The Impact of Divergent Algal Hydrocolloids Addition on the Physicochemical, Viscoelastic, Textural, and Organoleptic Properties of Cream Cheese Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials Used for the Manufacture of the Cream Cheese Samples
2.2. Manufacture of the Cream Cheese Samples
2.3. Basic Physicochemical Analysis of the Cream Cheese Samples
2.4. Rheological Analysis of the Cream Cheese Samples
2.5. Texture Profile Analysis and Spreadability Determination of the Cream Cheese Samples
2.6. Instrumental Determination of the Color and Emulsion Stability of the Cream Cheese Samples
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Basic Physicochemical Analysis of the Cream Cheese Samples
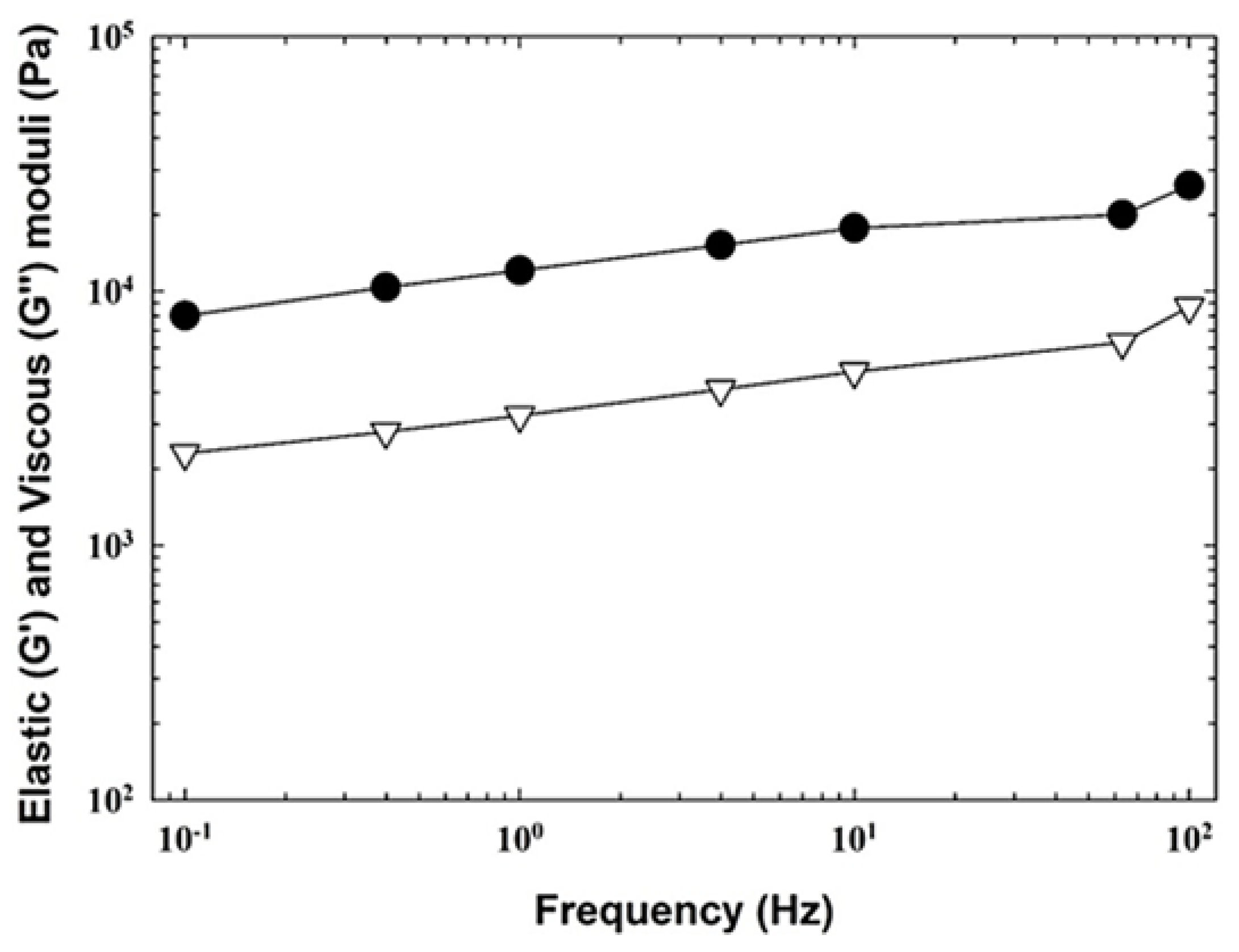
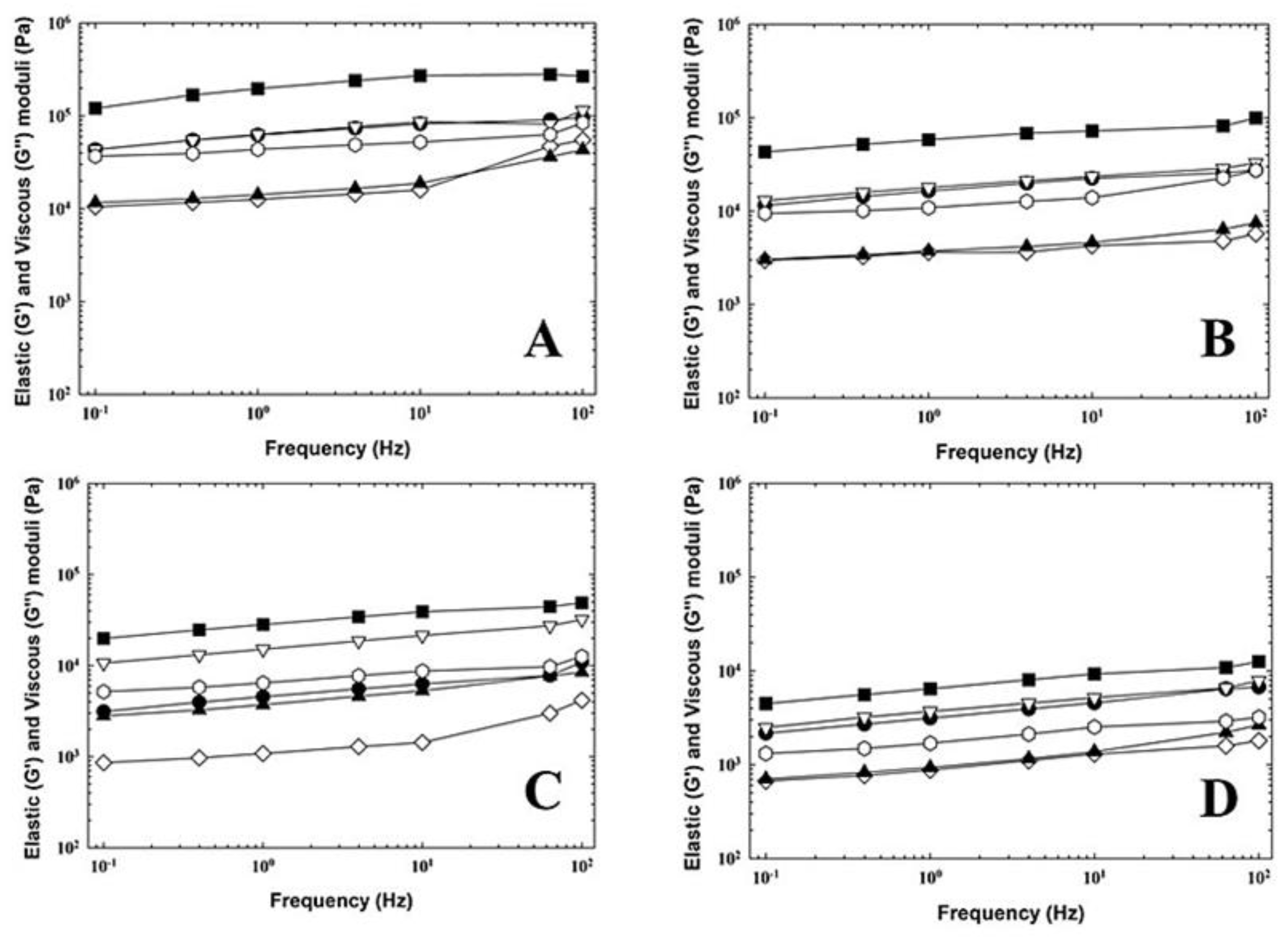
3.2. Rheological Analysis of the Cream Cheese Samples
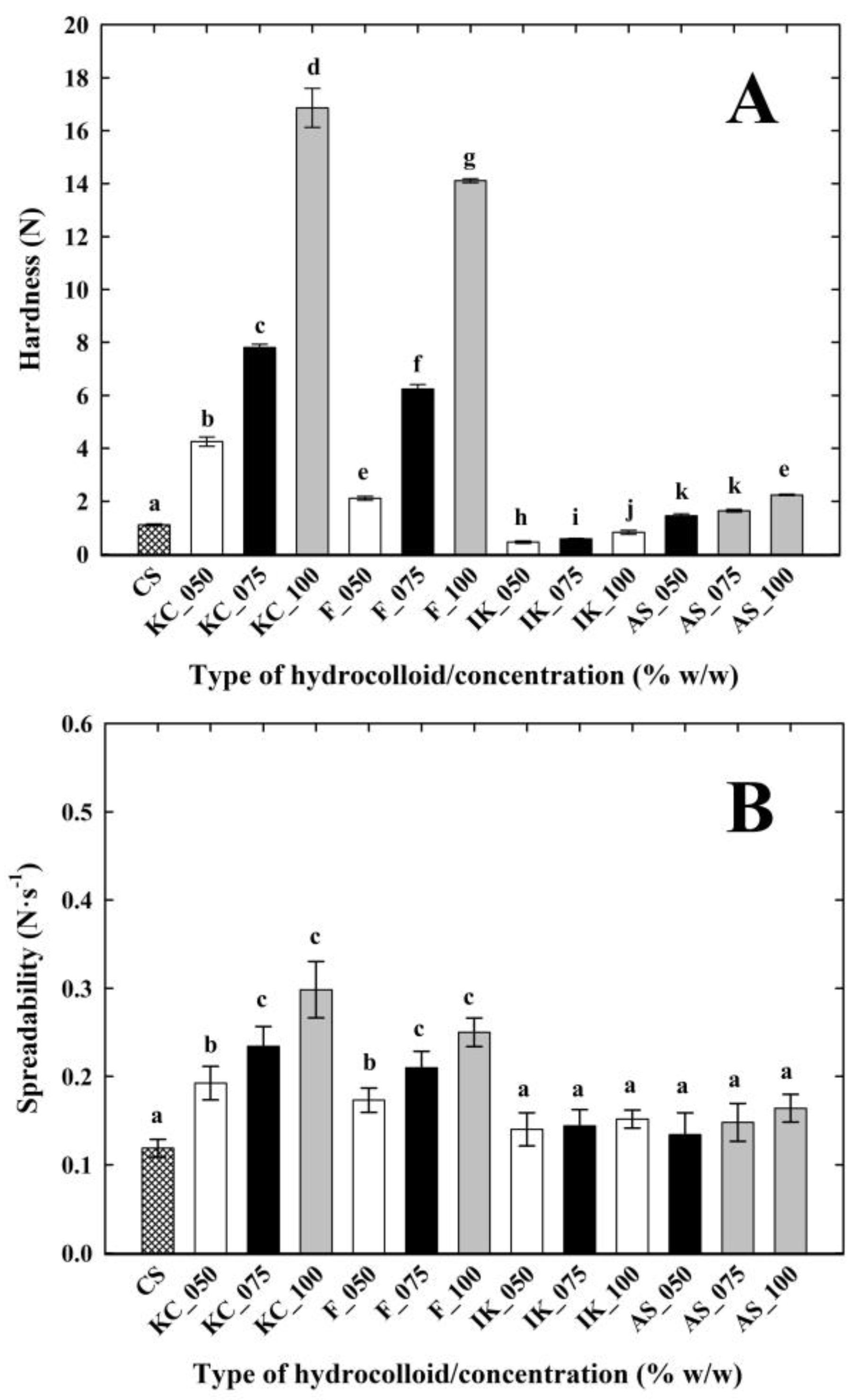
3.3. Texture Profile Analysis and Spreadability of the Cream Cheese Samples
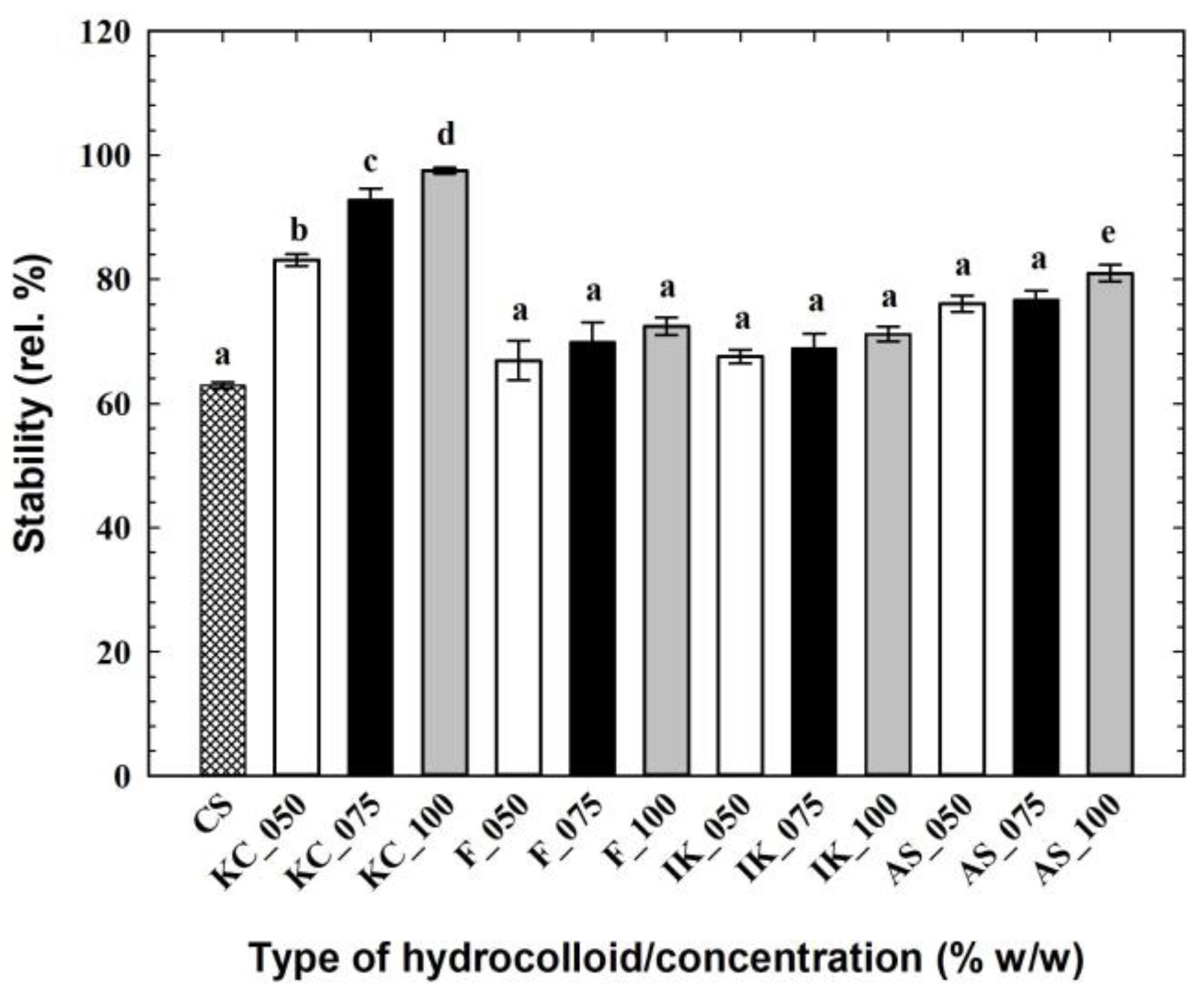
3.4. Instrumental Color and Emulsion Stability of the Cream Cheese Samples
3.5. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez-Méndez, N.; Balderrama-Carmona, A.; García-Sandoval, S.E.; Ramírez-Vigil, P.; Leal-Ramos, M.Y.; García-Triana, A. Proteolysis and rheological properties of cream cheese made with a plant-derived coagulant from Solanum elaeagnifolium. Foods 2019, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. Effect of homogenisation of cheese milk and high-shear mixing of the curd during cream cheese manufacture. Int. J. Dairy Technol. 2018, 71, 417–431. [Google Scholar] [CrossRef]
- Sainani, M.R.; Vyas, H.K.; Tong, P.S. Characterization of particles in cream cheese. J. Dairy Sci. 2004, 87, 2854–2863. [Google Scholar] [CrossRef] [PubMed]
- Fuquay, J.W.; Fox, P.F.; McSweeney, P.L.H. Encyclopedia of Dairy Sciences, 2nd ed.; Elsevier: London, UK, 2011; ISBN 978-0-12-374402-9. [Google Scholar]
- Monteiro, R.R.; Tavares, D.Q.; Kindstedt, P.S.; Gigante, M.L. Effect of pH on microstructure and characteristics of cream cheese. J. Food Sci. 2009, 74, C112–C117. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.Y. Handbook of Fermented Functional Foods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 593–594. [Google Scholar]
- Kim, J.; Watkinson, P.; Matia-Merino, L.; Smith, J.R.; Golding, M. Evaluation of formulation design on the physical and structural properties of commercial cream cheeses. Int. J. Food Sci. Technol. 2022, 57, 6422–6434. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid gel particles: Formation, characterization, and application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.O.; Williams, P.A. Introduction to food hydrocolloids. In Handbook of Hydrocolloids, 2nd rev. ed.; Phillips, G.O., Ed.; Woodhead Publishing: Cambridge, UK, 2009; ISBN 978-184-5695-873. [Google Scholar]
