Abstract
The antibiotic resistance phenomenon horizontally involves numerous bacteria cultured from fresh or processed seafood matrix microbiomes. In this study, the identified bacteria from food-producing processes and industrial environments were screened for phenotypic and genotypic resistance determinants. A total of 684 bacterial strains [537 from processed codfish (Gadus morhua and Gadus macrocephalus) products as salted and seasoned and soaked and 147 from environmental samples] were isolated. Antibiotic susceptibility tests showed resistance against tetracycline, oxacillin, and clindamycin in the Staphylococcus genus (both from food and environmental samples) and against beta-lactams (cefotaxime, carbapenems, etc.) and nitrofurans (nitrofurantoin) from E. coli and Salmonella enterica serovar. Enteritidis isolates. One-thousand and ten genetic determinants—tetracycline tetC (25.17%), tetK (21.06%), tetL (11.70%), clindamycin ermC (17.23%), ermB (7.60%), linezolid cfr (8.22%), optrA (3.62%), poxtA (2.05%), and oxacillin mecA (17.37%)—were amplified from Gram-positive resistant and phenotypically susceptible bacteria. Concerning Gram-negative bacteria, the beta-lactam-resistant genes (blaTEM, blaCIT, blaCTX-M, blaIMP, blaKPC, blaOXA-48-like) represented 57.30% of the amplified ARGs. This study found high antibiotic resistance genes in circulation in the fish food industry chain from the macro- to microenvironment. The obtained data confirmed the diffusion of the “antibiotic resistance phenomenon” and its repercussions on the One-health and food-producing systems.
1. Introduction
The increased failure of antibiotics for therapeutic purposes in human and animal medicines represents a crucial public health concern. Among bacterial pathogens, the zoonotic strains, both for fish and human species, have widely demonstrated (during their evolutive iter) consistent sanitary and economic implications in numerous countries. Relevant microbiological noxae, such as Listeria monocytogenes, Salmonella spp., Escherichia coli, and Staphylococcus aureus, acquired new resistance strategies against the commonly administrated molecules to treat their infections. The EFSA report denounced the critical increase of intoxication cases responsible for human death, and the notable numbers of panresistant nosocomial strains isolated from human hospital environments [1]. The strict correlation of resistance determinants between different bacteria (from food, animal, or human skin microbiomes) and antimicrobial treatment failures have led to a critical reduction of pharma efficacy. This condition has induced the survival of certain strains, which have also resulted in not being susceptible to modern, critically important antimicrobials [2]. Indeed, the wide administration or misuse of antimicrobial molecules in food-producing animals, and more specifically in conventional farming systems, have also contributed as further stimulus to the growth of this phenomenon [3]. The diffusion of the aquaculture zootechnic sector has led to high animal densities, and consequently, antibiotic therapies have become necessary to control potential infectious outbreaks. Indeed, due to the administrated antimicrobial molecules, many finfish species, i.e., Salmo salar, Oreochromis niloticus, Sparus aurata, Dicentrarchus labrax, and Gadidae Family (Gadus macrocephalus and G. morhua, etc.) have been widely farmed. Secondly, finfish farming has also had repercussions on the health of wild fish species, especially in the cases of improper wastewater management and improper administration of mariculture environments [4]. The lack of physical barriers, in combination with oceanic currents, involves a crucial role in the environmental diffusion of nucleotide-resistant forms (i.e., integrons, plasmids, etc.). In these conditions, microorganisms result as gene drivers. They were also defined as mobilized reservoirs by Loayza et al. [5] in the so-called antibiotic resistance genes’ (ARGs) environmental life cycle. A fascinating biochemical environmental aspect was explained by researchers demonstrating that high salt contents (i.e., marine waters, salted food matrices, etc.) are responsible for improved phenotypic expression to many antibiotics. This chemical language is translated into different membrane proteins, inducing structural modifications to the receptors (i.e., ion pumps) becoming not susceptible [6]. Indeed, ARGs were largely amplified from many fresh and salted fish products obtained from caught animals, i.e., Salmo salar and Oreochromis niloticu, and successively handled in many food production chains. This last consideration suggests that the microbiological impacts on food spoilage are strongly influenced by cross-contamination due to the improper application of hygienic measures (in agreement with EU Reg. No. 852/2004) [7]. The European Food Safety Authority [1] highlighted that food matrices and their relative microbiomes could represent potential sources for the horizontal transmission of