0.20 ± 0.01 mg CY 3-GLU Eq/g DM CY 3-GLU: 0.03 ± 0.01 mg/g DM | ||
| Guangfu red rice | Outer RB: Pro-ACN: 17.41 ± 0.63 mg CE/g DM Total ACN: 0.38 ± 0.03 mg CY 3-GLU Eq/g DM CY 3-GLU: 0.20 ± 0.04 mg/g DM Vitamin E total: 166.93 ± 3.65 µg/g DM γ-oryzanol: 3.59 ± 0.23 mg/g DM Inner RB: Pro-ACN: 4.31 ± 0.77 mg CE/g DM Total ACN: 0.22 ± 0.01 mg CY 3-GLU Eq/g DM CY 3-GLU: 0.07 ± 0.01 mg/g DM | ||
| Red rice Thailand | Outer RB: Pro-ACN: 12.16 ± 0.43 mg CE/g DM Total ACN: 0.35 ± 0.02 mg CY 3-GLU Eq/g DM CY 3-GLU: 0.15 ± 0.04 mg/g DM PN 3-GLU: 0.03 ± 0.00 mg/g DM Vitamin E total: 50.65 ± 5.07 µg/g DM γ-oryzanol: 3.69 ± 1.07 mg/g DM Inner RB: Pro-ACN: 0.75 ± 0.40 mg CE/g DM Total ACN: 0.28 ± 0.01 mg CY 3-GLU Eq/g DM CY 3-GLU: 0.08 ± 0.02 mg/g DM PN 3-GLU: 0.01 ± 0.00 mg/g DM | ||
| RB of KDML105 | TPC: Raw: 3.52 ± 0.06 mg GAE/g DW Hot air: 3.58 ± 0.03 mg GAE/g DW FIR: 4.05 ± 0.03 mg GAE/g DW Cellulase: 3.05 ± 0.03 mg GAE/g DW TFC: Raw: 3.88 ± 0.09 mg RE/g DW Hot air: 3.08 ± 0.10 mg RE/g DW FIR: 3.59 ± 0.16 mg RE/g DW Cellulase: 3.72 ± 0.10 mg RE/g DW γ-Oryzanol: Raw: 5.701 ± 0.022 mg/g of RFRB Hot air: 5.281 ± 0.018 mg/g of RFRB FIR: 5.612 ± 0.006 mg/g of RFRB Cellulase: 5.698 ± 0.012 mg/g of RFRB α-Tocopherol: Raw: 82.15 ± 2.84 µg/g of RFRB Hot air: 63.50 ± 2.56 µg/g of RFRB FIR: 95.78 ± 3.81 µg/g of RFRB Cellulase: 83.42 ± 5.26 µg/g of RFRB γ-Tocopherol: Raw: 5.04 ± 0.02 µg/g of RFRB Hot air: 5.03 ± 0.07 µg/g of RFRB FIR: 5.14 ± 0.09 µg/g of RFRB Cellulase: 5.04 ± 0.03 µg/g of RFRB δ-Tocopherol FIR: 7.84 ± 0.12 µg/g of RFRB | Hot air, far-infrared radiation, cellulase treatment. | [75] |
| IR64 | Oryzanol: Control: 267.3 ± 0.75 mg/100 g of RB Cellulase: 276.8 ± 0.49 mg/100 g of RB Xylanase: 286.3 ± 1.34 mg/100 g of RB Cellulase + Xylanase: 299.2 ± 1.14 mg/100 g of RB Flavonoid: Control: 32.2 ± 0.61 mg/100 g of RB Cellulase: 40.5 ± 0.50 mg CE/100 g of RB Xylanase: 38.1 ± 0.61 mg CE/100 g of RB Cellulase + Xylanase: 44.4 ± 0.61 mg CE/100 g of RB Soluble Polyphenol: Control: 278 ± 14 mg FA/100 g of RB Cellulase: 314 ± 6 mg FA/100 g of RB Xylanase: 304 ± 4 mg FA/100 g of RB Cellulase + Xylanase: 324 ± 3 mg FA/100 g Bound Polyphenol: Control: 255 ± 17 mg FA/100 g of RB Cellulase: 272 ± 4 mg FA/100 g of RB Xylanase: 305 ± 4 mg FA/100 g of RB Cellulase + Xylanase: 285 ± 2 mg FA/100 g of RB | For oryzanol extraction: Petroleum ether For soluble and bound polyphenol extraction: Methanol in 1% HCL. For flavonoid extraction: Petroleum ether and 1% HCl methanol | [76] |