- Glass, K.; Doyle, M.E. Safety of Processed Cheese; FRI Briefings, Food Research Institute, University of Wiskonsin: Madison, WI, USA, 2005. [Google Scholar]
- Wadhwani, R.; McMahon, D.J. Color of low-fat cheese influences flavor perception and consumer liking. J. Dairy Sci. 2012, 95, 2336–2346. [Google Scholar] [CrossRef]
- Eha, K.; Pehk, T.; Heinmaa, I.; Kaleda, A.; Laos, K. Impact of short-term heat treatment on the structure and functional properties of commercial furcellaran compared to commercial carrageenans. Heliyon 2021, 7, E06640. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P.; Balková, R.; Hynek, D.; Bytesnikova, Z.; Gagic, M.; Milosavljevic, V.; Adam, V. Intelligent and active composite films based on furcellaran: Structural characterization, antioxidant and antimicrobial activities. Food Packag. Shelf Life 2019, 22, 100405. [Google Scholar] [CrossRef]
- Wurm, F.; Nussbaumer, F.; Pham, T.; Bechtold, T. Structural elucidation of mixed carrageenan gels using rheometry. Food Hydrocoll. 2019, 95, 533–539. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Rheological characterization of polysaccharides extracted from brown seaweed. J. Sci. Food Agric. 2007, 87, 1630. [Google Scholar] [CrossRef]
- Venugopal, V. Polysaccharides from Seaweed and Microalgae. In Marine Polysaccharides; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9780429136429. [Google Scholar]
- ISO Standard No. 5534; Cheese and Processed Cheese—Determination of the Total Solid Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO Standard No. 1735; Cheese and Processed Cheese Products—Determination of Fat Content—Gravimetric Method (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- Winter, H.H.; Chambon, F. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Černíková, M.; Nebesářová, J.; Salek, R.N.; Popková, R.; Buňka, F. The effect of rework content addition on the microstructure and viscoelastic properties of processed cheese. J. Dairy Sci. 2018, 101, 2956–2962. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramírez, J.; Arnau, J.; Serra, X.; Gou, P. Effect of pH, NaCl content and proteolysis index on the relationship between water content and texture parameters in biceps femoris and semimembranosus muscles in dry-cured ham. Meat Sci. 2006, 72, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Sun, D.W. Assessment of cheese browning affected by baking conditions using computer vision. J. Food Eng. 2003, 56, 339–345. [Google Scholar] [CrossRef]
