Effect of Steaming and Microwave Heating on Taste of Clear Soup with Split-Gill Mushroom Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Standards and Reagents
2.1.2. Ingredients
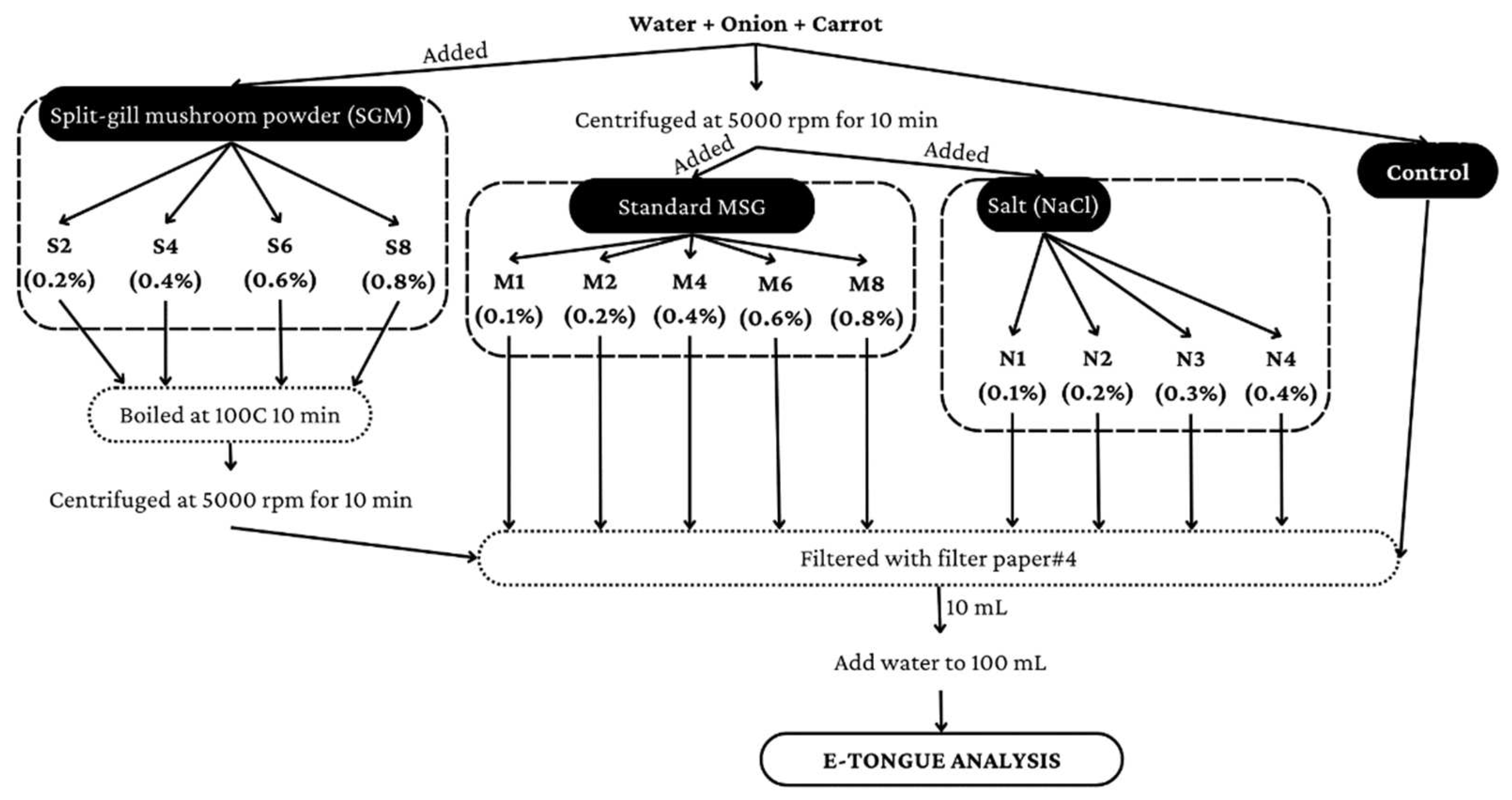
2.2. Sample Preparation
2.2.1. Clear Soup (without Seasoning and Spices) Preparation for Analysis with E-Tongue
2.2.2. Clear Soup (with Seasoning and Spices) Preparation for E-Tongue Analysis
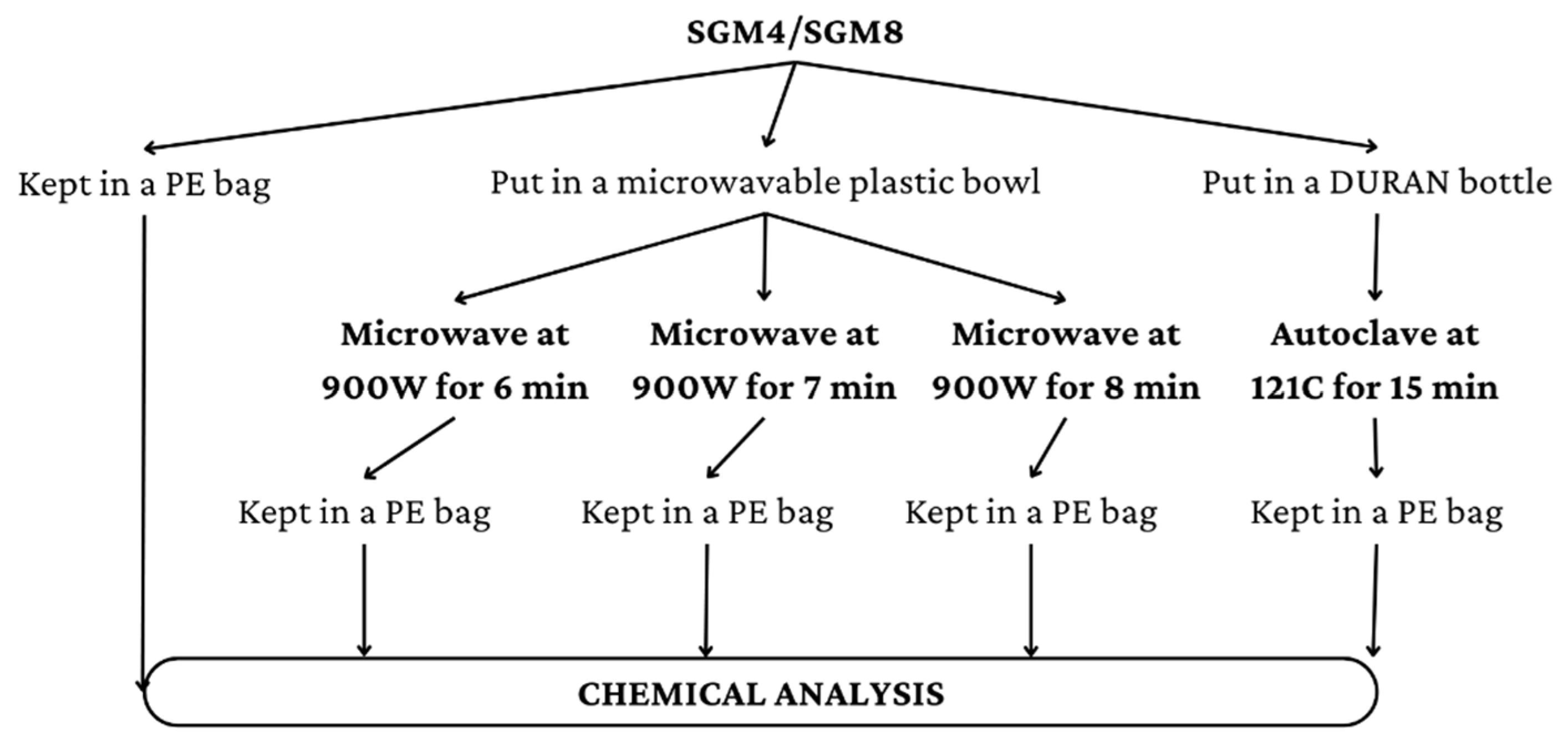
2.2.3. Clear Soup (with Seasoning and Spices) Preparation for Chemical Analysis