| Jyothi | Oryzanol: Control: 159.24 ± 1.13 mg/100 g of RB Cellulase: 164.04 ± 0.49 mg/100 g of RB Xylanase: 167.01 ± 0.95 mg/100 g of RB Cellulase + Xylanase: 174.45 ± 1.31 mg/100 g of RB Flavonoid: Control: 113.7 ± 0.60 mg/100 g of RB Cellulase: 119.3 ± 0.60 mg CE/100 g of RB Xylanase: 116.8 ± 0.61 mg CE/100 g of RB Cellulase + Xylanase: 127.7 ± 0.61 mg CE/100 g of RB Soluble Polyphenol: Control: 502.5 ± 13 mg FA/100 g of RB Cellulase: 741.5 ± 11 mg FA/100 g of RB Xylanase: 752.5 ± 22 mg FA/100 g of RB Cellulase + Xylanase: 794.9 ± 05 mg FA/100 g of RB Bound Polyphenol: Control: 1451 ± 19 mg FA/100 g of RB Cellulase: 1534 ± 16 mg FA/100 g of RB Xylanase: 1588 ± 15 mg FA/100 g of RB Cellulase + Xylanase: 1567 ± 15 mg FA/100 g of RB | ||
| DML105 | γ-Oryzanol: Microwave 60 °C: 8.94 mg/g of DRB; 90 °C: 9.08 mg/g of DRB; 110 °C: 8.82 mg/g of DRB Hot air 70 °C: 9.26 mg/g of DRB; 100 °C: 8.93 mg/g of DRB; 180 °C: 8.82 mg/g of DRB. Roasting 60 °C: 8.81 mg/g of DRB; 80 °C: 9.10 mg/g of DRB Parboiling 70 °C: 9.76 mg/g of DRB. Autoclave 121 °C: 8.86 mg/g of DRB. Enzyme 50 °C: 8.31 mg/g of DRB. | Maceration method with pre-treatment processes such as microwave heating, hot air heating, roasting, parboiling, autoclave heating, and enzyme. | [77] |
| RB of Khao Bahn Nah and Thai jasmine | Tocopherol: Khao Bahn Nah blank: 3.69 ± 0.29 mg/L of RBE Khao Bahn Nah with SSF by L. casei: 8.75 ± 1.11 mg/L of RBE Khao Bahn Nah with SSF by L. plantarum: 4.09 ± 0.17 mg/L of RBE Thai jasmine blank: 3.35 ± 0.97 mg/L of RBE Thai jasmine with SSF by L. casei: 4.51 ± 0.38 mg/L of RBE Thai jasmine with SSF by L. plantarum: 7.10 ± 0.23 mg/L of RBE γ-Oryzanol: Khao Bahn Nah blank: 1.55 ± 0.74 mg/L of RBE Khao Bahn Nah with SSF by L. casei: 2.57 ± 0.56 mg/L of RBE Khao Bahn Nah with SSF by L. plantarum: 1.44 ± 0.36 mg/L of RBE Thai jasmine blank: 1.69 ± 0.35 mg/L of RBE Thai jasmine with SSF by L. casei: 3.16 ± 0.15 mg/L of RBE Thai jasmine with SSF by L. plantarum: 2.31 ± 0.65 mg/L of RBE Coumaric acid: Thai jasmine with SSF by L. casei: 14.47 ± 1.20 mg/L of RBE Ferulic acid: Khao Bahn Nah blank: 18.91 ± 0.60 mg/L of RBE Khao Bahn Nah with SSF by L. casei: 30.93 ± 0.81 mg/L of RBE Khao Bahn Nah with SSF by L. plantarum: 19.39 ± 0.56 mg/L of RBE Thai jasmine blank: 18.86 ± 1.05 mg/L of RBE Thai jasmine with SSF by L. casei: 35.23 ± 0.82 mg/L of RBE Thai jasmine with SSF by L. plantarum: 21.61 ± 0.66 mg/L of RBE | Solid state fermentation by Lactobacillus casei TISTR 1463 and Lactobacillus plantarum TISTR 1465. HPLC analysis of the phenolic compounds. | [78] |
