Smilax china L. Polysaccharide Alleviates Dextran Sulphate Sodium-Induced Colitis and Modulates the Gut Microbiota in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Isolation and Purification of Polysaccharides
2.3. Molecular Weight and Monosaccharide Composition Determination
2.4. UV and FT-IR Spectra Analysis
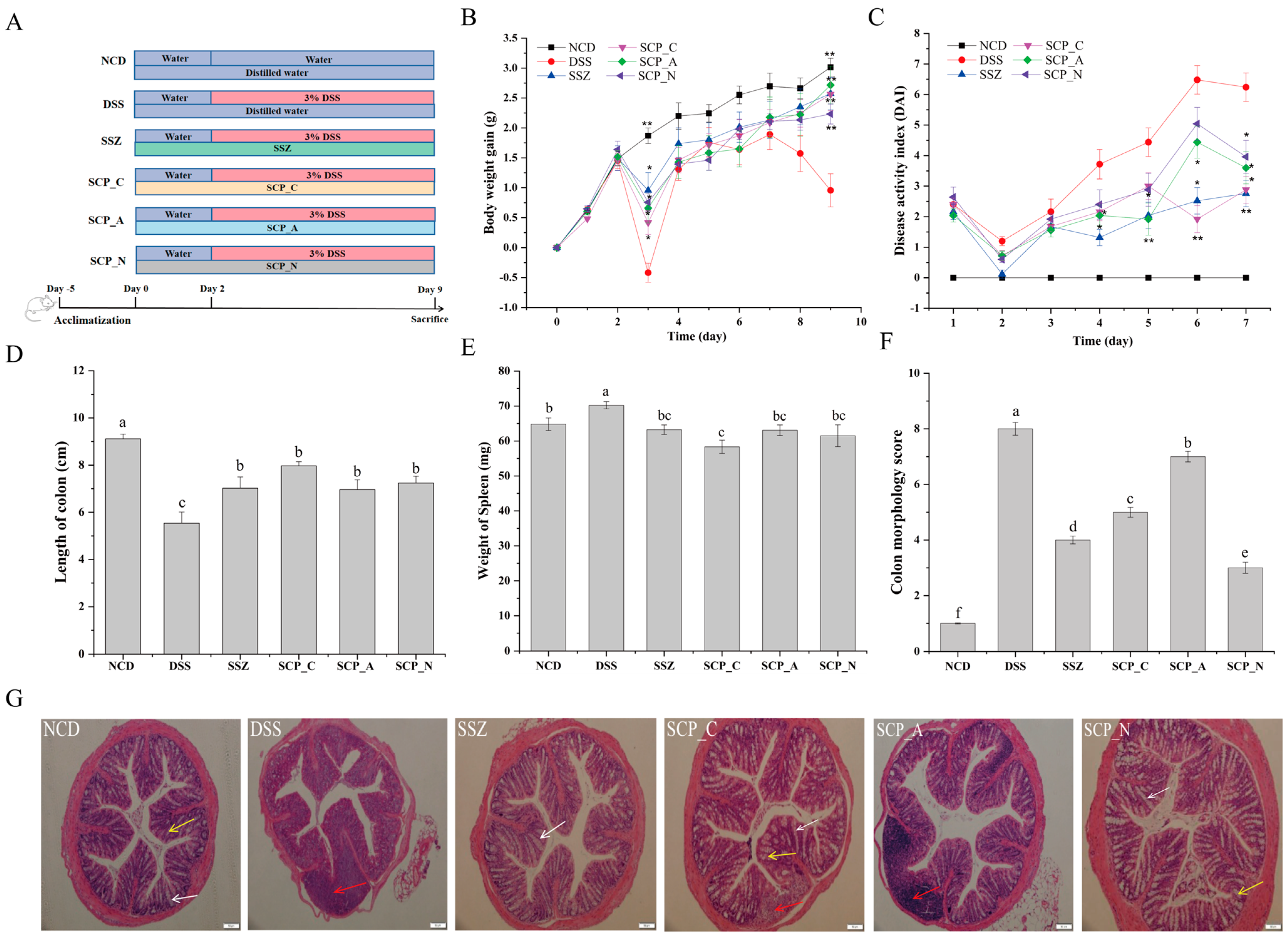
2.5. Experimental Design
2.6. Disease Activity Index (DAI) and Colonic Damage
2.7. Histopathologic Analysis of Colon Tissue
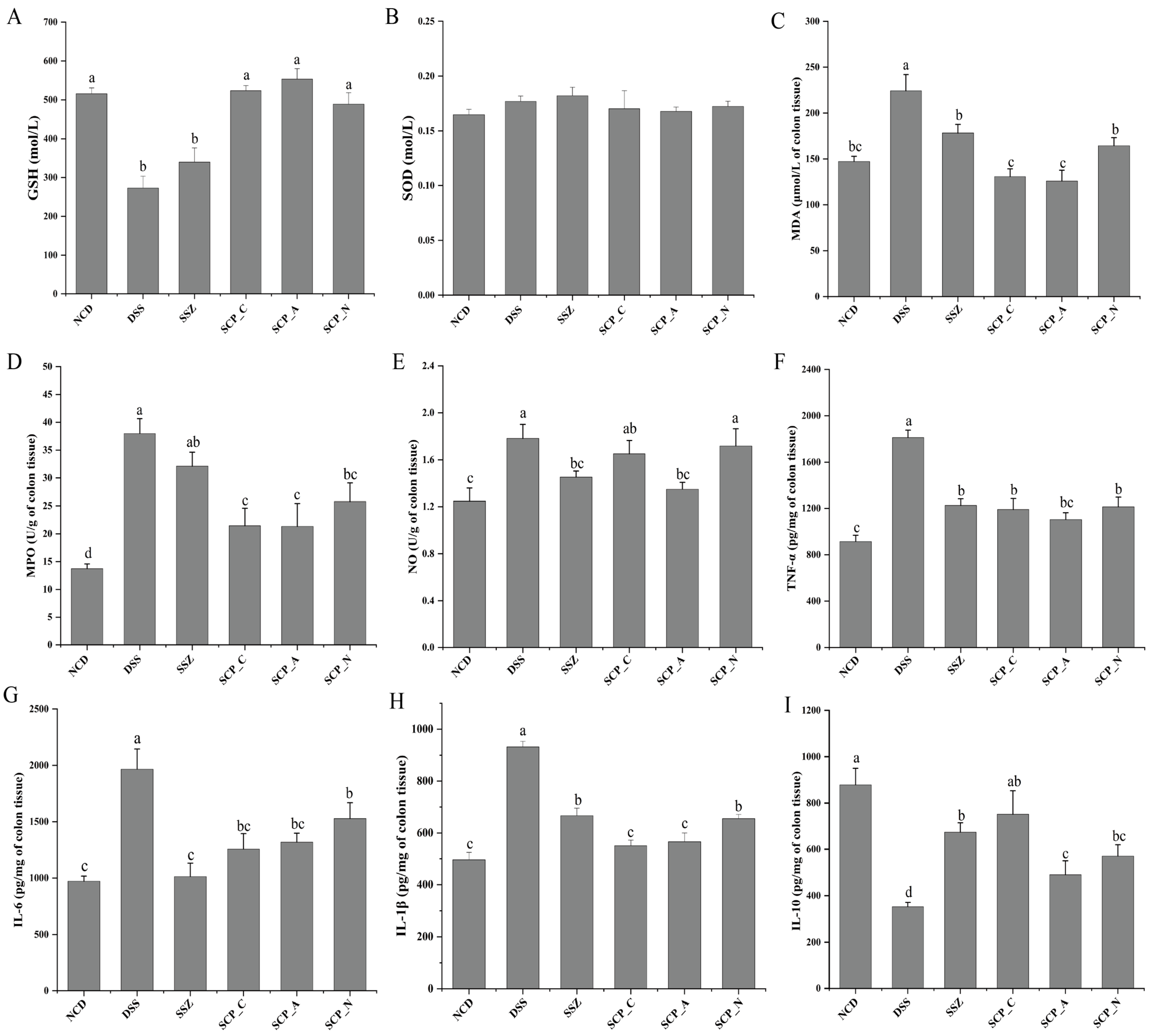
2.8. Analysis of the Oxidative Stress Index
2.9. Measurements of Inflammation Cytokines Levels
2.10. 16S rDNA Gene Sequencing
2.11. Statistics
3. Results and Discussion
3.1. Structural Analysis of SCP_N and SCP_A
3.1.1. Purification and Identification
3.1.2. Chemical Compositions of SCP_A and SCP_N
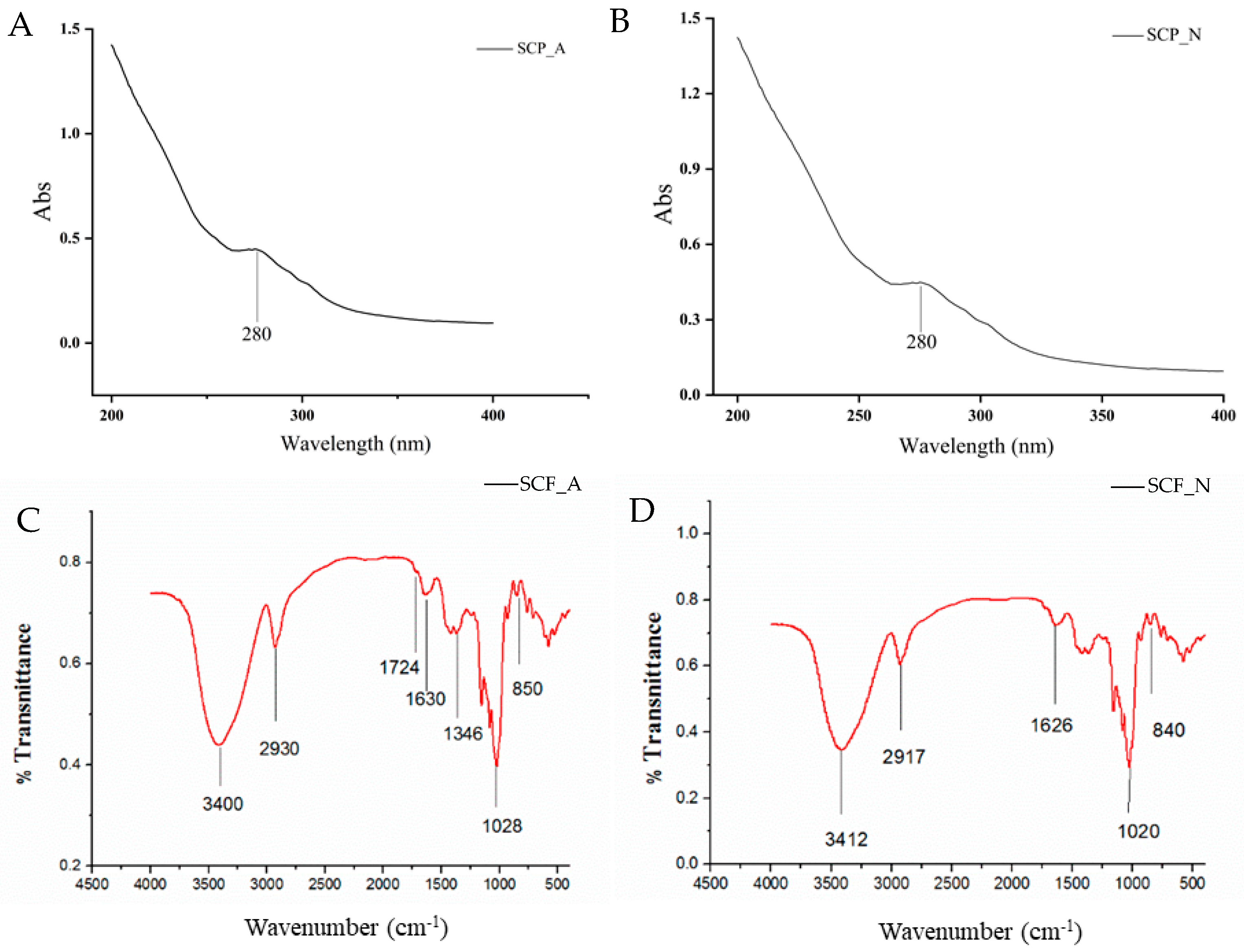
3.1.3. UV Visible Spectra and FT-IR Spectra
3.2. Polysaccharide Alleviates the Clinical Symptoms of DSS-Induced Colitis
3.3. Impact of Polysaccharide on Oxidative Stress in DSS-Induced Mice
3.4. Effect of Polysaccharide on Inflammatory Cytokine Levels
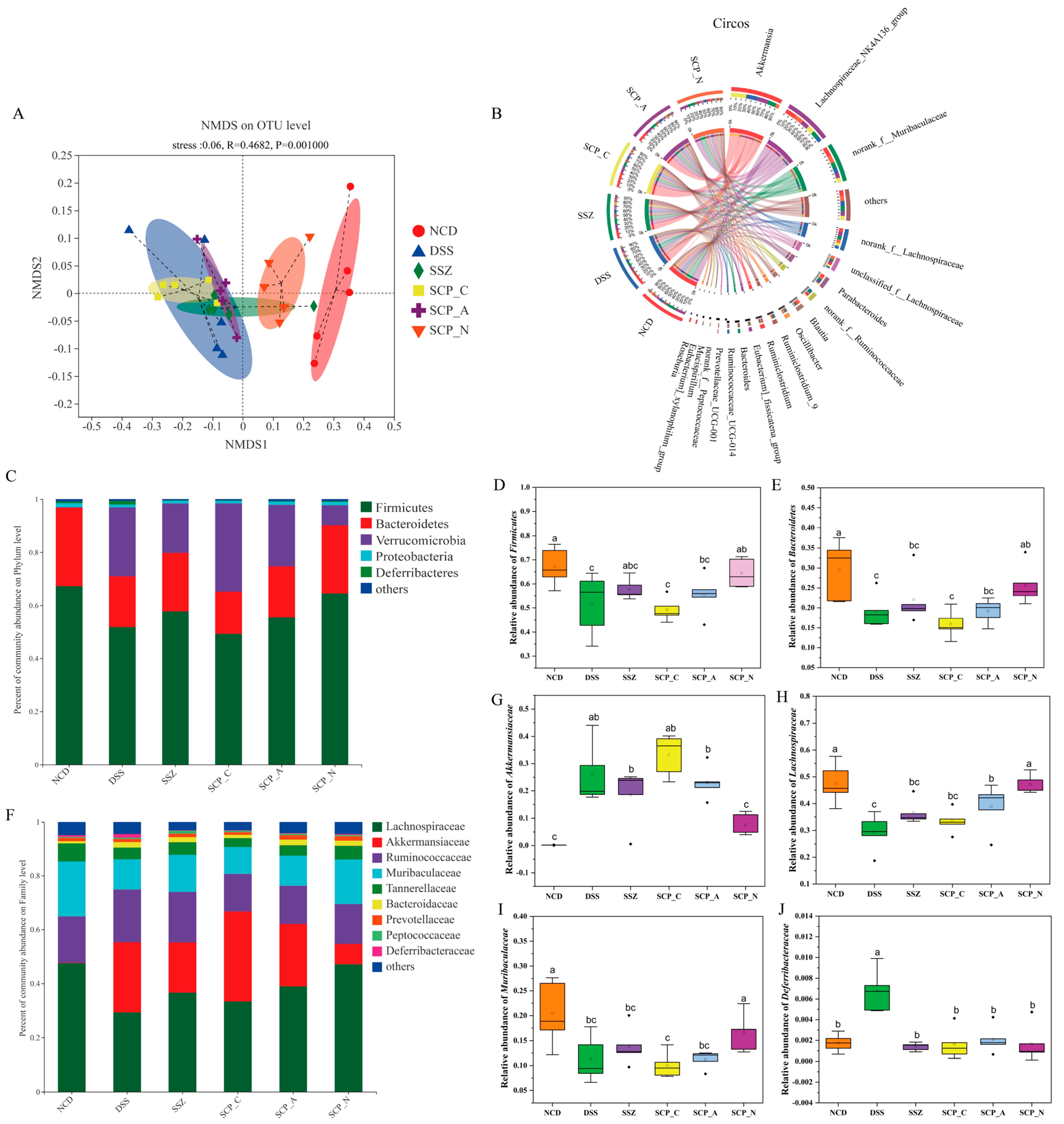
3.5. Effect of Polysaccharide on the Diversity of Gut Microbiota
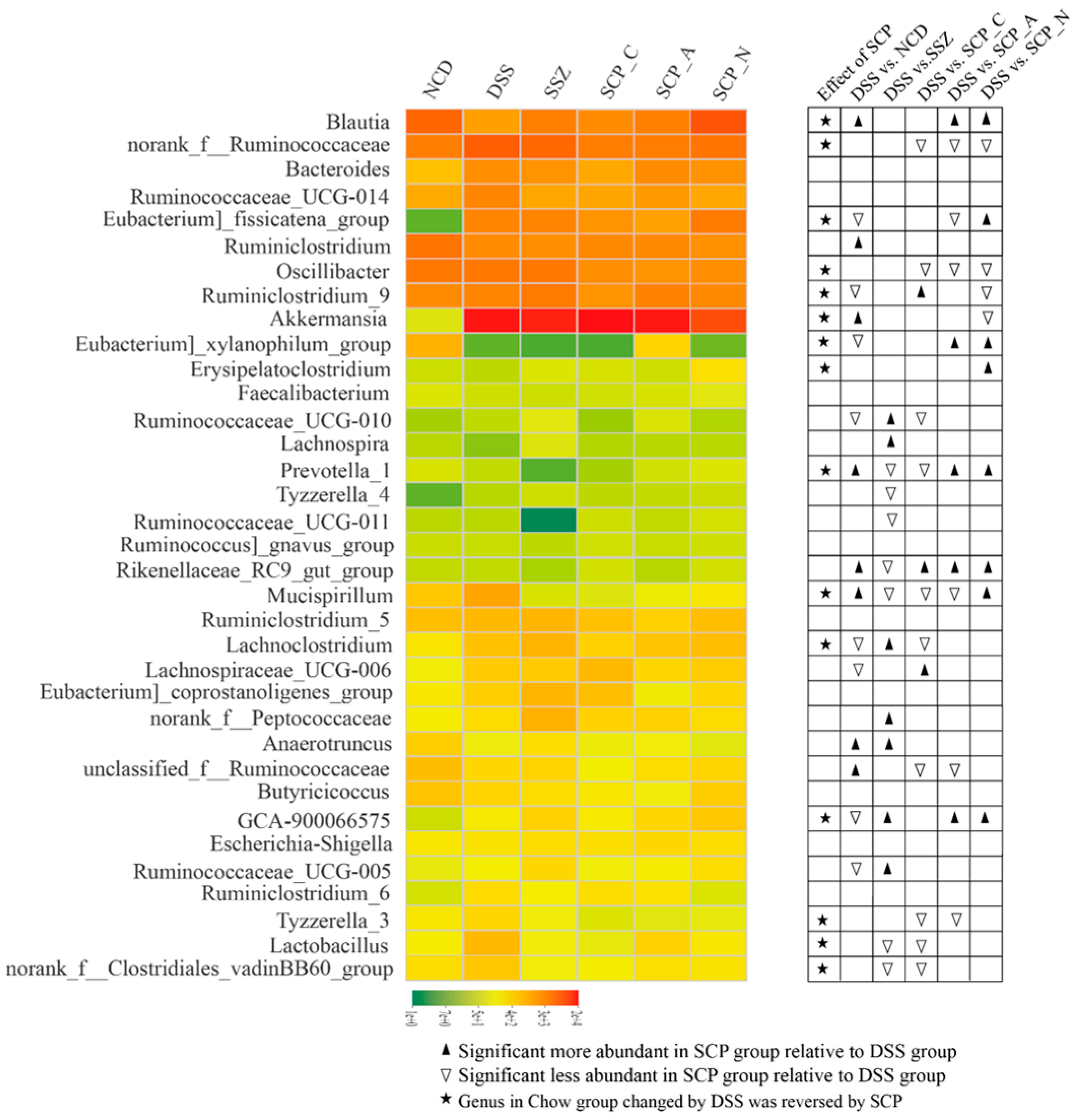
3.6. Effect of Polysaccharide on the Structure of Gut Microbiota
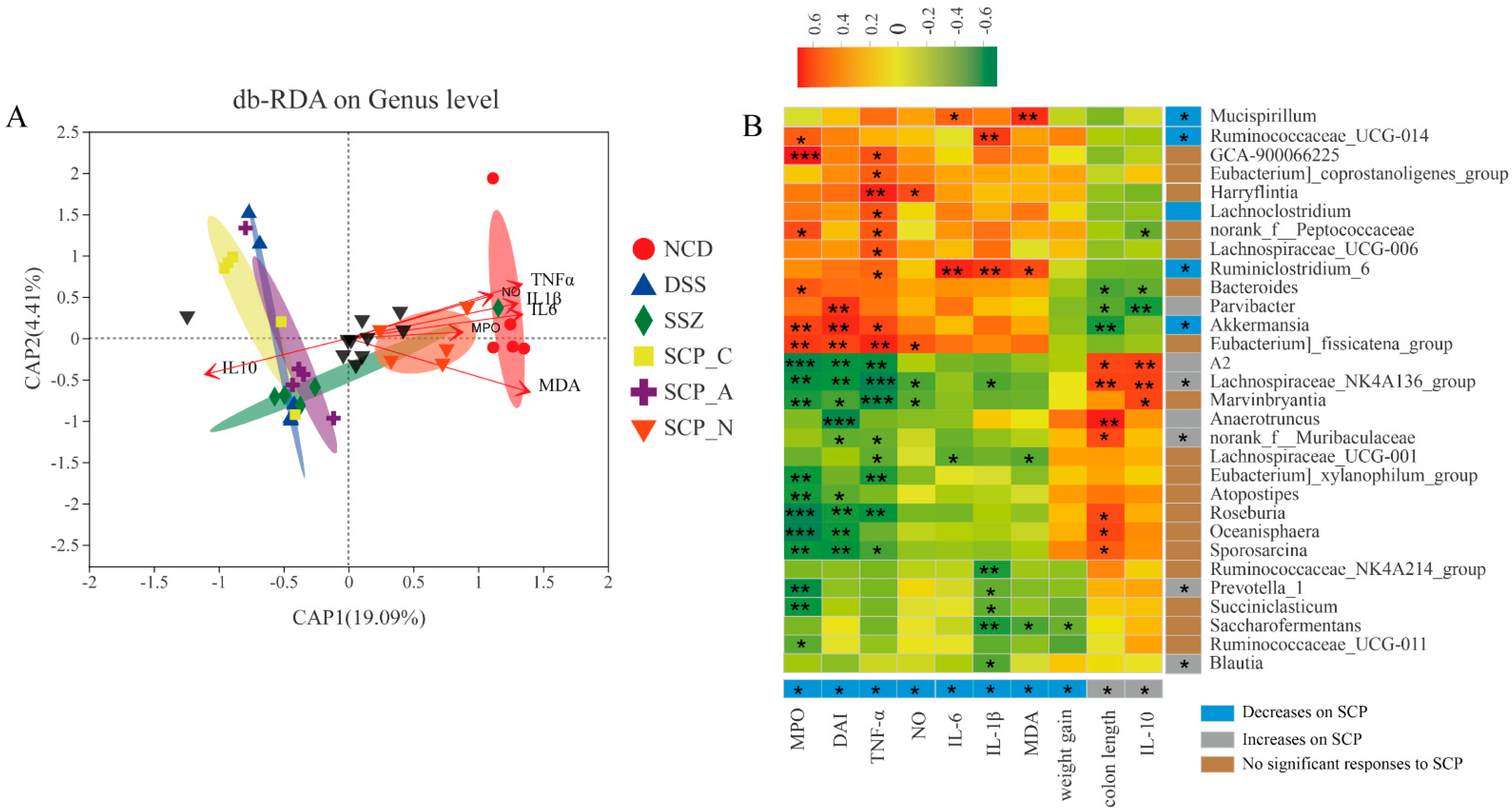
3.7. Correlation between UC-Related Parameters and Key Phylotypes of Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hirten, R.P.; Iacucci, M.; Shah, S.; Ghosha, S.; Colombel, J.F. Combining biologics in inflammatory bowel disease and other immune mediated inflammatory disorders. Clin. Gastroenterol. Hepatol. 