Metagenomic and Untargeted Metabolomic Analysis of the Effect of Sporisorium reilianum Polysaccharide on Improving Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Polysaccharides from S. reilianum
2.2. Experimental Animals and Study Design
2.3. Serum Lipids and Liver Enzyme Function Analysis
2.4. Histological Analysis
2.5. DNA Extraction, Sequencing, and Analysis of Fecal Sample
2.6. Sample Preparation and LC-HRMS Analysis
2.7. Statistical Analysis
3. Results
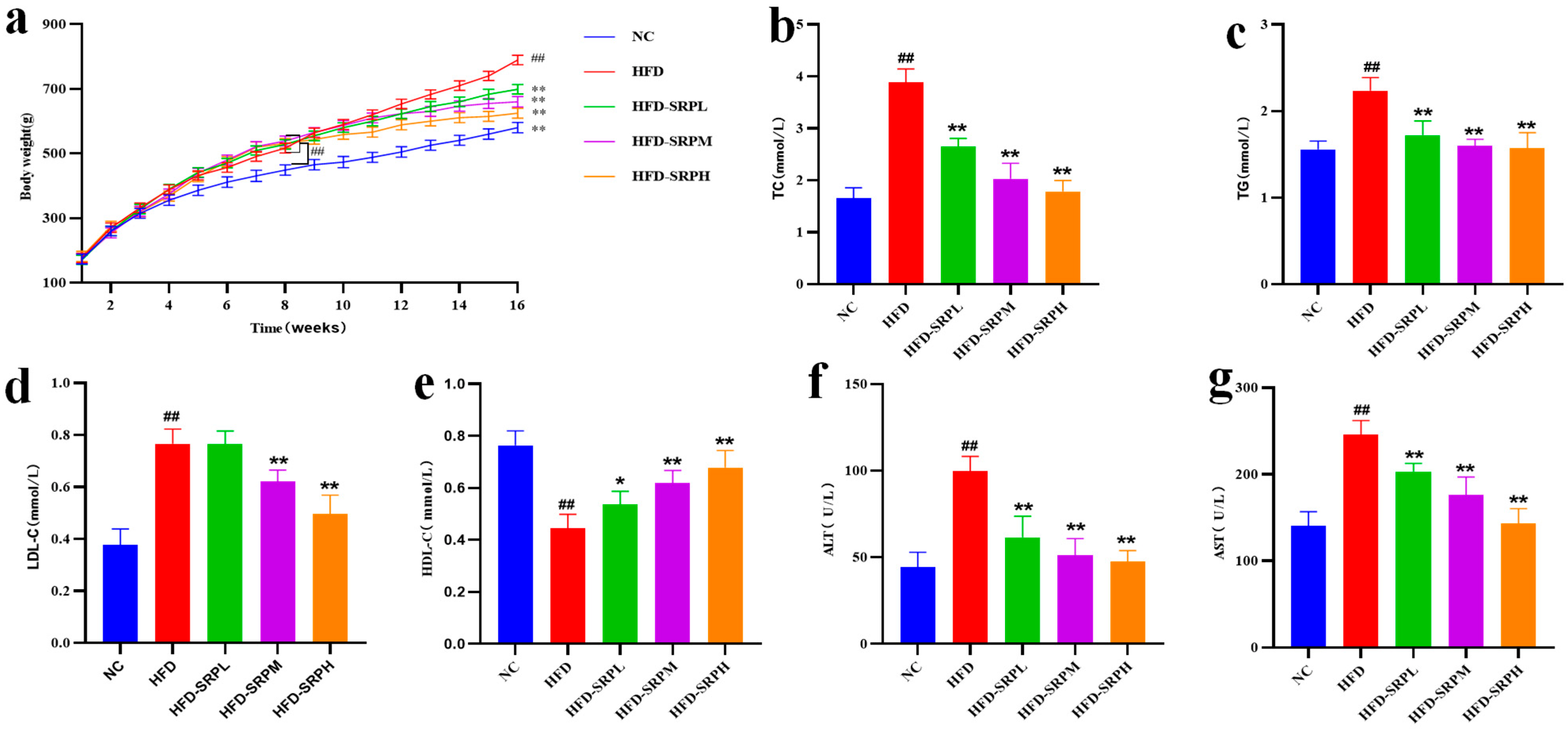
3.1. The Effect of SRP on the Weight of Obese Rats
3.2. Effect of SRP on Serum Lipid Levels and Liver Enzyme Functions
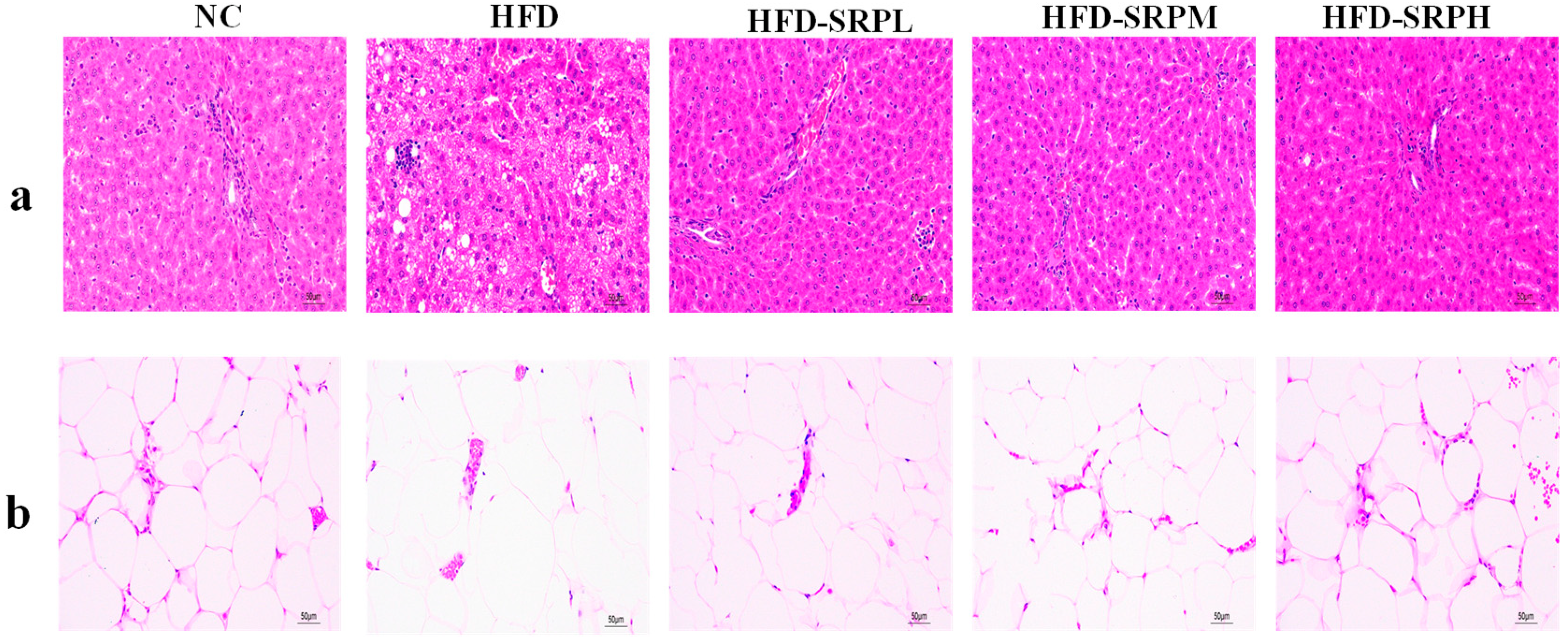
3.3. Pathological Analysis of Rat Liver and White Adipose Tissue Sections
3.4. Quality Control of Fecal Metagenomics
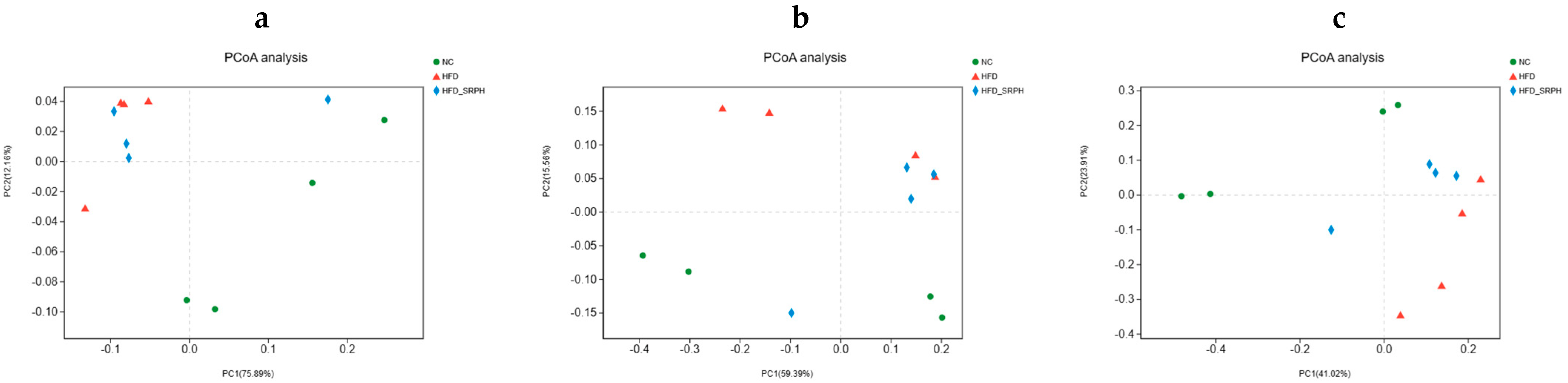
3.5. Effect of SRP on Gut Microbiota
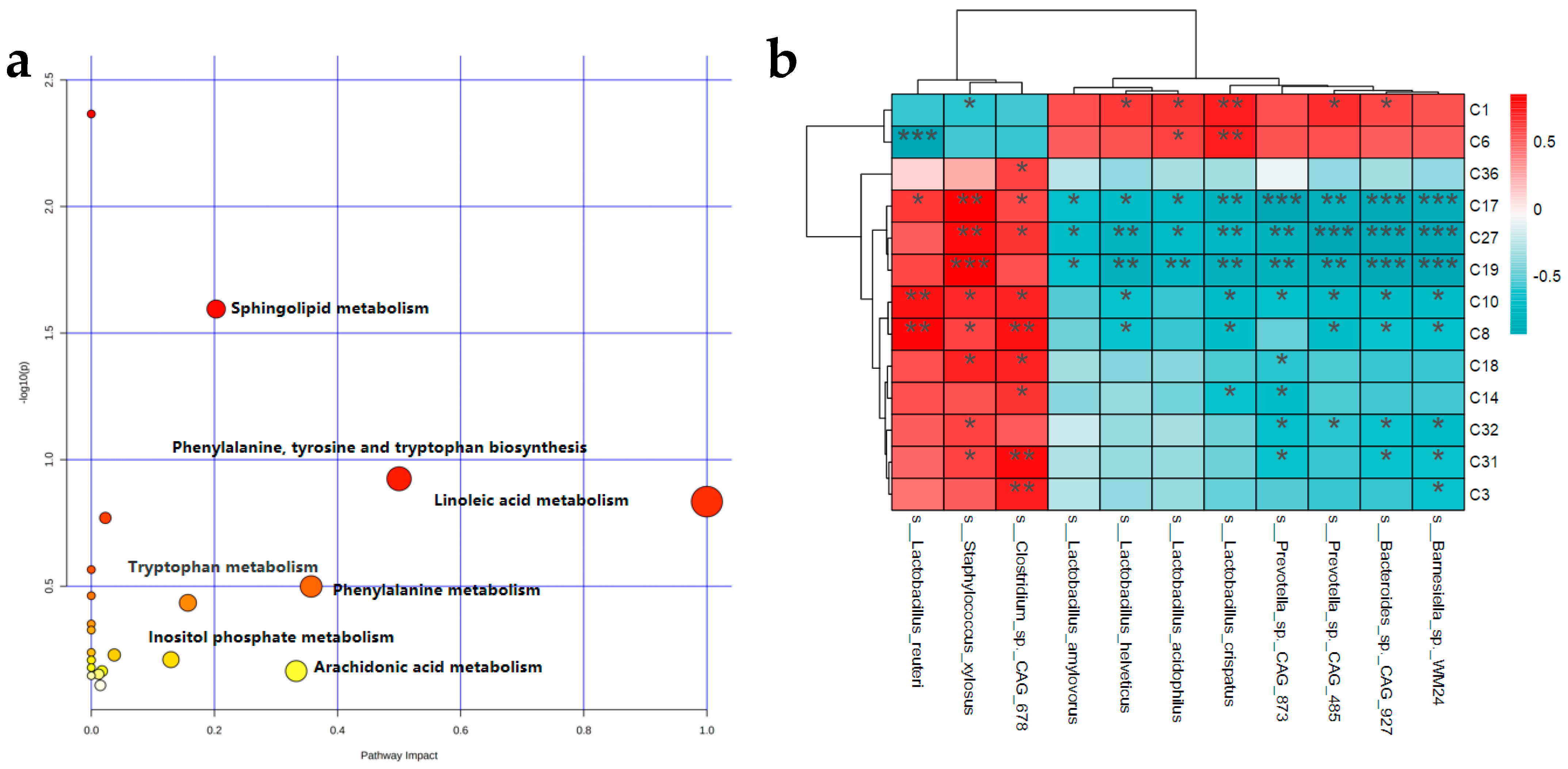
3.6. Analysis of Metabolic Profile of Untargeted Metabolomics in Feces
3.7. Correlation between Gut Microbial Species and Differential Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Paczkowska-Abdulsalam, M.; Kretowski, A. Obesity, metabolic health and omics: Current status and future directions. World J. Diabetes 2021, 12, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in children and adolescents: Epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Pathogenesis and treatment. Lancet 2008, 371, 2153–2156. [Google Scholar] [CrossRef]
- Ravussin, E.; Smith, S.R. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2002, 967, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.F.; Tuero, C.; Valenti, V.; Bilbao, I.; de la Higuera, M.; Fruhbeck, G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients 2019, 11, 2664. [Google Scholar] [CrossRef] [Green Version]
- Boden, G.; Lebed, B.; Schatz, M.; Homko, C.; Lemieux, S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 2001, 50, 1612–1617. [Google Scholar] [CrossRef] [Green Version]
- Lahey, R.; Khan, S.S. Trends in Obesity and Risk of Cardiovascular Disease. Curr. Epidemiol. Rep. 2018, 5, 243–251. [Google Scholar] [CrossRef]
- Fan, J.; Song, Y.; Chen, Y.; Hui, R.; Zhang, W. Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013, 168, 4761–4768. [Google Scholar] [CrossRef]