| RB | γ-Oryzanol: Unfermented: 954.47 ± 21.23 µg/mL of RBE Fermented with P. acidilactici: 1148.38 ± 48.20 µg/mL of RBE Fermented with L. lactis: 522.26 ± 59.11 µg/mL of RBE Fermented with P. pentoseous: 761.82 ± 22.10 µg/mL of RBE α-Tocopherol: Unfermented: 92.25 ± 10.06 µg/mL of RBE Fermented with P. acidilactici: 182.37 ± 20.02 µg/mL of RBE Fermented with L. lactis: 138.37 ± 15.89 µg/mL of RBE Fermented with P. pentoseous: 135.60 ± 12.45 µg/mL of RBE Ferulic acid: Unfermented: 6.19 ± 0.75 µg/mL of RBE Fermented with P. acidilactici: 8.56 ± 0.99 µg/mL of RBE Fermented with P. pentoseous: 6.96 ± 0.76 µg/mL of RBE Coumaric acid: Unfermented: 10.77 ± 0.52 µg/mL of RBE Fermented with P. acidilactici: 3.51 ± 0.14 µg/mL of RBE Fermented with L. lactis: 7.29 ± 0.21 µg/mL of RBE Fermented with P. pentoseous: 3.79 ± 0.24 µg/mL of RBE TPC: Unfermented: 212.5 ± 0.7 µg GAE/mL of RBE Fermented with P. acidilactici: 246 ± 8.5 µg GAE/mL of RBE Fermented with L. lactis: 214 ± 16.3 µg GAE/mL of RBE Fermented with P. pentoseous: 230 ± 15.6 µg GAE/mL of RBE | Rice bran fermented with Pediococcus acidilactici, Lactococcus lactis, and Pediococcus pentoseous at 30 °C for 48 h. | [79] |
| Kum Akha 1′s RB extracts | TPC: 341.31 ± 6.88 mg GAE/g of RBE TFC: 155.21 ± 3.53 mg CAE/g of RBE TA: 132.43 ± 1.69 mg/g of RBE CY 3-GLU: 106.76 ± 2.94 mg/g of RBE PN 3-GLU: 14.48 ± 0.40 mg/g of RBE | Ethanol extraction/ Total flavonoid assay/Total phenolic assay/Total anthocyanin assay and quantification by HPLC | [80] |
| Sang 5 | TPC: 280.56 ± 4.49 mg GAE/g of RBE TFC: 132.39 ± 5.79 mg CAE/g of RBE TA: 126.19 ± 3.36 mg/g of RBE CY 3-GLU: 80.47 ± 5.49 mg/g of RBE PN 3-GLU: 11.42 ± 0.69 mg/g of RBE | ||
| Pieisu 1 | TPC: 306.25 ± 2.05 mg GAE/g of RBE TFC: 138.43 ± 3.62 mg CAE/g of RBE TA: 124.61 ± 1.43 mg/g of RBE CY 3-GLU: 100.68 ± 2.17 mg/g of RBE PN 3-GLU: 13.07 ± 0.80 mg/g of RBE | ||
| Kum Doi Saket | TPC: 290.12 ± 1.23 mg GAE/g of RBE TFC: 119.39 ± 3.53 mg CAE/g of RBE TA: 98.63 ± 9.86 mg/g of RBE CY 3-GLU: 40.53 ± 3.12 mg/g of RBE PN 3-GLU: 32.37 ± 1.10 mg/g of RBE | ||
| Kum Chao Morchor 107 | TPC: 166.19 ± 3.75 mg GAE/g of RBE TFC: 79.76 ± 2.95 mg CAE/g of RBE TA: 22.32 ± 3.82 mg/g of RBE CY 3-GLU: 4.61 ± 0.10 mg/g of RBE PN 3-GLU: 6.40 ± 0.22 mg/g of RBE | ||
| Bien Koo 5 | TPC: 306.34 ± 11.15 mg GAE/g of RBE TFC: 133.35 ± 6.94 mg CAE/g of RBE TA: 114.19 ± 2.04 mg/g of RBE CY 3-GLU: 89.68 ± 6.60 mg/g of RBE PN 3-GLU: 13.21 ± 0.72 mg/g of RBE | ||
| K2 | TPC: 157.12 ± 5.67 mg GAE/g of RBE TFC: 75.76 ± 1.72 mg CAE/g of RBE TA: 35.49 ± 5.09 mg/g of RBE CY 3-GLU: 6.84 ± 0.07 mg/g of RBE PN 3-GLU: 6.67 ± 0.25 mg/g of RBE | ||