- Nikzade, V.; Tehrani, M.M.; Saadatmand-Tarzjan, M. Optimization of low-cholesterol–low-fat mayonnaise formulation: Effect of using soy milk and some stabilizer by a mixture design approach. Food Hydrocoll. 2012, 28, 344–352. [Google Scholar] [CrossRef]
- ISO Standard No. 8586; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO Standard No. 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- Weiserová, E.; Doudová, L.; Galiová, L.; Žák, L.; Michálek, J.; Janiš, R.; Buňka, F. The effect of combinations of sodium phosphates in binary mixtures on selected texture parameters of processed cheese spreads. Int. Dairy J. 2011, 21, 979–986. [Google Scholar] [CrossRef]
- Tadeu da Veiga Correia, V.; D’Angelis, D.F.; Neris dos Santos, A.; Silva Roncheti, E.F.; Vieira Queiroz, V.A.; Fontes Figueiredo, J.E.; Azevedo da Silva, W.; Ferreira, A.A.; Fante, C.A. Tannin-sorghum flours in cream cheese: Physicochemical, antioxidant and sensory characterization. LWT 2022, 154, 112672. [Google Scholar] [CrossRef]
- Schulz-Collins, D.; Senge, B. Acid- and acid/rennet-curd cheeses part A: Quark, cream cheese and related varieties. In Cheese: Chemistry, Physics and Microbiology: Major Cheese Groups; Elsevier: Amsterdam, The Netherlands, 2004; pp. 301–328. [Google Scholar] [CrossRef]
- Ruusunen, M.; Vainionpää, J.; Puolanne, E.; Lyly, M.; Lähteenmäki, L.; Niemistö, M.; Ahvenainen, R. Effect of sodium citrate, carboxymethyl cellulose and carrageenan levels on quality characteristics of low-salt and low-fat bologna type sausages. Meat Sci. 2003, 64, 371–381. [Google Scholar] [CrossRef]
- Møller, S.M.; Hansen, T.B.; Andersen, U.; Lillevang, S.K.; Rasmussen, A.; Bertram, H.C. Water properties in cream cheeses with variations in pH, fat, and salt content and correlation to microbial survival. J. Agric. Food Chem. 2012, 60, 1635–1644. [Google Scholar] [CrossRef]
- Joyner (Melito), H.S. Explaining food texture through rheology. Curr. Opin. Food Sci. 2018, 21, 7–14. [Google Scholar] [CrossRef]
- Schädle, C.N.; Bader-Mittermaier, S.; Sanahuja, S. The effect of corn dextrin on the rheological, tribological, and aroma release properties of a reduced-fat model of processed cheese spread. Molecules 2022, 27, 1864. [Google Scholar] [CrossRef]
- Kůrová, V.; Salek, R.N.; Vašina, M.; Vinklárková, K.; Zálešáková, L.; Gál, R.; Adámek, R.; Buňka, F. The effect of homogenization and addition of polysaccharides on the viscoelastic properties of processed cheese sauce. J. Dairy Sci. 2022, 105(8), 6563–6577. [Google Scholar] [CrossRef]