- Okwan-Duodu, D.; Umpierrez, G.E.; Brawley, O.W.; Diaz, R. Obesity-driven inflammation and cancer risk: Role of myeloid derived suppressor cells and alternately activated macrophages. Am. J. Cancer Res. 2013, 3, 21–33. [Google Scholar]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech Dis. 2016, 11, 421–449. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Feng, C.P.; Li, X.; Chang, M.C.; Meng, J.L.; Xu, L.J. Immunomodulatory and antioxidative activity of Cordyceps militaris polysaccharides in mice. Int. J. Biol. Macromol. 2016, 86, 594–598. [Google Scholar] [CrossRef]
- Deng, C.; Shang, J.; Fu, H.; Chen, J.; Liu, H.; Chen, J. Mechanism of the immunostimulatory activity by a polysaccharide from Dictyophora indusiata. Int. J. Biol. Macromol. 2016, 91, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, H.; Zong, X.; Li, S.; Wang, J.; Wang, Y.; Jin, M. Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities. Carbohydr. Polym. 2020, 247, 116750. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Udchumpisai, W.; Bangyeekhun, E. Purification, Structural Characterization, and Biological Activity of Polysaccharides from Lentinus velutinus. Mycobiology 2020, 48, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Yang, W.; Fang, Y.; Ma, N.; Pei, F.; Zhao, L.; Hu, Q. Antioxidant and cytotoxicites of Pleurotus eryngii residue polysaccharides obtained by ultrafiltration. LWT 2016, 73, 108–116. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, X.; Li, C.; Li, J.; Huang, Y.; Han, L.; Wei, J.A.; Huang, H. Inhibition of urinary bladder carcinogenesis by aqueous extract of sclerotia of Polyporus umbellatus fries and polyporus polysaccharide. Am. J. Chin. Med. 2011, 39, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, C.; Wang, X.; Jia, D.; Lu, W.; Sun, X.; Liu, Y.; Yuan, L. Hpyerglycemic and anti-diabetic nephritis activities of polysaccharides separated from Auricularia auricular in diet-streptozotocin-induced diabetic rats. Exp. Ther. Med. 2017, 13, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, B.; Ibrahim, S.A.; Gao, S.S.; Yang, H.; Huang, W. Purification, characterization and antioxidant activity of polysaccharides from Flammulina velutipes residue. Carbohydr. Polym. 2016, 145, 71–77. [Google Scholar] [CrossRef]