| K4 | TPC: 174.42 ± 1.64 mg GAE/g of RBE TFC: 77.33 ± 7.18 mg CAE/g of RBE TA: 50.57 ± 3.55 mg/g of RBE CY 3-GLU: 8.40 ± 0.19 mg/g of RBE PN 3-GLU: 7.83 ± 0.15 mg/g of RBE |
| Model | Intervention | Dose and Duration | Results | Ref. |
|---|---|---|---|---|
| Male Wistar rats | DAG-enriched RB oil (20 and 40%) | 10% in the diet for 12 weeks | ↓ Serum TG, TC, and BF ↑ Fecal cholesterol excretion ↓ C-RP, TNF-α, platelet aggregation ↓ Expression of iNOS, COX-2, and VCAM-1 | [86] |
| Obese Zucker rats | RB enzymatic extract (RBEE) | 1% or 5% RBEE in the diet for 20 weeks | ↓ TNF-α, IL-6, IL-1β, iNOS in visceral abdominal adipose tissue. ↑ IL-6 and iNOS in visceral epididymal adipose tissue ↓ Adipocyte size | [87] |
| Obese Zucker rats | RBEE | 1% or 5% RBEE in the diet for 20 weeks | ↓ Vascular hyperreactivity ↑eNOS ↓ Vascular inflammation (iNOS, TNF-α) ↓ Superoxide, and NADPH oxidase subunits | [88] |
| C57BL/6J mice | RBEE | 1% or 5% RBEE in the diet for 20 weeks | ↓ Insulin resistance Improved the TG, TC, glucose, insulin, adiponectin, and nitrates levels ↓ Adipocyte size ↓ IL-6, and IL-1β in WAT Improved the PPARγ, TNF-α, and Emr1 levels in WAT | [90] |
| Male albino rats | Diacylglycerol-rich rice bran oil | 28 days | ↓ TC, non-HDL-C in plasma ↓ TL, TC, TG, and phospholipids in the mesentery ↓ TC, TG, and phospholipids in the liver ↓ TG in erythrocyte membrane ↑ Phospholipids in erythrocyte membrane ↓ HMG-CoA: Mevalonate ratio in liver | [91] |
| C57BL/6J mice | Triterpene alcohol and sterol from rice bran | 0.5, 2.5, 5, and 12.5 μg of cycloartenol/g BW, 23 weeks | ↓ Secretion of diet-induced GIP ↓ Weight gain ↑ Fatty acid oxidation-associated gene expression, fatty acid utilization ↓ Fatty acid synthesis-associated gene expression | [92] |
| Male Sprague–Dawley rats | γ-Oryzanol (OZ) and ferulic acid (FA) | 0.05% FA or 0.16% OZ for 13 weeks | Improved obesity, insulin resistance, and lipid profile ↓ TG, C-RP, IL-6 ↑ Adiponectin | [93] |
| C57BL/6J mice | Rice bran unsaponifiable matter (USM) | 10 or 20, or 50 mg/kg BW/day for 6 weeks. | ↓ Weight gain, food efficiency ratio, epididymal fat tissue size ↓ TG, TC, LDL-C, cardiac risk factor, and atherogenic index | [94] |
| Male Sprague–Dawley rats | Rice bran water extract (RBWE) | 2205 mg/kg/day for 4 weeks. | ↓ Body weight, visceral fat tissue weights, BGL, TC, and malondialdehyde levels ↑ Expression of eNOS ↓ Expression of NF-kB p65 and CD36 | [95] |