2018, 16, 1374–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznarić, Ž.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology 2020, 158, 562–572.e12. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, G.G.; Ng, S.C. Globalisation of inflammatory bowel disease: Perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 2016, 1, 307–316. [Google Scholar] [CrossRef]
- Lendrum, R.; Walker, J.G.; West, B.; Hill, M.J. Proceedings: Effects of sulphasalazine (Salazopyrin) on faecal flora in patients with inflammatory bowel disease. Gut 1974, 15, 344. [Google Scholar]
- Zhang, X.J.; Yuan, Z.W.; Qu, C.; Yu, X.T.; Huang, T.; Chen, P.V.; Su, Z.R.; Dou, Y.X.; Wu, J.Z.; Zeng, H.F.; et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota. Pharmacol. Res. 2018, 137, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, X.; Zheng, X.; Nie, S.; Xu, X. Inhibition of dextran sodium sulfate-induced colitis in mice by baker’s yeast polysaccharides. Carbohydr. Polym. 2019, 207, 371–381. [Google Scholar] [CrossRef]
- Gilardi, D.; Fiorino, G.; Genua, M.; Allocca, M.; Danese, S. Complementary and alternative medicine in inflammatory bowel diseases: What is the future in the field of herbal medicine? Expert. Rev. Gastroenterol. Hepatol. 2014, 8, 835–846. [Google Scholar] [CrossRef]
- Pan, X.; Wang, H.; Zheng, Z.; Huang, X.; Yang, L.; Liu, J.; Wang, K.; Zhang, Y. Pectic polysaccharide from Smilax china L. ameliorated ulcerative colitis by inhibiting the galectin-3/NLRP3 inflammasome pathway. Carbohydr. Polym. 