- Blakemore, W.R.; Harpell, A.R. Carrageenan. In Food Stabilisers, Thickeners and Gelling Agents; Imeson, A., Ed.; Wiley-Blackwell Publishing: Hoboken, NJ, USA, 2010; pp. 73–94. ISBN 978-140-5132-671. [Google Scholar]
- Černíková, M.; Buňka, F.; Pavlínek, V.; Březina, P.; Hrabě, J.; Valášek, P. Effect of carrageenan type on viscoelastic properties of processed cheese. Food Hydrocoll. 2008, 22, 1054–1061. [Google Scholar] [CrossRef]
- Míšková, Z.; Salek, R.N.; Křenková, B.; Kůrová, V.; Němečková, I.; Pachlová, V.; Buňka, F. The effect of κ- and ι-carrageenan concentrations on the viscoelastic and sensory properties of cream desserts during storage. LWT 2021, 145, 111539. [Google Scholar] [CrossRef]
- Macků, I.; Buňka, F.; Voldánová, B.; Pavlínek, V. Effect of addition of selected solid cosolutes on viscoelastic properties of model processed cheese containing pectin. Food Hydrocoll. 2009, 23, 2078–2084. [Google Scholar] [CrossRef]
- Langendorff, V.; Cuvelier, G.; Launay, B.; Michon, C.; Parker, A.; De Kruif, C.G. Casein micelle/iota carrageenan interactions in milk: Influence of temperature. Food Hydrocoll. 1999, 13, 211–218. [Google Scholar] [CrossRef]
- Nagyová, G.; Buňka, F.; Salek, R.N.; Černíková, M.; Mančík, P.; Grůber, T.; Kuchař, D. Use of sodium polyphosphates with different linear lengths in the production of spreadable processed cheese. J. Dairy Sci. 2014, 97, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V. Marine Polysaccharides: Food Applications; CRC Press: Boca Raton, FL, USA, 2011; p. 377. ISBN 978-1-4398-1526-7. [Google Scholar]
- Piska, I.; Štěnina, J.; Ipsen, R.H.; Qwist, K.B. Mikrostruktura a reologické vlastnosti vysokotučného taveného sýry. In Sborník Celostátní Přehlídky Sýrů 2002; Česká společnost chemická: Praha, Czech Republic, 2002; pp. 192–196. ISBN 80-86238-21-0. [Google Scholar]
- Cunha, C.R.; Grimaldi, R.; Alcântara, M.R.; Viotto, W.H. Effect of the type of fat on rheology, functional properties and sensory acceptance of spreadable cheese analogue. Int. J. Dairy Technol. 2013, 66, 54–62. [Google Scholar] [CrossRef]
- Salek, R.N.; Černíková, M.; Lorencová, E.; Pachlová, V.; Kůrová, V.; Šenkýřová, J.; Buňka, F. The impact of Cheddar or white brined cheese with various maturity degrees on the processed cheese consistency: A comparative study. Int. Dairy J. 2020, 111, 104816. [Google Scholar] [CrossRef]
- Nickerson, M.T.; Paulson, A.T.; Hallett, F.R. Dilute solution properties of κ-carrageenan polysaccharides: Effect of potassium and calcium ions on chain conformation. Carbohydr. Polym. 2004, 58, 25–33. [Google Scholar] [CrossRef]