| Male Sprague–Dawley rats | RBWE | 2.205 or 4.410 g/kg/day for 4 weeks | ↓ Expression of SREBP-1c ↑ Expression of IRS-2, GLUT-2, and GK in the pancreas ↓ Fat droplets in acinar cells ↓ HFD-induced obesity and hyperglycemia Improved glucose tolerance and TG level | [96] |
| Mice | Red rice bran extract (RRBE) | 0.5 or 1 g/kg of RRBE for 6 weeks | ↓ Adipocyte hypertrophy, lipid accumulation, and inflammation ↓ Expression of CCAAT/enhancer binding protein-alpha, sterol regulatory element-binding protein-1c, hormone-sensitive lipase, macrophage marker F4/80, NF-kB p65, monocyte chemoattractant protein-1, TNF-α, and iNOS | [97] |
| Male ICR mice | Red rice bran ethanolic extract (RRBEE) | 0.5 or 1 g/kg BW for 12 weeks | ↑ Expression of IRS and GLUT in the adipose tissue ↑ Expression of GLUT in the muscles ↓ Serum insulin level ↓ Expression of IDE in muscles ↓ Expression of pancreatic insulin and pancreatic islet size. | [98] |
| Mice | Rice bran (RB) or fermented rice bran (FRB) | High-fat diet with 5% of FRB or RB for 10 weeks | ↓ Body weight, TG, TC, Non-HDL-C, fat cell ↑ HDL-C, adiponectin level ↓ C/EBPα, SREBP-1c, FAS, ACC | [99] |
| C57BL/6 male mice | Rice bran oil (RBO) | 170 g of RBO/Kg of food (no changes in food consumption between groups); 10 weeks | ↓ Epididymal white adipose tissue (EWAT) weights ↓ Expression of SREBP-1c and PPAR-γ in EWAT ↓ Expression of M2-macrophage markers (iNOS, COX-2, and f4/80) in EWAT ↑ Expression of arg1 and ym1 in EWAT Altered the expression of surface M2 makers (CD206 and CD11c) ↓ Expression of pro-inflammatory cytokines (IL-6 and TNF-α) ↑ Expression of anti-inflammatory cytokine (IL-10) | [100] |
| Male Sprague–Dawley rats | Rice bran | 2 or 4 or 8% in food for 8 weeks | ↓ Body weight and adipocyte size ↓ TG and TC levels in liver ↓ Glucose and uric acid in serum ↑ Phosphatidylcholine, cholesteryl ester, glycerol-1-2-hexadecanoate 3-octadecanoate levels in the liver | [101] |
| Male Sprague–Dawley rats | IR-64 rice bran extract | 100 or 150, or 200 mg/kg BW of RBE for 6 weeks | ↓ Body weight, TG, and MDA | [102] |
| Male ICR mice | RBWE | 220 or 1100 mg/kg BW/day for 8 weeks | ↓ Diastolic blood pressure ↓ Serum and liver TNF-α and MDA levels ↓ NF-κB levels in the liver and heart ↓ Lipid accumulation in the liver ↓ Myocardial COX-2, and MMP-9 ↓ Adipose tissue mass ↓ VEGF and MMP-2 expressions in visceral fat tissue | [103,104] |
| Male Wistar rats | Anthocyanin-rich black rice bran extract | 100 or 200 mg/kg BW/day for 8 weeks | ↓ Body weight and visceral fat weight ↓ Plasma glucose, TC, and TG levels ↓ Serum creatinine and renal cortical MDA levels Attenuates kidney injury | [105] |