2.3. E-Tongue Analysis
2.4. Determination of Moisture, Protein, and Sodium Contents
2.5. 5′-Nucleotide Assay
2.6. Free Amino Acid Assay
2.7. Equivalent Umami Concentration (EUC)
2.8. Statistical Analysis
3. Results and Discussion
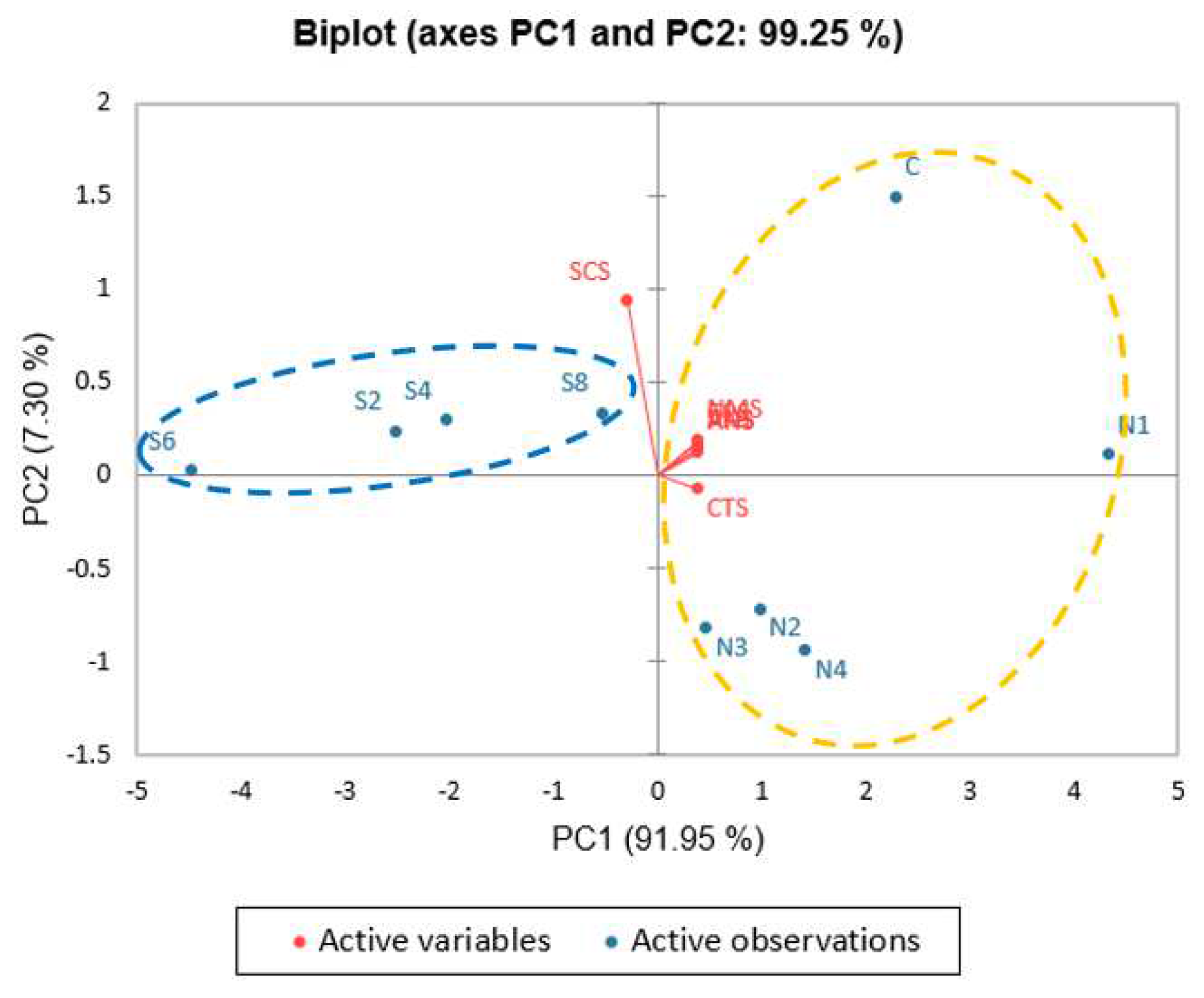
3.1. Taste Profile of Different Concentration of SGM in Clear Soup
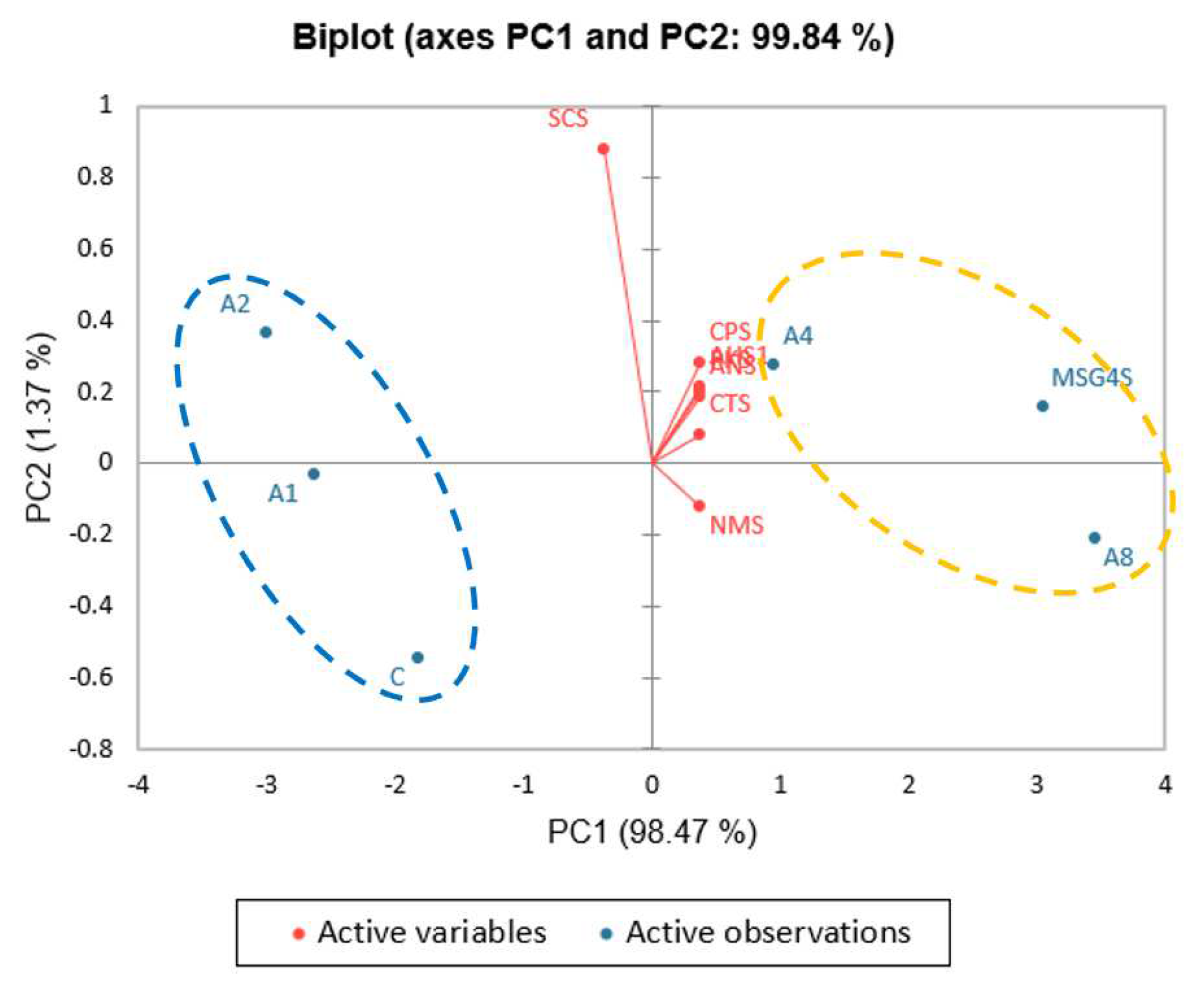
3.2. Moisture, Protein, and Sodium Contents and Taste Compounds of Clear Soups
3.3. Effect of Microwave Heating and Steaming under Pressure on Protein and Sodium Contents and Taste Compounds of Clear Soups
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Zhong, K.; Zhang, D.; Shi, B.; Wang, H.; Shi, J.; Battino, M.; Wang, G.; Zou, X.; Zhao, L. The enhancement of the perception of saltiness by umami sensation elicited by flavor enhancers in salt solutions. Food Res. Int. 2022, 157, 111287. [Google Scholar] [CrossRef]
- Salt Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 30 December 2022).