- Polášek, Z.; Salek, R.N.; Vašina, M.; Lyčková, A.; Gál, R.; Pachlová, V.; Buňka, F. The effect of furcellaran or κ-carrageenan addition on the textural, rheological and mechanical vibration damping properties of restructured chicken breast ham. LWT 2021, 138, 110623. [Google Scholar] [CrossRef]
- Trius, A.; Sebranek, J.G.; Lanier, T. Carrageenans and their use in meat products. Crit. Rev. Food Sci. Nutr. 2009, 36, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.M.; Stanley, D.V. Microstructural Principles of Food Processing and Engineering, 2nd ed.; Aspen Publishers: Gaithersburg, MD, USA, 1999; pp. 93–108. ISBN 0-8342-1256-0. [Google Scholar]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780849308505. [Google Scholar]
- Lee, S.K.; Klostermeyer, H. The effect of pH on the rheological properties of reduced-fat model processed cheese spreads. LWT 2001, 34, 288–292. [Google Scholar] [CrossRef]
- Milovanovic, B.; Djekic, I.; Miocinovic, J.; Djordjevic, V.; Lorenzo, J.M.; Barba, F.J.; Mörlein, D.; Tomasevic, I. What is the color of milk and dairy products and how is it measured? Foods 2020, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
| Raw Materials and Processing Parameters | Ingredients Composition * (% w/w) | |||
|---|---|---|---|---|
| CS | CC1 | CC2 | CC3 | |
| Raw materials | ||||
| Quark-type cheese | 46.38 | 45.88 | 45.63 | 45.38 |
| Sour cream | 39.86 | 39.86 | 39.86 | 39.86 |
| Water | 13.04 | 13.04 | 13.04 | 13.04 |
| NaCl | 0.72 | 0.72 | 0.72 | 0.72 |
| Hydrocolloid | - | 0.50 | 0.75 | 1.00 |
| Processing parameters | ||||
| Stirring speed (rpm) | 3000 | 3000 | 3000 | 3000 |
| Target temperature (°C) | 80 | 80 | 80 | 80 |
| Holding time (min) ** | 10 | 10 | 10 | 10 |
| Total time (min) | 16 | 16 | 16 | 16 |
| Sample* | Hydrocolloid Concentration | Dry Matter | pH | aw |
|---|---|---|---|---|
| (% w/w) | (% w/w) | (-) | (-) | |
| CS | 29.74 a,A ± 0.07 | 4.18 a,A ± 0.07 | 0.9982 a,A ± 0.001 | |
| KC | 0.50 | 29.51 a,A ± 0.05 | 4.16 a,A ± 0.08 | 0.9817 a,A ± 0.001 |
| 0.75 | 29.85 a,A ± 0.08 | 4.18 a,A ± 0.04 | 0.9819 a,A ± 0.001 | |
| 1.00 | 30.27 a,A ± 0.15 | 4.21 a,A ± 0.01 | 0.9829 a,A ± 0.001 | |
| F | 0.50 | 30.66 a,A ± 0.16 | 4.21 a,A ± 0.06 | 0.9925 a,A ± 0.002 |
| 0.75 | 30.01 a,A ± 0.12 | 4.22 a,A ± 0.05 | 0.9911 a,A ± 0.003 | |
| 1.00 | 29.64 e,A ± 0.09 | 4.23 a,A ± 0.06 | 0.9923 a,A ± 0.001 | |
| IK | 0.50 | 29.85 a,A ± 0.12 | 4.18 a,A ± 0.05 | 0.9938 a,A ± 0.002 |
| 0.75 | 29.96 a,A ± 0.09 | 4.22 a,A ± 0.01 | 0.9919 a,A ± 0.001 | |