| Male Wistar rats | Rice bran | 11% rice bran in the diet for 20 weeks | ↓ Body weight, body fat, and adiposity index ↓ IL-6, MDA and TNF-α ↑ SOD and CAT activities in the myocardium ↓ TG, Insulin, HOMA-IR Improved the structural and functional properties of the heart | [106] |
| Female C57BL/6J mice | Fermented rice bran | 0.239% of FRB in the diet for 8 weeks | ↓ Weight gain ↓ Abundances of Enterococcus and Peptostreptococcaceae ↓ Fecal succinic acid concentration ↑ Fumaric acid in the blood ↓ Xylitol, sorbitol, uracil, glutamic acid, and malic acid levels in the blood | [107] |
| Subjects | Intervention | Dose and Duration | Results | Ref. |
|---|---|---|---|---|
| Obese Japanese men | RB-ASG | 30–50 mg/day for 12 weeks | ↓ TC ↓ LDL-C ↓ Non-HDL-C ↓ LDL/HDL ratio ↓ HbA1c ↓ Abdominal circumference ↓ Subcutaneous fat area | [21] |
| Borderline hypercholesterolemic Chinese subjects | Refined olive oil (ROO), blended oil 1 (BO1) *, and blended oil 2 (BO2) ** | 30 g of ROO or BO1 or BO2 for 8 weeks | ↓ TC ↓ LDL-C ↓ TG ↓ HDL-C ↓ apoB-to-apoA1 ratio ↓ Blood pressure ↓ Serum glucose ↑ Body weight | [22] |
| Overweight and obese adults on a calorie-restricted diet | Pigmented rice bran (PRB) or PRB with plant sterols (PRB + PS) | 30 g per day for 8 weeks | ↑ Body weight loss ↓ TC ↓ LDL-C ↓ Blood pressure ↓ Serum leptin ↓ F2-isoprostane | [23] |
| Overweight and obese adults on an energy-restriction diet | Rice bran (RB) or rice husk (RH) | 70 g of RB/day or 25 of RH/day for 12 weeks | ↓ Serum hs-CRP ↓ Serum IL-6 | [24] |
| Supplements | Model | Dose and Duration | Changes in Microbiome | Ref. |
|---|---|---|---|---|
| BBBLBAFRB | High-fat-induced obese C57BL/6J mice | 0.239% fermented rice bran for 8 weeks | ↓ Unclassified family Peptostreptococcaceae and Enterococcus | [107] |
| RB or SLFRB | High sucrose and no-fiber-fed ICR mice | 20% in the diet for 2 weeks | ↑ Bacteroidetes and Firmicutes ↓ Lachnospiraceae and Enterorhabdus mucosicola ↑ α-diversity of microbiota | [108] |
| RBO | Daidzein and RBO-supplemented mice | 10% RBO for 30 days | ↑ Abundance of Lactobacillales | [109] |
| RB-HBP | High-fat-diet-fed mice | 100 mg/kg/day for 14 weeks | ↑ α-diversity of microbiota ↑ Bacteroidetes/Firmicutes ratio ↑ Bacteroides, Allobaculum, Rikenellaceae_RC9_gut_group and Faecalibaculum ↓ Alistipes, Odoribacter, Butyricimonas, Parabacteroides, unclassified_f_Lachnospiraceae, Ruminiclostridium_9, Romboutsia and norank_f_Erysipelotrichaceae | [110] |