- Hu, Y.N.; Sung, T.J.; Chou, C.H.; Liu, K.L.; Hsieh, L.P.; Hsieh, C.W. Characterization and Antioxidant Activities of Yellow Strain Flammulina velutipes (Jinhua Mushroom) Polysaccharides and Their Effects on ROS Content in L929 Cell. Antioxidants 2019, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.M.; Wang, J.; Wu, Z.G.; Yang, J.M.; Li, W.; Shen, L.X. Extraction, purification and anti-proliferative activities of polysaccharides from Lentinus edodes. Int. J. Biol. Macromol. 2016, 93, 136–144. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Sarker, M.; Yan, X.; Yang, C.; Zhao, L.; Lv, X.; Liu, B.; Zhao, C. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr. Polym. 2018, 198, 452–461. [Google Scholar] [CrossRef]
- Zhu, K.X.; Nie, S.P.; Li, C.; Gong, D.; Xie, M.Y. Ganoderma atrum polysaccharide improves aortic relaxation in diabetic rats via PI3K/Akt pathway. Carbohydr. Polym. 2014, 103, 520–527. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yao, X.-P.; Liu, B.; Reheman, H.N.; Gao, Y.; Sun, Z.; Ma, Q. Auricularia auricular-judae polysaccharide attenuates lipopolysaccharide-induced acute lung injury by inhibiting oxidative stress and inflammation. Biomed. Rep. 2015, 3, 478–482. [Google Scholar]
- Yue, Y.; Yadav, S.K.; Wang, C.; Zhao, Y.; Zhang, X.; Wu, Z. Nonabsorbable polysaccharide-functionalized polyethylenimine for inhibiting lipid absorption. Carbohydr. Polym. 2018, 197, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chater, P.I.; Wilcox, M.D.; Houghton, D.; Pearson, J.P. The role of seaweed bioactives in the control of digestion: Implications for obesity treatments. Food Funct. 2015, 6, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Guo, Y.; Liu, R.; Wang, K.; Zhang, M. Black tea polyphenols and polysaccharides improve body composition, increase fecal fatty acid, and regulate fat metabolism in high-fat diet-induced obese rats. Food Funct. 2016, 7, 2469–2478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, S.; Ai, C.; Wen, C.; Liu, Z.; Wang, L.; Jiang, L.; Shen, P.; Zhang, G.; Song, S. Galactofucan from Laminaria japonica is not degraded by the human digestive system but inhibits pancreatic lipase and modifies the intestinal microbiota. Int. J. Biol. Macromol. 2021, 166, 611–620. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2017, 13, 149–160. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef]