ARGs to the final consumers [5,8,9]. Many authors, based on the biomolecular ARG trades between food matrices and human microbiota, performed PCR assays, amplifying numerous genetic determinants from many bacterial species isolated in fresh fillets (fish and mammalian species). Molecular tests were designed to discover nucleotide sequences, which codify resistance against the most frequently administrated antibiotic molecules in veterinary and human medicine (i.e., tetracyclines, beta-lactams, and quinolones) [10,11,12,13]. They discovered the phenotypic expression of many antibiotic resistance patterns against the veterinary legally permitted molecules and the so-called Critical Important Antimicrobials (CIA), whose usage is strongly indicated for humans only [2]. Among the different finfish species, G. macrocephalus and G. morhua are largely caught wild; however, the aquaculture systems, mainly located in Northern Europe (i.e., Norway, Scotland, etc.), involve a strategic role. Indeed, they have improved production volumes in an attempt to satisfy the high demand for animal-origin protein for human and animal feeding. More specifically, the Gadidae Family has specific nutritional characteristics: high protein and low-fat contents. Their processed products, known as salted and seasoned codfish in European and South American countries, can provide the necessary nutrients to the human diet [14,15]. These processed fish matrices were poorly screened for antibiotic susceptibility or ARG detection (with special regard to horizontal gene transmission). Therefore, the objective of this research study was to provide antibiotic resistance profiles as the phenotypic expression of resistance genes amplified from isolated bacterial strains. These were obtained from differently processed codfish products (belonging to the fish species G. macrocephalus and G. morhua) and the environment along an integrated industrial supply food chain. This biomolecular study wants to provide preliminary data regarding antibiotic susceptibility tests and ARG detection starting from a culture-dependent investigation of bacterial strains isolated in the codfish industry.
2. Materials and Methods
2.1. Samples Collection
2.1.1. Food Matrices
A total of 450 finfish products belonging to the Gadidae Family were involved in the sampling activities. Half of them were G. macrocephalus caught in the FAO zone 67 (Northern Pacific Ocean) and the others were G. morhua caught in the FAO zone 27 subarea IIa (Norway Sea, Atlantic Ocean). In accordance with European legal requirements EU Reg No. 1276/2011 and EU Reg. 625/2017, the primary producers immediately deheated and eviscerated all fish after catching. This last measure is considered necessary to reduce the migration of Anisakis spp. larval forms (L3 stage) from fish intestine to muscle tissues. These fish were successively salted and seasoned. After these steps, samples were imported by an industrial producer and were sectioned, producing fillets (muscle and skin tissues) characterized by an average weight of 400 ± 20 g/fillet. In accordance with consumer requests, many fish industries apply innovative technological processes, i.e., the soaking process and the subsequent exposure to High-Pressure Procedures (HPPs), to provide ready-to-cook and microbiologically safe food matrices [16]. These measures have resulted in being able to prolong the shelf life of products [17]. More specifically, 225 specimens collected from the two screened fish species were composed of three groups formed by 75 salted and seasoned (SD), 75 soaked (SP) and 75 HPP-treated samples. In agreement with international standardized methods, the soaking process was successively performed by the industrial fish producer in steel tanks using refrigerated waters +3 °C for 5–6 days. During this step, water was changed three times per day at regular intervals, as reported by Rode and Rotabaak [15]. After the soaking process, the same products were microbiologically stabilized by exposing them to the HPP technology. It was performed using the QFP 2L-700 system manufactured by Avure TechnologiesTM—HPP (Middletown, OH, USA) at the machine setting of 600 MPa for 5 min due to its high bactericidal impact, as previously described [15].
2.1.2. Process Samples
One hundred processing samples (as food operator hands and industrial surface swabs) were also included in this study. More specifically, 50 specimens were collected from hand and 50 from surfaces, which usually were in contact with fish food products. Samples collections were performed after cleaning, washing, and disinfection procedures, as mentioned in the HACCP industrial document. The swabbing procedures were applied for their microbiological screenings (Sterile Swab Stick—Genorex Medsolutions, Suzhou City, Jiangsu Province, China) covering a total area of 100 cm2, in accordance with the UNI EN ISO 18593:2018. After collection, all samples were transported under refrigerated conditions and processed until 8 h from receipting.