| RRB, RRBS, IRRB, IRRBS | High-fat-diet-fed C57BL/6 mice | 300 mg/kg BW/day for 39 days | ↑ Bacteroidetes and Bacteroidetes/Firmicutes ratio ↓ Desulfovibrio ↑ Akkermansia and Lachnospiraceae | [111] |
| RB-AX | In vitro fecal fermentation | 100 mg of AX | ↑ Collinsella, Blautia and Bifidobacterium ↓ Sutterella, Bilophila and Parabacteroides | [121] |
| PRBA | BALB/c mice | 50, 100, and 200 mg/kg BW for 7 days | ↑ Lachnospiraceae, Bacteroidaceae, Ruminococcaceae ↓ Shigella | [112] |
| LFRB | STZ-induced diabetic C57BL/6J mice | 0.5 or 1.0 g/kg BW for 7 days | Improved the abundance of Dubosiella and Lactobacillus. | [113] |
| BFRB or HSRB | BALB/c mice | 10% of the diet for 15 weeks | Improved the abundance of Roseburia, Lachnospiraceae and Clostridiales | [114] |
| Rice berry bran oil exerts | Male Wistar rats | 100 mg/kg BW of -γ-oryzanol 5 days/week for 10 weeks | ↑ Firmicutes/Bacteroidetes ratio. Improved the gut microbiota | [115] |
| HSRB | Healthy adult subjects | 30 g/day in the diet for 4 weeks | Bifidobacterium and Ruminococcus | [116] |
| Rice bran | Adults with a high risk of colorectal cancer | 30 g/day in the diet for 24 weeks | ↑ The abundance of Firmicutes and Lactobacillus ↑ Firmicutes/Bacteroidetes ratio. ↑ Prevotella_9, Lactobacillales, and Bifidobacteria | [117] |
| HSRB | Malian and Nicaraguan infants | 1-to-5 g/day for 6 months | ↑ α-diversity | [118] |
| HSRB | Nicaraguan and Malian weaning infants | 1-to-5 g/day for 6 months | Improved the gut microbiota | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivamaruthi, B.S.; Alagarsamy, K.; Thangaleela, S.; Bharathi, M.; Kesika, P.; Chaiyasut, C. Composition, Microbiota, Mechanisms, and Anti-Obesity Properties of Rice Bran. Foods 2023, 12, 1300. https://doi.org/10.3390/foods12061300
Sivamaruthi BS, Alagarsamy K, Thangaleela S, Bharathi M, Kesika P, Chaiyasut C. Composition, Microbiota, Mechanisms, and Anti-Obesity Properties of Rice Bran. Foods. 2023; 12(6):1300. https://doi.org/10.3390/foods12061300
Chicago/Turabian StyleSivamaruthi, Bhagavathi Sundaram, Karthikeyan Alagarsamy, Subramanian Thangaleela, Muruganantham Bharathi, Periyanaina Kesika, and Chaiyavat Chaiyasut. 2023. "Composition, Microbiota, Mechanisms, and Anti-Obesity Properties of Rice Bran" Foods 12, no. 6: 1300. https://doi.org/10.3390/foods12061300
APA StyleSivamaruthi, B. S., Alagarsamy, K., Thangaleela, S., Bharathi, M., Kesika, P., & Chaiyasut, C. (2023). Composition, Microbiota, Mechanisms, and Anti-Obesity Properties of Rice Bran. Foods, 12(6), 1300. https://doi.org/10.3390/foods12061300