2022, 277, 118864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Z.Z.; He, Y.; Yang, Y.; Liu, L.; Lin, Q.; Nie, Y.; Li, M.; Zhi, F.; Liu, S.; et al. Gut Microbiota Offers Universal Biomarkers across Ethnicity in Inflammatory Bowel Disease Diagnosis and Infliximab Response Prediction. mSystems 2018, 3, e00188-17. [Google Scholar] [CrossRef] [Green Version]
- Ha, C.W.Y.; Martin, A.; Sepich-Poore, G.D.; Shi, B.; Wang, Y.; Gouin, K.; Humphrey, G.; Sanders, K.; Ratnayake, Y.; Chan, K.S.L.; et al. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 2020, 183, 666–683.e17. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Chen, H.; Fu, Y.; Wang, W.; Li, Q.; Li, X.; Wang, X.; Fan, G.; Zhang, Y. The Underlying Molecular Mechanisms Involved in Traditional Chinese Medicine Smilax china L. For the Treatment of Pelvic Inflammatory Disease. Evid Based Complement Alternat Med. 2021, 2021, 5552532. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, J.Y.; Zou, Z.M. Studies on chemical constituents of rhizomes of Smilax China L. Zhongguo Zhong Yao Za Zhi 2008, 33, 2497–2499. (In Chinese) [Google Scholar]
- Li, X.; Chu, L.; Liu, S.; Zhang, W.; Lin, L.; Zheng, G. Smilax china L. flavonoid alleviates HFHS-induced inflammation by regulating the gut-liver axis in mice. Phytomedicine 2022, 95, 153728. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Li, J.; Lin, L.; Zheng, G. A flavonoid-rich Smilax china L. extract prevents obesity by upregulating the adiponectin-receptor/AMPK signalling pathway and modulating the gut microbiota in mice. Food Funct. 2021, 12, 5862–5875. [Google Scholar] [CrossRef]