- Tang, C.; Ding, R.; Sun, J.; Liu, J.; Kan, J.; Jin, C. The impacts of natural polysaccharides on intestinal microbiota and immune responses—A review. Food Funct. 2019, 10, 2290–2312. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Ma, L.; Ma, Y.; Zhang, F.; Zhao, C.; Nie, Y. Insights into the role of gut microbiota in obesity: Pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell. 2018, 9, 397–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, M.; Sun, X.; Ma, N.; Liu, Y.; Luo, T.; Song, S.; Ai, C. Polysaccharides from Laminaria japonica alleviated metabolic syndrome in BALB/c mice by normalizing the gut microbiota. Int. J. Biol. Macromol. 2019, 121, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Liu, M.; Zhang, P.; Fan, S.; Huang, J.; Yu, S.; Zhang, C.; Li, H. Fucoidan and galactooligosaccharides ameliorate high-fat diet-induced dyslipidemia in rats by modulating the gut microbiota and bile acid metabolism. Nutrition 2019, 65, 50–59. [Google Scholar] [CrossRef]
- Shi, L.; Wang, J.; Wang, Y.; Feng, Y. MDG-1, an Ophiopogon polysaccharide, alleviates hyperlipidemia in mice based on metabolic profile of bile acids. Carbohydr. Polym. 2016, 150, 74–81. [Google Scholar] [CrossRef]
- Kan, L.; Chai, Y.; Li, X.; Zhao, M. Structural analysis and potential anti-tumor activity of Sporisorium reilianum (Fries) polysaccharide. Int. J. Biol. Macromol. 2020, 153, 986–994. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Wang, C.; Lou, Y.; Xia, X.; Xu, H. Whole and polysaccharide powdered Sporisorium reilianum improves DSS-induced colitis in BALB/c mice by modulating gut microbiota. J. Funct. Food. 2021, 79, 104409. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, H.; Park, J.; Takagi, T. MetaGene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [Green Version]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Method. 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Cairns, R.; Fischer, A.W.; Blanco-Munoz, P.; Alvarez-Guaita, A.; Meneses-Salas, E.; Egert, A.; Buechler, C.; Hoy, A.J.; Heeren, J.; Enrich, C.; et al. Altered hepatic glucose homeostasis in AnxA6-KO mice fed a high-fat diet. PLoS ONE 2018, 13, e201310. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Yang, J.; Liu, X.; Zhang, Y.; Lei, A.; Yi, R.; Tan, F.; Zhao, X. Preventive effect of small-leaved Kuding tea (Ligustrum robustum) on high-diet-induced obesity in C57BL/6J mice. Food Sci. Nutr. 2020, 8, 4512–4522. [Google Scholar] [CrossRef]