| 1.00 | 30.29 a,A ± 0.02 | 4.23 a,A ± 0.01 | 0.9942 a,A ± 0.002 | |
| AS | 0.50 | 30.01 a,A ± 0.08 | 4.19 a,A ± 0.02 | 0.9933 a,A ± 0.001 |
| 0.75 | 29.87 a,A ± 0.04 | 4.21 a,A ± 0.01 | 0.9935 a,A ± 0.002 | |
| 1.00 | 30.06 a,A ± 0.04 | 4.18 a,A ± 0.07 | 0.9929 a,A ± 0.001 |
| Sample * | Hydrocolloid Concentration | AF | z | G′ (kPa) | G″ (kPa) | G* (kPa) | tan δ (-) |
|---|---|---|---|---|---|---|---|
| (% w/w) | (Pa·s1/z) | (-) | |||||
| CS | 2261.1 a,A ± 124.5 | 5.69 a,A ± 0.02 | 21.6 a,A ± 1.5 | 6.1 a,A ± 0.5 | 22.4 a,A ± 1.3 | 0.28 a,A ± 0.01 | |
| KC | 0.50 | 62,002.1 b,B ± 354.7 | 7.26 b,B ± 0.03 | 658.3 b,B ± 20.4 | 131.2 b,B ± 11.3 | 671.3 b,B ± 19.8 | 0.20 b,B ± 0.01 |
| 0.75 | 73,085.8 c,C ± 245.7 | 7.91 c,C ± 0.05 | 674.6 c,B ± 35.7 | 149.2 c,B ± 18.6 | 690.9 c,B ± 21.7 | 0.22 c,C ± 0.02 | |
| 1.00 | 194,112.1 d,D ± 145.3 | 9.95 d,C ± 0.04 | 1972.1 d,C ± 50.7 | 437.9 d,C ± 28.9 | 2020.1 d,C ± 68.8 | 0.22 d,C ± 0.01 | |
| F | 0.50 | 17,575.9 e,E ± 114.2 | 8.41 e,D ± 0.02 | 164.6 e,D ± 11.8 | 36.3 e,D ± 2.7 | 168.6 e,D ± 20.1 | 0.22 e,C ± 0.01 |
| 0.75 | 17,926.6 f,F ± 247.8 | 9.44 f,C ± 0.01 | 178.9 f,D ± 12.7 | 37.3 f,D ± 3.3 | 182.7 f,D ± 15.7 | 0.21 f,D ± 0.02 | |
| 1.00 | 71,381.8 g,G ± 456.7 | 9.57 g,C ± 0.04 | 583.3 g,E ± 25.9 | 108.9 g,E ± 12.7 | 593.4 g,E ± 26.4 | 0.19 g,E ± 0.01 | |
| IK | 0.50 | 4614.7 h,H ± 85.8 | 6.57 h,E ± 0.03 | 45.5 h,F ± 2.6 | 10.8 h,F ± 0.9 | 46.7 h,F ± 5.1 | 0.24 h,F ± 0.01 |
| 0.75 | 20,357.2 I,I ± 114.6 | 6.96 i,E ± 0.02 | 151.5 i,D ± 16.4 | 37.2 i,D ± 2.2 | 155.9 i,D ± 2.8 | 0.25 i,H ± 0.01 | |
| 1.00 | 28,772.5 j,J ± 158.8 | 7.52 j,B ± 0.03 | 282.6 j,F ± 21.3 | 64.6 j,G ± 6.4 | 289.9 j,G ± 8.7 | 0.23 j,I ± 0.02 | |
| AS | 0.50 | 3258.2 k,K ± 256.7 | 6.50 k,E ± 0.01 | 31.4 k,G ± 5.1 | 8.8 k,H ± 0.9 | 32.60 k,H ± 6.7 | 0.28 k,A ± 0.02 |
| 0.75 | 3769.4 l,L ± 54.7 | 6.25 l,E ± 0.04 | 64.7 l,H ± 6.4 | 16.9 l,I ± 1.3 | 66.9 l,I ± 3.7 | 0.26 l,J ± 0.02 | |
| 1.00 | 6661.7 m,M ± 147.6 | 6.73 m,E ± 0.05 | 86.4 m,I ± 4.7 | 83.7 m,J ± 14.5 | 120.2 m,J ± 11.8 | 0.97 m,K ± 0.01 |
| Sample * | Hydrocolloid Concentration | Adhesiveness | Stickiness | Cohesiveness | Gumminess |
|---|---|---|---|---|---|
| (% w/w) | (N·s) | (N) | (-) | (N) | |
| CS | −0.01 a,A ± 0.01 | −0.04 a,A ± 0.01 | 2.83 a,A ± 0.01 | 3.14 a,A ± 0.02 | |
| KC | 0.50 | −0.01 a,A ± 0.01 | −0.01 a,A ± 0.01 | 3.06 b,B ± 0.01 | 13.01 b,B ± 0.01 |
| 0.75 | −0.01 a,A ± 0.01 | −0.01 a,A ± 0.01 | 3.09 c,B ± 0.01 | 24.11 c,C ± 0.01 | |
| 1.00 | −0.01 a,A ± 0.01 | −0.01 a,A ± 0.01 | 3.52 d,B ± 0.01 | 59.25 d,D ± 0.03 | |