- Li, X.; Jin, W.; Zhang, W.; Zheng, G. The inhibitory kinetics and mechanism of quercetin-3-O-rhamnoside and chlorogenic acid derived from Smilax china L. EtOAc fraction on xanthine oxidase. Int. J. Biol. Macromol. 2022, 213, 447–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Ran, S.; Wang, K. Purification, structural elucidation and anti-inflammatory activity in vitro of polysaccharides from Smilax china L. Int. J. Biol. Macromol. 2019, 139, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Navarini, L.; Gilli, R.; Gombac, V.; Abatangelo, A.; Bosco, M.; Toffanin, R. Polysaccharides from hot water extracts of roasted coffea arabica beans: Isolation and characterization. Carbohydr. Polym. 1999, 40, 71–81. [Google Scholar] [CrossRef]
- Qin, X.S.; Chen, S.S.; Li, X.J.; Luo, S.Z.; Zhong, X.Y.; Jiang, S.T.; Zhao, Y.Y.; Zheng, Y. Gelation properties of transglutaminase-induced soy protein isolate and wheat gluten mixture with ultrahigh pressure pretreatment. Food Bioprocess. Technol. 2017, 10, 866–874. [Google Scholar] [CrossRef]
- Hu, J.; Huang, H.; Che., Y.; Ding, C.; Zhang, L.; Wang, Y.; Hao, H.; Shen, H.; Cao, L. Qingchang huashi formula attenuates DSS-induced colitis in mice by restoring gut microbiota-metabolism homeostasis and goblet cell function. J. Ethnopharmacol. 2021, 266, 113394. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.R. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: Updated experimental and clinical evidence. Exp. Biol. Med. 2012, 237, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, Y.; Shi, M.; Xu, X.; Zhao, Y.; Wu, X.; Zhang, Y. Gegen Qinlian decoction alleviates experimental colitis via suppressing TLR4/NF-κB signaling and enhancing antioxidant effect. Phytomedicine 2016, 23, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Kristjánsson, G.; Venge, P.; Wanders, A. Clinical