- Kotsis, V.; Stabouli, S.; Toumanidis, S.; Papamichael, C.; Lekakis, J.; Germanidis, G.; Hatzitolios, A.; Rizos, Z.; Sion, M.; Zakopoulos, N. Target organ damage in “white coat hypertension” and “masked hypertension”. Am. J. Hypertens. 2008, 21, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.J.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Collantes, R.S.; Ong, J.P.; Younossi, Z.M. The metabolic syndrome and nonalcoholic fatty liver disease. Panminerva Med. 2006, 48, 41–48. [Google Scholar]
- Wang, Q.S.; Li, M.; Li, X.; Zhang, N.W.; Hu, H.Y.; Zhang, L.L.; Ren, J.N.; Fan, G.; Pan, S.Y. Protective effect of orange essential oil on the formation of non-alcoholic fatty liver disease caused by high-fat diet. Food Funct. 2022, 13, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Z.; Huang, J.; Luo, G.; Liang, Q.; Wang, D.; Ye, X.; Wu, C.; Wang, L.; Hu, J. Anti-obesity and hypolipidemic effects of Fuzhuan brick tea water extract in high-fat diet-induced obese rats. J. Sci. Food Agric. 2013, 93, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Romano, A.D.; Lo, B.A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Gruninger, R.J.; Ribeiro, G.O.; Cameron, A.; McAllister, T.A. Invited review: Application of meta-omics to understand the dynamic nature of the rumen microbiome and how it responds to diet in ruminants. Animal 2019, 13, 1843–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhang, N.; Kan, J.; Sun, R.; Tang, S.; Wang, Z.; Chen, M.; Liu, J.; Jin, C. Anti-inflammatory activity of alkali-soluble polysaccharides from Arctium lappa L. and its effect on gut microbiota of mice with inflammation. Int. J. Biol. Macromol. 2020, 154, 773–787. [Google Scholar] [CrossRef]
- Candela, M.; Maccaferri, S.; Turroni, S.; Carnevali, P.; Brigidi, P. Functional intestinal microbiome, new frontiers in prebiotic design. Int. J. Food Microbiol. 2010, 140, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.Y.; Korivi, M.; Tsai, C.H.; Yang, J.H.; Tsai, Y.C. Supplementation of Lactobacillus plantarum K68 and Fruit-Vegetable Ferment along with High Fat-Fructose Diet Attenuates Metabolic Syndrome in Rats with Insulin Resistance. Evid. Based Complement Altern. Med. 2013, 2013, 943020. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, N.; Yin, B.; Fang, D.; Jiang, T.; Fang, S.