- Wang, C.; Lee, Y.; Lee, S.-Y. Consumer acceptance of model soup system with varying levels of herbs and salt. J. Food Sci. 2014, 79, S2098–S2106. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Song, H.; Zou, T.; Raza, A.; Li, P.; Li, K.; Xiong, J. Reduction of sodium chloride: A review. J. Sci. Food Agric. 2022, 102, 3931–3939. [Google Scholar] [CrossRef] [PubMed]
- Bobowski, N.; Vickers, Z. Determining sequential difference thresholds for sodium chloride reduction. J. Sens. Stud. 2012, 27, 168–175. [Google Scholar] [CrossRef]
- Maluly, H.D.B.; Arisseto-Bragotto, A.P.; Reyes, F.G.R. Monosodium glutamate as a tool to reduce sodium in foodstuffs: Technological and safety aspects. Food Sci. Nutr. 2017, 5, 1039–1048. [Google Scholar] [CrossRef]
- Wang, S.; Adhikari, K. Consumer perceptions and other influencing factors about monosodium glutamate in the United States. J. Sens. Stud. 2018, 33, e12437. [Google Scholar] [CrossRef]
- Selani, M.M.; Ramos, P.H.B.; Patinho, I.; França, F.; Harada-Padermo, S.d.S.; Contreras-Castillo, C.J.; Saldaña, E. Consumer’s perception and expected liking of labels of burgers with sodium reduction and addition of mushroom flavor enhancer. Meat Sci. 2022, 185, 108720. [Google Scholar] [CrossRef]
- Wang, S.; Tonnis, B.D.; Wang, M.L.; Zhang, S.; Adhikari, K. Investigation of monosodium glutamate alternatives for content of umami substances and their enhancement effects in chicken soup compared to monosodium glutamate. J. Food Sci. 2019, 84, 3275–3283. [Google Scholar] [CrossRef]
- Banerjee, A.; Mukherjee, S.; Maji, B.K. Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: An overview. Toxicol. Rep. 2021, 8, 938–961. [Google Scholar] [CrossRef]
- Wijayasekara, K.N.; Wansapala, J. Comparison of a flavor enhancer made with locally available ingredients against commercially available Mono Sodium Glutamate. Int. J. Gastron. Food Sci. 2021, 23, 100286. [Google Scholar] [CrossRef]
- Sun, L.-b.; Zhang, Z.-y.; Xin, G.; Sun, B.-x.; Bao, X.-j.; Wei, Y.-y.; Zhao, X.-m.; Xu, H.-r. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Harada-Padermo, S.d.S.; Dias-Faceto, L.S.; Selani, M.M.; Conti-Silva, A.C.; Vieira, T.M.F.d.S. Umami Ingredient, a newly developed flavor enhancer from shiitake byproducts, in low-sodium products: A study case of application in corn extruded snacks. LWT 2021, 138, 110806. [Google Scholar] [CrossRef]
- Guinard, J.-X.; Myrdal Miller, A.; Mills, K.; Wong, T.; Lee, S.M.; Sirimuangmoon, C.; Schaefer, S.E.; Drescher, G. Consumer acceptance of dishes in which beef has been partially substituted with mushrooms and sodium has been reduced. Appetite 2016, 105, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Mongkontanawat, N.; Thumrongchote, D. Effect of strains and extraction methods on β-glucan production, antioxidant properties, and FTIR Spectra from mushroom fruiting bodies of Schizophyllum commune Fr. in Thailand. Food Res. 2021, 5, 410–415. [Google Scholar] [CrossRef]
- Garcia, J.; Rodrigues, F.; Saavedra, M.J.; Nunes, F.M.; Marques, G. Bioactive polysaccharides from medicinal mushrooms: A review on their isolation, structural characteristics and antitumor activity. Food Biosci. 2022, 49, 101955. [Google Scholar] [CrossRef]
- Smirnou, D.; Knotek, P.; Nesporova, K.; Smejkalova, D.; Pavlik, V.; Franke, L.; Velebny, V. Ultrasound-assisted production of highly-purified β-glucan schizophyllan and characterization of its immune properties. Process Biochem. 2017, 58, 313–319. [Google Scholar] [CrossRef]