and subclinical intestinal inflammation assessed by the mucosal patch technique: Studies of mucosal neutrophil and eosinophil activation in inflammatory bowel diseases and irritable bowel syndrome. Gut 2004, 53, 1806–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Dong, W.; Liu, W.; Yan, Y.; Wan, P.; Peng, Y.; Xu, Y.; Zeng, X.; Cao, Y. 2-O-beta-d-Glucopyranosyl-l-ascorbic Acid, an Ascorbic Acid Derivative Isolated from the Fruits of Lycium Barbarum L. Modulates Gut Microbiota and Palliates Colitis in Dextran Sodium Sulfate-Induced Colitis in Mice. J. Agric. Food Chem. 2019, 67, 11408–11419. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2017, 18, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coccia, M.; Harrison, O.J.; Schiering, C.; Asquith, M.J.; Becher, B.; Powrie, F.; Maloy, K.J. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J. Exp. Med. 2012, 209, 1595–1609. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.L.; Wei, Z.; Min, W.; Hu, M.M.; Chen, W.L.; Xu, X.J.; Lu, C.J. Polysaccharides from Smilax glabra inhibit the pro-inflammatory mediators via ERK1/2 and JNK pathways in LPS-induced RAW264.7 cells. Carbohydr. Polym. 2015, 122, 428–436. [Google Scholar]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Larabi, A.; Barnich, N.; Nguyen, H. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henn, M.; O’Brien, E.; Diao, L.; Feagan, B.; Sandborn, W.; Huttenhower, C.; Wortman, J.; McGovern, B.; Wang-Weigand, S.; Lichter, D.; et al. A phase 1b safety study of SER-287, a spore-based microbiome therapeutic, for active mild to moderate ulcerative colitis. Gastroenterology 2021, 160, 115–127.e30. [Google Scholar] [CrossRef]