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J. Appl. Microbiol. 2016, 121, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Opazo, M.C.; Ortega-Rocha, E.M.; Coronado-Arrazola, I.; Bonifaz, L.C.; Boudin, H.; Neunlist, M.; Bueno, S.M.; Kalergis, A.M.; Riedel, C.A. Intestinal Microbiota Influences Non-intestinal Related Autoimmune Diseases. Front. Microbiol. 2018, 9, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr. Clin. Pract. 2015, 30, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Thuny, F.; Angelakis, E.; Casalta, J.P.; Giorgi, R.; Habib, G.; Raoult, D. Lactobacillus reuteri and Escherichia coli in the human gut microbiota may predict weight gain associated with vancomycin treatment. Nutr. Diabetes 2013, 3, e87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Jin, H.; Oh, S.Y.; Ji, G.E. Anti-obese effects of two Lactobacilli and two Bifidobacteria on ICR mice fed on a high fat diet. Biochem. Biophys. Res. Commun. 2016, 480, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Bell, R.; Klag, K.A.; Lee, S.H.; Soto, R.; Ghazaryan, A.; Buhrke, K.; Ekiz, H.A.; Ost, K.S.; Boudina, S.; et al. T cell-mediated regulation of the microbiota protects against obesity. Science 2019, 365, eaat9351. [Google Scholar] [CrossRef]
- Park, S.S.; Lee, Y.J.; Kang, H.; Yang, G.; Hong, E.J.; Lim, J.Y.; Oh, S.; Kim, E. Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARgamma signaling. Sci. Rep. 2019, 9, 20152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.; Mao, G.; Wu, D.; Yu, C.; Cheng, H.; Xiao, H.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Highly Branched RG-I Domain Enrichment Is Indispensable for Pectin Mitigating against High-Fat Diet-Induced Obesity. J. Agric. Food Chem. 2020, 68, 8688–8701. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Platten, M.; Nollen, E.; Rohrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Lee, H.S.; Nam, Y.; Chung, Y.H.; Kim, H.R.; Park, E.S.; Chung, S.J.; Kim, J.H.; Sohn, U.D.; Kim, H.C.; Oh, K.W.; et al. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 2014, 118, 7–14. [Google Scholar] [CrossRef]