- Yelithao, K.; Surayot, U.; Lee, C.; Palanisamy, S.; Prabhu, N.M.; Lee, J.; You, S. Studies on structural properties and immune-enhancing activities of glycomannans from Schizophyllum commune. Carbohydr. Polym. 2019, 218, 37–45. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.-H.; Claver, I.P.; Zhu, K.-X.; Peng, W.; Zhou, H.-M. Effect of different cooking methods on the flavour constituents of mushroom (Agaricus bisporus (Lange) Sing) soup. Int. J. Food Sci. Technol. 2011, 46, 1100–1108. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, F.; Tian, J.; Ye, X.q.; Chen, J.; Sun, P. Domestic cooking methods affect nutrient, phytochemicals, and flavor content in mushroom soup. Food Sci. Nutr. 2019, 7, 1969–1975. [Google Scholar] [CrossRef]
- Zhang, Y.; Venkitasamy, C.; Pan, Z.; Wang, W. Recent developments on umami ingredients of edible mushrooms–A review. Trends Food Sci. Technol. 2013, 33, 78–92. [Google Scholar] [CrossRef]
- Ghawi, S.K.; Rowland, I.; Methven, L. Enhancing consumer liking of low salt tomato soup over repeated exposure by herb and spice seasonings. Appetite 2014, 81, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.; Brunton, N.P.; Wilkinson, M.G. Impact of salt reduction on the instrumental and sensory flavor profile of vegetable soup. Food Res. Int. 2011, 44, 1036–1043. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Toda, N.; Tamura, Y.; Terakado, S.; Ueno, M.; Otsuka, K.; Numabe, A.; Kawabata, Y.; Uehara, Y. Japanese traditional miso soup attenuates salt-induced hypertension and its organ damage in Dahl salt-sensitive rats. Nutrition 2012, 28, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Roininen, K.; Lähteenmäki, L.; Tuorilla, H. Effect of umami taste on pleasantness of low-salt soups during repeated testing. Physiol. Behav. 1996, 60, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Phat, C.; Moon, B.; Lee, C. Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system. Food Chem. 2016, 192, 1068–1077. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhu, Y.; Ben, A.; Qi, J. A novel strategy for discriminating different cultivation and screening odor and taste flavor compounds in Xinhui tangerine peel using E-nose, E-tongue, and chemometrics. Food Chem. 2022, 384, 132519. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Official Journal of the European Communities. L 257; Official Journal of the European Communities: Luxembourg, 16 September 1983; pp. 1–33. [Google Scholar]
- Çevikkalp, S.A.; Löker, G.B.; Yaman, M.; Amoutzopoulos, B. A simplified HPLC method for determination of tryptophan in some cereals and legumes. Food Chem. 2016, 193, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S. Basic properties of umami and effects on humans. Physiol. Behav. 1991, 49, 833–841. [Google Scholar] [CrossRef]
- Yang, F.; Lv, S.; Liu, Y.; Bi, S.; Zhang, Y. Determination of umami compounds in edible fungi and evaluation of salty enhancement effect of Antler fungus enzymatic hydrolysate. Food Chem. 2022, 387, 132890. [Google Scholar] [CrossRef]
- Wang, S.; Dermiki, M.; Methven, L.; Kennedy, O.B.; Cheng, Q. Interactions of umami with the four other basic tastes in equi-intense aqueous solutions. Food Qual. Prefer. 2022, 98, 104503. [Google Scholar] [CrossRef]
- Mleczek, M.; Budka, A.; Siwulski, M.; Mleczek, P.; Gąsecka, M.; Jasińska, A.; Kalač, P.; Sobieralski, K.; Niedzielski, P.; Proch, J.; et al. Investigation of differentiation of metal contents of Agaricus bisporus, Lentinula edodes and Pleurotus ostreatus sold commercially in Poland between 2009 and 2017. J. Food Compos. Anal. 2020, 90, 103488. [Google Scholar] [CrossRef]