- Guo, M.; Li, Z. Polysaccharides isolated from Nostoc commune Vaucher inhibit colitis-associated colon tumorigenesis in mice and modulate gut microbiota. Food Funct. 2019, 10, 6873–6881. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guan, X.; Ding, W.; Luo, Y.; Wang, W.; Bu, W.; Song, J.; Tan, X.; Sun, E.; Ning, Q.; et al. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol. 2021, 166, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Deng, Z.; Lu, L.; Ouyang, Y.; Zhong, S.; Luo, T.; Fan, Y.; Zheng, L. Polysaccharides from soybean residue fermented by Neurospora crassa alleviate DSS-induced gut barrier damage and microbiota disturbance in mice. Food Funct. 2022, 13, 5739–5751. [Google Scholar] [CrossRef]
- Han, Y.; Song, M.; Gu, M.; Ren, D.; Zhu, X.; Cao, X.; Li, F.; Wang, W.; Cai, X.; Yuan, B.; et al. Dietary Intake of Whole Strawberry Inhibited Colonic Inflammation in Dextran-Sulfate-Sodium-Treated Mice via Restoring Immune Homeostasis and Alleviating Gut Microbiota Dysbiosis. J. Agric. Food Chem. 2019, 67, 9168–9177. [Google Scholar] [CrossRef]
- Johansson, M.E.; Gustafsson, J.K.; Holmén-Larsson, J.; Jabbar, K.S.; Xia, L.; Xu, H.; Ghishan, F.K.; Carvalho, F.A.; Gewirtz, A.T.; Sjövall, H.; et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 2014, 63, 281–291. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [Green Version]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Volk, J.K.; Nyström, E.E.L.; van der Post, S.; Abad, B.M.; Schroeder, B.O.; Johansson, Å.; Svensson, F.; Jäverfelt, S.; Johansson, M.E.V.; Hansson, G.C.; et al. The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function. J. Exp. Med. 2019, 216, 2602–2618. [Google Scholar] [CrossRef]