| F | 0.50 | −0.03 a,A ± 0.01 | −0.01 a,A ± 0.01 | 3.32 e,C ± 0.01 | 7.01 e,E ± 0.01 |
| 0.75 | −0.03 a,A ± 0.01 | −0.01 a,A ± 0.01 | 3.01 f,B ± 0.01 | 18.76 f,F ± 0.02 | |
| 1.00 | −0.01 a,A ± 0.01 | −0.01 a,A ± 0.01 | 3.15 g,B ± 0.01 | 44.45 g,G ± 0.01 | |
| IK | 0.50 | −0.01 a,A ± 0.01 | −0.03 a,A ± 0.01 | 3.17 h,B ± 0.01 | 1.45 h,H ± 0.01 |
| 0.75 | −0.02 a,A ± 0.01 | −0.03 a,A ± 0.01 | 3.29 i,C ± 0.01 | 1.93 i,I ± 0.01 | |
| 1.00 | −0.03 a,A ± 0.01 | −0.03 a,A ± 0.01 | 3.49 j,C ± 0.01 | 2.87 j,J ± 0.02 | |
| AS | 0.50 | −0.02 a,A ± 0.01 | −0.04 a,A ± 0.01 | 3.01 k,B ± 0.01 | 4.37 k,K ± 0.01 |
| 0.75 | −0.01 a,A ± 0.01 | −0.04 a,A ± 0.01 | 3.53 l,C ± 0.01 | 5.78 l,L ± 0.01 | |
| 1.00 | −0.04 a,A ± 0.01 | −0.04 a,A ± 0.01 | 3.20 m,C ± 0.01 | 47.17 m,M ± 0.03 |
| Sample * | Hydrocolloid Concentration | L* | a* | b* | C* | h (°) | ΔΕ12 | WI |
|---|---|---|---|---|---|---|---|---|
| (% w/w) | ||||||||
| CS | 92.27 a,A ± 0.12 | −0.61 a,A ± 0.01 | 14.39 a,A ± 0.02 | 14.41 a,A ± 0.05 | 92.40 a,A ± 0.23 | - | 83.66 a,A ± 0.01 | |
| KC | 0.50 | 92.63 b,B ± 0.11 | −0.61 b,A ± 0.01 | 13.94 b,B ± 0.03 | 13.96 b,B ± 0.02 | 92.50 a,A ± 0.24 | 0.57 a,A ± 0.01 | 83.22 a,A ± 0.02 |
| 0.75 | 92.67 c,B ± 0.06 | −0.45 c,B ± 0.02 | 13.73 c,B ± 0.02 | 13.74 c,B ± 0.05 | 91.90 a,A ± 0.31 | 0.79 b,B ± 0.02 | 83.43 a,A ± 0.03 | |
| 1.00 | 92.24 d,A ± 0.03 | −0.53 d,C ± 0.01 | 13.90 d,B ± 0.04 | 13.91 d,B ± 0.04 | 92.20 a,A ± 0.19 | 0.49c,C ± 0.01 | 84.07 a,A ± 0.01 | |
| F | 0.50 | 91.96 e,C ± 0.02 | −0.35 e,D ± 0.01 | 14.21 e,A ± 0.04 | 14.22 e,A ± 0.03 | 91.42 a,A ± 0.21 | 0.43 d,D ± 0.01 | 83.67 a,A ± 0.01 |
| 0.75 | 91.70 f,C ± 0.14 | −0.21 f,E ± 0.02 | 14.06 f,A ± 0.04 | 14.06 f,A ± 0.06 | 90.86 a,A ± 0.07 | 0.77 e,B ± 0.01 | 83.67 a,A ± 0.01 | |
| 1.00 | 91.25 g,D ± 0.08 | −0.15 g,F ± 0.01 | 14.00 g,A ± 0.03 | 14.00 g,A ± 0.08 | 90.63 a,A ± 0.13 | 1.18 f,E ± 0.01 | 83.49 a,A ± 0.02 | |
| IK | 0.50 | 91.49 h,E ± 0.04 | −0.52 h,G ± 0.01 | 15.16 h,C ± 0.01 | 15.17 h,C ± 0.04 | 91.97 a,A ± 0.24 | 1.10 g,F ± 0.02 | 83.61 a,A ± 0.02 |
| 0.75 | 91.81 i,C ± 0.02 | −0.47 i,B ± 0.01 | 14.06 i,A ± 0.03 | 14.07 i,A ± 0.03 | 91.80 a,A ± 0.18 | 0.58 h,A ± 0.02 | 83.72 a,A ± 0.01 | |
| 1.00 | 91.71 j,C ± 0.05 | −0.35 j,D ± 0.01 | 13.88 j,B ± 0.01 | 13.89 j,B ± 0.04 | 91.46 a,A ± 0.05 | 0.79 i,B ± 0.01 | 83.83 a,A ± 0.01 | |
| AS | 0.50 | 92.17 k,A ± 0.03 | −0.63 k,A ± 0.02 | 14.82 k,A ± 0.02 | 14.83 k,A ± 0.05 | 92.45 a,A ± 0.15 | 0.44 j,C ± 0.01 | 83.23 a,A ± 0.02 |
| 0.75 | 92.27 l,A ± 0.07 | −0.59 l,A ± 0.02 | 14.45 l,A ± 0.02 | 14.46 l,A ± 0.06 | 92.35 a,A ± 0.05 | 0.06 k,G ± 0.02 | 83.61 a,A ± 0.01 | |