- Zsigmond, A.R.; Varga, K.; Kántor, I.; Urák, I.; May, Z.; Héberger, K. Elemental composition of wild growing Agaricus campestris mushroom in urban and peri-urban regions of Transylvania (Romania). J. Food Compos. Anal. 2018, 72, 15–21. [Google Scholar] [CrossRef]
- Rotola-Pukkila, M.; Yang, B.; Hopia, A. The effect of cooking on umami compounds in wild and cultivated mushrooms. Food Chem. 2019, 278, 56–66. [Google Scholar] [CrossRef]
- Li, X.; Feng, T.; Zhou, F.; Zhou, S.; Liu, Y.; Li, W.; Ye, R.; Yang, Y. Effects of drying methods on the tasty compounds of Pleurotus eryngii. Food Chem. 2015, 166, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gu, Z.; Yang, Y.; Zhou, S.; Liu, Y.; Zhang, J. Non-volatile taste components of several cultivated mushrooms. Food Chem. 2014, 143, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.; Muniz, A.; Du, X. The impact of roasting and steaming on savory flavors contributed by amino acids, 5′-nucleotides, and volatiles in Agaricus bisporus mushrooms. Int. J. Gastron. Food Sci. 2022, 30, 100590. [Google Scholar] [CrossRef]
- Koutchma, T. Chapter 4-Microwave heating and quality of food. In Microwave and Radio Frequency Heating in Food and Beverages; Koutchma, T., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 81–111. [Google Scholar]
- Wang, X.; Feng, T.; Wang, X.; Xia, S.; Yu, J.; Zhang, X. Microwave heating and conduction heating pork belly: Non-volatile compounds and their correlation with taste characteristics, heat transfer modes, and matrix microstructure. Meat Sci. 2022, 192, 108899. [Google Scholar] [CrossRef]
- Nguyen, E.; Jones, O.; Kim, Y.H.B.; San Martin-Gonzalez, F.; Liceaga, A.M. Impact of microwave-assisted enzymatic hydrolysis on functional and antioxidant properties of rainbow trout Oncorhynchus mykiss by-products. Fish. Sci. 2017, 83, 317–331. [Google Scholar] [CrossRef]
- Hu, Q.; He, Y.; Wang, F.; Wu, J.; Ci, Z.; Chen, L.; Xu, R.; Yang, M.; Lin, J.; Han, L.; et al. Microwave technology: A novel approach to the transformation of natural metabolites. Chin. Med. 2021, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Jiang, C.; Yan, Y.; Li, C.; Fang, Z.; Hu, B.; Wang, C.; Chen, S.; Wu, W.; Li, X.; et al. Effect of different cooking methods on the nutrients, antioxidant and hypoglycemic activities of Pleurotus cornucopiae in vitro simulated digestion. Food Res. Int. 2022, 162, 112199. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Huang, H.; Huang, S.; Yang, M.; Wu, J.; Ci, Z.; He, Y.; Wu, Z.; Han, L.; Zhang, D. Insight into the incredible effects of microwave heating: Driving changes in the structure, properties and functions of macromolecular nutrients in novel food. Front. Nutr. 2022, 9, 941527. [Google Scholar] [CrossRef]
- Kutzli, I.; Weiss, J.; Gibis, M. Glycation of Plant Proteins Via Maillard Reaction: Reaction Chemistry, Technofunctional Properties, and Potential Food Application. Foods 2021, 10, 376. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Álvarez, R.G.; Vaka, M.R.; Rustad, T.; Sone, I.; Fernández, E.N. Potential of innovative pre-treatment technologies for the revalorisation of residual materials from the chicken industry through enzymatic hydrolysis. Innov. Food Sci. Emerg. Technol. 2020, 64, 102377. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci. Biotechnol. 2017, 27, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Asghari, L.; Zeynali, F.; Sahari, M.A. Effects of boiling, deep-frying, and microwave treatment on the proximate composition of rainbow trout fillets: Changes in fatty acids, total protein, and minerals. J. Appl. Ichthyol. 2013, 29, 847–853. [Google Scholar] [CrossRef]