- Poudyal, H.; Panchal, S.K.; Diwan, V.; Brown, L. Omega-3 fatty acids and metabolic syndrome: Effects and emerging mechanisms of action. Prog. Lipid Res. 2011, 50, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Mao, Y.; Xiang, Z.; Zhu, J.; Wang, H.; Wang, A.; Jiang, M.; Gu, Y. Traditional Chinese Medicine Formula Jian Pi Tiao Gan Yin Reduces Obesity in Mice by Modulating the Gut Microbiota and Fecal Metabolism. Evid. Based Complement Altern. Med. 2022, 2022, 9727889. [Google Scholar] [CrossRef]
- Lake, A.D.; Novak, P.; Shipkova, P.; Aranibar, N.; Robertson, D.G.; Reily, M.D.; Lehman-McKeeman, L.D.; Vaillancourt, R.R.; Cherrington, N.J. Branched chain amino acid metabolism profiles in progressive human nonalcoholic fatty liver disease. Amino Acids 2015, 47, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Y.; Liang, X.; Zou, L.; Ong, C.N.; Yuan, J.M.; Koh, W.P.; Pan, A. Serum Amino Acids in Association with Prevalent and Incident Type 2 Diabetes in A Chinese Population. Metabolites 2019, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Cui, M.; Trimigno, A.; Castro-Mejia, J.L.; Reitelseder, S.; Bulow, J.; Bechshoft, R.L.; Nielsen, D.S.; Holm, L.; Engelsen, S.B.; Khakimov, B. Human Fecal Metabolome Reflects Differences in Body Mass Index, Physical Fitness, and Blood Lipoproteins in Healthy Older Adults. Metabolites 2021, 11, 717. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Liu, M.; Liu, X.; Zheng, M.; Xu, X.; Liu, X.; Gong, J.; Liu, H.; Liu, J. Metagenomic and Untargeted Metabolomic Analysis of the Effect of Sporisorium reilianum Polysaccharide on Improving Obesity. Foods 2023, 12, 1578. https://doi.org/10.3390/foods12081578
Guo Y, Liu M, Liu X, Zheng M, Xu X, Liu X, Gong J, Liu H, Liu J. Metagenomic and Untargeted Metabolomic Analysis of the Effect of Sporisorium reilianum Polysaccharide on Improving Obesity. Foods. 2023; 12(8):1578. https://doi.org/10.3390/foods12081578
Chicago/Turabian StyleGuo, Yunlong, Meihong Liu, Xin Liu, Mingzhu Zheng, Xiuying Xu, Xiaokang Liu, Jiyu Gong, Huimin Liu, and Jingsheng Liu. 2023. "Metagenomic and Untargeted Metabolomic Analysis of the Effect of Sporisorium reilianum Polysaccharide on Improving Obesity" Foods 12, no. 8: 1578. https://doi.org/10.3390/foods12081578
APA StyleGuo, Y., Liu, M., Liu, X., Zheng, M., Xu, X., Liu, X., Gong, J., Liu, H., & Liu, J. (2023). Metagenomic and Untargeted Metabolomic Analysis of the Effect of Sporisorium reilianum Polysaccharide on Improving Obesity. Foods, 12(8), 1578. https://doi.org/10.3390/foods12081578