- Meng, J.; Banerjee, S.; Zhang, L.; Sindberg, G.; Moidunny, S.; Li, B.; Robbins, D.J.; Girotra, M.; Segura, B.; Ramakrishnan, S.; et al. Opioids Impair Intestinal Epithelial Repair in HIV-Infected Humanized Mice. Front. Immunol. 2020, 10, 2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagkouvardos, I.; Lesker, T.R.; Hitch, T.C.A.; Gálvez, E.J.C.; Smit, N.; Neuhaus, K.; Wang, J.; Baines, J.F.; Abt, B.; Stecher, B.; et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Tang, X.; Shao, X.; Han, D.; Zhang, H.; Shan, Y.; Gooneratne, R.; Shi, L.; Wu, X.; Hosseininezhad, M. Mulberry (morus atropurpurea roxb.) leaf protein hydrolysates ameliorate dextran sodium sulfate-induced colitis via integrated modulation of gut microbiota and immunity. J. Funct. Foods 2021, 84, 104575. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, H.C.; Lee, J.Y.; Kim, T.I.; Kim, W.H.; Kim, S.W.; Cheon, J.H. Lactobacillus plantarum CBT LP3 ameliorates colitis via modulating T cells in mice. Int. J. Med. Microbiol. 2020, 310, 151391. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, Å.; Tormo-Badia, N.; Baridi, A.; Xu, J.; Molin, G.; Hagslätt, M.L.; Karlsson, C.; Jeppsson, B.; Cilio, C.M.; Ahrné, S. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin. Exp. Med. 2015, 15, 107–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Wu, Y.; Hu, Y.; Zhao, L.; Zhang, C. Initial gut microbiota structure affects sensitivity to DSS-induced colitis in a mouse model. Sci.China Life Sci. 2018, 61, 762–769. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, M.S.; Bae, J.W. Blautia faecis sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 2, 599–603. [Google Scholar] [CrossRef]