| 1.00 | 91.97 m,C ± 0.04 | −0.63 m,A ± 0.01 | 14.79 m,A ± 0.02 | 14.80 m,A ± 0.05 | 92.45 a,a ± 0.02 | 0.50 l,C ± 0.02 | 83.16 a,A ± 0.02 |
| Sample * | Hydrocolloid Concentration (% w/w) | Appearance | Consistency | Hardness | Spreadability | Flavor | Off-Flavor |
|---|---|---|---|---|---|---|---|
| CS | 1 a,A | 1 a,A | 3 a,A | 4 a,A | 1 a,A | 1 a,A | |
| KC | 0.50 | 1 a,A | 2 b,B | 4 b,B | 3 b,B | 1 a,A | 1 a,A |
| 0.75 | 1 a,A | 3 c,C | 4 c,B | 4 c,A | 1 a,A | 1 a,A | |
| 1.00 | 1 a,A | 4 d,D | 6 d,C | 4 d,A | 1 a,A | 1 a,A | |
| F | 0.50 | 1 a,A | 2 e,B | 3 e,A | 3 e,B | 1 a,A | 1 a,A |
| 0.75 | 1 a,A | 3 f,C | 3 f,A | 3 f,B | 1 a,A | 1 a,A | |
| 1.00 | 1 a,A | 3 g,C | 4 g,B | 4 g,A | 1 a,A | 1 a,A | |
| IK | 0.50 | 1 a,A | 5 h,E | 2 h,D | 6 h,C | 1 a,A | 1 a,A |
| 0.75 | 1 a,A | 5 i,E | 2 i,D | 6 i,C | 1 a,A | 1 a,A | |
| 1.00 | 1 a,A | 5 j,E | 2 j,D | 6 j,C | 1 a,A | 1 a,A | |
| AS | 0.50 | 1 a,A | 6 k,F | 6 k,E | 6 k,C | 1 a,A | 1 a,A |
| 0.75 | 1 a,A | 6 l,F | 5 l,F | 6 l,C | 1 a,A | 1 a,A | |
| 1.00 | 1 a,A | 6 m,F | 4 m,B | 6 m,C | 1 a,A | 1 a,A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincová, A.; Šantová, K.; Kůrová, V.; Kratochvílová, A.; Halámková, V.; Suchánková, M.; Lorencová, E.; Sumczynski, D.; Salek, R.N. The Impact of Divergent Algal Hydrocolloids Addition on the Physicochemical, Viscoelastic, Textural, and Organoleptic Properties of Cream Cheese Products. Foods 2023, 12, 1602. https://doi.org/10.3390/foods12081602
Vincová A, Šantová K, Kůrová V, Kratochvílová A, Halámková V, Suchánková M, Lorencová E, Sumczynski D, Salek RN. The Impact of Divergent Algal Hydrocolloids Addition on the Physicochemical, Viscoelastic, Textural, and Organoleptic Properties of Cream Cheese Products. Foods. 2023; 12(8):1602. https://doi.org/10.3390/foods12081602
Chicago/Turabian StyleVincová, Anna, Kristýna Šantová, Vendula Kůrová, Alena Kratochvílová, Veronika Halámková, Markéta Suchánková, Eva Lorencová, Daniela Sumczynski, and Richardos Nikolaos Salek. 2023. "The Impact of Divergent Algal Hydrocolloids Addition on the Physicochemical, Viscoelastic, Textural, and Organoleptic Properties of Cream Cheese Products" Foods 12, no. 8: 1602. https://doi.org/10.3390/foods12081602
APA StyleVincová, A., Šantová, K., Kůrová, V., Kratochvílová, A., Halámková, V., Suchánková, M., Lorencová, E., Sumczynski, D., & Salek, R. N. (2023). The Impact of Divergent Algal Hydrocolloids Addition on the Physicochemical, Viscoelastic, Textural, and Organoleptic Properties of Cream Cheese Products. Foods, 12(8), 1602. https://doi.org/10.3390/foods12081602