- Poojary, M.M.; Orlien, V.; Passamonti, P.; Olsen, K. Improved extraction methods for simultaneous recovery of umami compounds from six different mushrooms. J. Food Compos. Anal. 2017, 63, 171–183. [Google Scholar] [CrossRef]
- Hu, S.; Feng, X.; Huang, W.; Ibrahim, S.A.; Liu, Y. Effects of drying methods on non-volatile taste components of Stropharia rugoso-annulata mushrooms. LWT 2020, 127, 109428. [Google Scholar] [CrossRef]
- Zhou, L.; Li, W.; Pan, W.; Sajid, H.; Wang, Y.; Guo, W.; Cai, Z.; Wang, D.; Yang, W.; Chen, Y. Effects of thermal processing on nutritional characteristics and non-volatile flavor components from Tricholoma lobayense. Emir. J. Food Agric. 2017, 29, 285–292. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, M.; Fan, H.; Liu, Y. Effect of microwave combined with ultrasonic pretreatment on flavor and antioxidant activity of hydrolysates based on enzymatic hydrolysis of bovine bone. Food Biosci. 2021, 44, 101399. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; San Martin-González, M.F.; Garcia-Bravo, J.; López Malo Vigil, A.; Liceaga, A.M. Physicochemical characteristics of chia seed (Salvia hispanica) protein hydrolysates produced using ultrasonication followed by microwave-assisted hydrolysis. Food Hydrocoll. 2019, 97, 105187. [Google Scholar] [CrossRef]
- Mau, J.L. The Umami Taste of Edible and Medicinal Mushrooms. Int. J. Med. Mushrooms 2005, 7, 113–119. [Google Scholar] [CrossRef]





| Samples | Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture (%wb.) | Mean Rank | Protein (%db.) | Mean Rank | NaCl (%wb.) | Mean Rank | Salinity (%wb.) | Mean Rank | ||
| SGM4 | Before heating | 98.24 ± 0.12 | 12.00 ab | 18.94 ± 0.36 | 8.00 ab | 0.22 ± 0.01 | 4.17 a | 1.30 ± 0.14 | 2.00 b |
| Microwave 6 min | 97.91 ± 0.03 | 8.00 ab | 20.15 ± 0.19 | 14.00 a | 0.26 ± 0.03 | 10.33 a | 1.80 ± 0.00 | 9.00 ab | |
| Microwave 7 min | 97.72 ± 0.03 | 5.00 ab | 18.63 ± 0.64 | 7.00 ab | 0.24 ± 0.06 | 9.33 a | 1.93 ± 0.11 | 12.00 a | |
| Microwave 8 min | 97.63 ± 0.02 | 2.00 b | 17.68 ± 0.38 | 2.00 b | 0.26 ± 0.00 | 10.00 a | 1.93 ± 0.11 | 12.00 a | |
| Steaming 15 min | 98.21 ± 0.00 | 13.00 a | 19.13 ± 0.12 | 9.00 ab | 0.23 ± 0.03 | 6.17 a | 1.60 ± 0.00 | 5.00 ab | |
| SGM8 | Before heating | 98.07 ± 0.02 | 14.00 a | 20.51 ± 0.25 | 2.00 b | 0.23 ± 0.01 | 3.33 b | 1.77 ± 0.06 | 2.33 b |
| Microwave 6 min | 97.72 ± 0.03 | 8.00 ab | 24.95 ± 0.16 | 11.67 ab | 0.24 ± 0.00 | 8.33 ab | 2.00 ± 0.00 | 9.50 ab | |
| Microwave 7 min | 97.66 ± 0.01 | 5.00 ab | 24.73 ± 0.10 | 8.00 ab | 0.24 ± 0.01 | 9.33 ab | 2.00 ± 0.00 | 9.50 ab | |
| Microwave 8 min | 97.57 ± 0.01 | 2.00 b | 25.15 ± 0.42 | 13.33 a | 0.26 ± 0.01 | 13.33 a | 2.10 ± 0.00 | 14.00 a | |
| Steaming 15 min | 97.92 ± 0.02 | 11.00 ab | 24.22 ± 0.16 | 5.00 ab | 0.23 ± 0.01 | 5.67 ab | 1.85 ± 0.07 | 4.67 ab | |
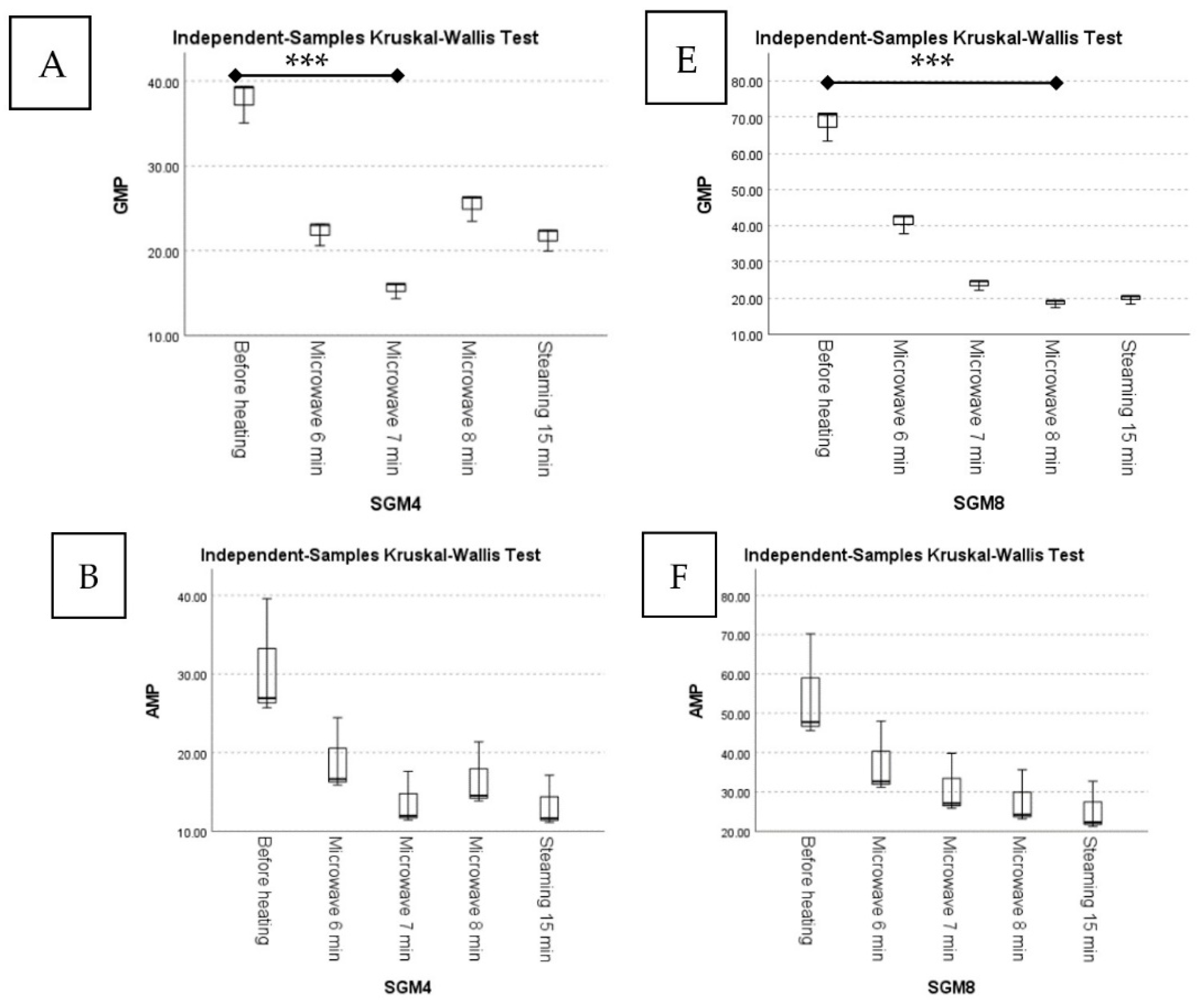
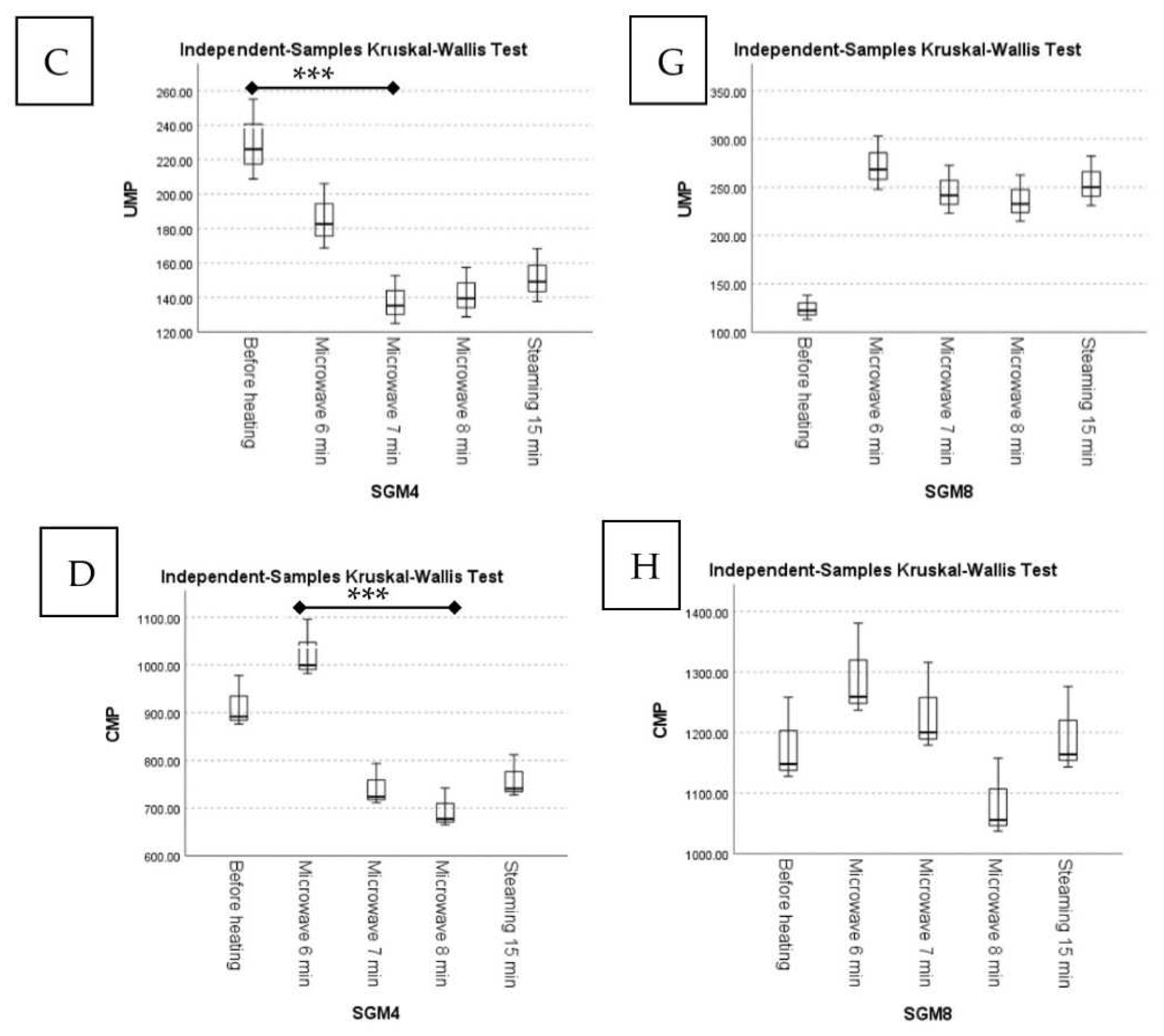
| Samples | 5′-Nucleotides (mg/100 g; Dry Weight) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5′-GMP | Mean Rank | 5′-AMP | Mean Rank | 5′-IMP | Mean Rank | 5′-UMP | Mean Rank | 5′-CMP | Mean Rank | Total Nucleotides | ||
| SGM4 | Before heating | 37.89 ± 2.45 | 14.00 a | 30.73 ± 7.68 | 14.00 a | n.d. | - | 229.98 ± 23.39 | 14.00 a | 915.12 ± 54.72 | 11.00 ab | 1213.72 |
| Microwave 6 min | 22.25 ± 1.44 | 7.33 ab | 18.99 ± 4.75 | 9.00 a | n.d. | - | 185.83 ± 18.90 | 11.00 ab | 1025.71 ± 61.33 | 14.00 a | 1252.78 | |
| Microwave 7 min | 15.51 ± 1.00 | 2.00 b | 13.67 ± 3.42 | 5.33 a | n.d. | - | 137.70 ± 14.00 | 3.67 b | 742.75 ± 44.41 | 5.00 ab | 909.63 | |