| Score | Weight Loss (%) | Stool Consistency | Occult/Gross Bleeding |
|---|---|---|---|
| 0 | <1 | normal | negative |
| 1 | 1~5 | loose | weakly positive |
| 2 | 6~10 | loose stool | positive |
| 3 | 11~15 | very soft, wet | strong positive |
| 4 | >15 | watery diarrhea | blood traces in stool visible |
| Properties | |||||||
|---|---|---|---|---|---|---|---|
| Total Sugar/% | Protein/% | Uronic Acid/% | Mw/kDa | ||||
| SCP_A | 88.5 ± 0.6 | 4.1 ± 1.0 | 24.8 ± 1.4 | 14.8 | |||
| SCP_N | 86.7 ± 0.7 | 2.8 ± 0.7 | n.d. a | 31.2 | |||
| Molar Ratio | |||||||
| Fuc | Rha | Glu | Gal | Xyl | Gala | Glca | |
| SCP_A | 1.01 | 3.45 | 1.45 | 7.41 | 0.12 | 1.47 | 1.45 |
| SCP_N | 0.18 | 0.37 | 42.89 | 1.11 | 0.79 | n.d. a | n.d. a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Qiao, G.; Chu, L.; Lin, L.; Zheng, G. Smilax china L. Polysaccharide Alleviates Dextran Sulphate Sodium-Induced Colitis and Modulates the Gut Microbiota in Mice. Foods 2023, 12, 1632. https://doi.org/10.3390/foods12081632
Li X, Qiao G, Chu L, Lin L, Zheng G. Smilax china L. Polysaccharide Alleviates Dextran Sulphate Sodium-Induced Colitis and Modulates the Gut Microbiota in Mice. Foods. 2023; 12(8):1632. https://doi.org/10.3390/foods12081632
Chicago/Turabian StyleLi, Xin, Gaoxiang Qiao, Lulu Chu, Lezhen Lin, and Guodong Zheng. 2023. "Smilax china L. Polysaccharide Alleviates Dextran Sulphate Sodium-Induced Colitis and Modulates the Gut Microbiota in Mice" Foods 12, no. 8: 1632. https://doi.org/10.3390/foods12081632