| Microwave 8 min | 25.37 ± 1.64 | 11.00 ab | 16.59 ± 4.15 | 7.33 a | n.d. | - | 141.89 ± 14.43 | 5.00 ab | 694.53 ± 41.53 | 3.33 b | 878.38 | |
| Steaming 15 min | 21.57 ± 1.39 | 5.67 ab | 13.31 ± 3.33 | 4.33 a | n.d. | - | 151.73 ± 15.43 | 6.33 ab | 759.78 ± 45.43 | 6.67 ab | 946.39 | |
| SGM8 | Before heating | 68.44 ±4.42 | 14.00 a | 54.53 ± 13.63 | 13.33 a | n.d. | - | 124.46 ± 12.66 | 2.00 a | 1177.94 ± 70.44 | 6.33 a | 1425.37 |
| Microwave 6 min | 41.01 ± 2.65 | 11.00 ab | 37.29 ± 9.32 | 9.67 a | n.d. | - | 273.05 ± 27.77 | 12.00 a | 1292.04 ± 77.26 | 12.33 a | 1643.39 | |
| Microwave 7 min | 23.88 ± 1.54 | 8.00 ab | 30.93 ± 7.73 | 7.33 a | n.d. | - | 245.70 ± 24.99 | 8.67 a | 1231.61 ± 73.65 | 10.33 a | 1532.12 | |
| Microwave 8 min | 18.58 ± 1.20 | 2.67 b | 27.67 ± 6.92 | 5.67 a | n.d. | - | 236.73 ± 24.07 | 7.33 a | 1083.39 ± 64.78 | 3.00 a | 1366.37 | |
| Steaming 15 min | 19.82 ± 1.28 | 4.33 ab | 25.40 ± 6.35 | 4.00 a | n.d. | - | 254.49 ± 25.88 | 10.00 a | 1194.55 ± 71.43 | 8.00 a | 1494.26 | |
| Amino Acid (mg/100 g; Dry Weight) | SGM4 | ||||
|---|---|---|---|---|---|
| Before Heating | Microwave 6 min | Steaming 15 min | χ2 (2) # | p-Value | |
| Glutamic acid | 1269.34 ± 21.59 | 1216.45 ± 5.33 | 1249.25 ± 28.96 | 3.714 | 0.156 |
| Aspartic acid | 602.34 ± 0.12 | 607.31 ± 0.19 | 552.75 ± 15.15 | 4.571 | 0.102 |
| Threonine | 208.83 ± 2.28 | 223.70 ± 0.62 | 201.71 ± 3.83 | 4.571 | 0.102 |
| Serine | 238.07 ± 6.26 | 255.63 ± 0.45 | 229.78 ± 3.92 | 4.571 | 0.102 |
| Glycine | 265.78 ± 4.02 | 280.13 ± 1.50 | 258.67 ± 10.90 | 3.714 | 0.156 |
| Alanine | 332.14 ± 12.02 | 339.73 ± 0.44 | 320.38 ± 9.13 | 2.000 | 0.368 |
| Cystine | n.d. | n.d. | n.d. | - | - |
| Valine | 233.88 ± 6.17 | 264.83 ± 4.64 | 240.78 ± 0.83 | 4.571 | 0.102 |
| Methionine | n.d. | n.d. | n.d. | - | - |
| Isoleucine | 163.54 ± 1.38 | 176.80 ± 3.16 | 160.62 ± 2.48 | 4.571 | 0.102 |
| Leucine | 316.23 ± 0.99 | 342.03 ± 0.81 | 307.64 ± 8.43 | 4.571 | 0.102 |
| Tyrosine | n.d. | <250 | n.d. | 5.000 | 0.082 |
| Phenylalanine | <250 | <250 | <250 | - | - |
| Histidine | 105.82 ± 8.04 | 110.28 ± 0.47 | <100 | 3.529 | 0.171 |
| Hydroxylysine | n.d. | n.d. | n.d. | - | - |
| Lysine | 207.22 ± 7.71 | 194.52 ± 6.70 | 153.90 ± 9.08 | 4.571 | 0.102 |
| Arginine | 687.21 ± 12.52 | 622.56 ± 2.97 | 438.99 ± 9.89 | 4.571 | 0.102 |
| Hydroxyproline | n.d. | n.d. | n.d. | - | - |
| Proline | 201.57 ± 3.63 | 222.39 ± 5.79 | <200 | 4.194 | 0.123 |
| Tryptophan | <150 | <150 | <150 | - | - |
| EUC (g/100 g) | 149.85 | 85.25 | 83.12 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiranpradith, V.; Therdthai, N.; Soontrunnarudrungsri, A. Effect of Steaming and Microwave Heating on Taste of Clear Soup with Split-Gill Mushroom Powder. Foods 2023, 12, 1685. https://doi.org/10.3390/foods12081685
Hiranpradith V, Therdthai N, Soontrunnarudrungsri A. Effect of Steaming and Microwave Heating on Taste of Clear Soup with Split-Gill Mushroom Powder. Foods. 2023; 12(8):1685. https://doi.org/10.3390/foods12081685
Chicago/Turabian StyleHiranpradith, Vimolpa, Nantawan Therdthai, and Aussama Soontrunnarudrungsri. 2023. "Effect of Steaming and Microwave Heating on Taste of Clear Soup with Split-Gill Mushroom Powder" Foods 12, no. 8: 1685. https://doi.org/10.3390/foods12081685
APA StyleHiranpradith, V., Therdthai, N., & Soontrunnarudrungsri, A. (2023). Effect of Steaming and Microwave Heating on Taste of Clear Soup with Split-Gill Mushroom Powder. Foods, 12(8), 1685. https://doi.org/10.3390/foods12081685