2.2. Qualitative Microbiological Screenings
2.2.1. Food Matrices
From each fish fillet sample type, two aliquots of 25 g and one of 10 g were sterilely collected using mono-usage scalpels (Monopec Scalpels, Thermo Fisher ScientificTM, Waltham, MA, USA). They were introduced in sterile stomacher bags (BagMixer®, Interscience, Puycapel, Cantal, France) in accordance with ISO 6887-3:2017. Thus, from each fish fillet, a total of three muscle tissue parts were collected for Enterobacteriaceae, Listeria monocytogenes, Pseudomonas spp., Staphylococcus spp., and Vibrio spp. qualitative detection. The first 25 g were diluted with 225 mL of Buffered Peptone Water (BPW) for Enterobacteriaceae, Enterococcus spp., and Pseudomonas spp. and the others in 225 mL Alkaline Peptone Water (APW) for halotolerant strains (Staphylococcus spp. and Vibrio spp.). The last 10 g were diluted with 10 mL of supplemented (Half Fraser Supplement, ThermoFisher Scientific, Waltham, MA, USA) and Half Fraser Broth (HFB) (Half Fraser Broth, ThermoFisher Scientific, Waltham, MA, USA) for Listeria monocytogenes selective culturing. All performed dilutions were performed following the ratio 1:10. After these steps, samples were stomached for 60 s and incubated at 37 °C for 24–36 h. From each broth, an aliquot was directly plated onto specific and supplemented agar media and successively incubated (in accordance with the culturing procedures mentioned in the referenced International Standards reported in Table 1). After this step, on the respective selective media, colonies’ morphological aspects were also considered as preliminary factors for identification, in agreement with the procedures (Table 1), but also supported by the following reported procedures.

Table 1.
Selective culturing agar and broth media for bacterial pathogens (according to the EU Reg. 2073/2005) used in the present investigation.
2.2.2. Environmental and Personnel Sample Collections
The swabbing method was used through sterile swabs and delimitators for 100 cm2 surfaces (Syntesys Disponsable Labware, Padova, Italy) and swabbing was performed directly from food operators’ hands. The collected samples were directly plated onto specific agar culture media for a qualitative investigation of Staphylococcus spp. and Enterobacteriaceae. These microbiological parameters were selected as quality and efficacy indicators of industrial hygienic preoperative sanitary measures (applied by the food operators along the productive lines). However, the other mentioned microorganisms (i.e., Listeria monocytogenes) were also considered. All strains were isolated according to the international standardized methods, as mentioned in detail in Table 1. After plating, specimens were incubated at 37 °C for 24–36 h.
2.3. Bacterial Identification and Antibiotic Susceptibility Tests (ASTs)
The bacterial identification and AST evaluations were performed using the biochemical automated method, VITEK® 2 system, following the manufacturer’s procedures (bioMérieux, Paris, France). Gram-negative and positive strains were identified, obtaining results after incubation periods of 8 h from sample processing. Specific cards, VITEK® ID-GN and VITEK® ID-GP (VITEK® 2 system, bioMérieux, Paris, France), were loaded and processed following the producer’s instructions. The biochemical assays were successively confirmed through the mass spectrometry MALDI-TOF (Matrix Assisted Laser Desorption Ionization—Time of Flight). The ASTs were also performed with the VITEK® 2 system (bioMérieux, Paris, France) as an automated device providing results between 22–24 h from samples’ loading. Parallelly to the antibiogram assays, following the same protocol, the Minimum Inhibitory Concentration (MIC) values were calculated for each resistant bacterial strain. Bacterial suspensions, with a final density of 0.5 McFarland standard, were realized. A final volume of 280 µL for Gram-negative strains and 145 µL for Gram-positive bacteria were collected from the suspensions and added to 3 mL of VITEK 0.45% saline solution. Gram-negative ASTs were performed using the card named AST-N379, which tested 16 antibiotic molecules (amikacin, amoxicillin/clavulanic acid, cefepime, cefotaxime, ceftazidime, ciprofloxacin, colistin, ertapenem, ESBL, fosfomycin, gentamycin, imipenem, meropenem, nitrofurantoin, and sulfamethoxazole) belonging to the most frequently administrated antibiotics in veterinary medicine and part of the CIA lists.
The AST-P658 and AST-P659 were used for Gram-positive strains, including 26 antibiotics (amoxicillin/clavulanic acid, ampicillin, ciprofloxacin, daptomycin, gentamicin, imipenem, kanamycin, levofloxacin, linezolid, nitrofurantoin, quinupristin/dalfopristin, streptomycin, teicoplanin, tigecycline, trimethoprim, trimethoprim/sulfamethoxazole, vancomycin, benzylpenicillin, cefoxitin, cefazoline, clindamycin, erythromycin, mupirocin, oxacillin, rifampicin, and tetracycline). The obtained susceptibility results were elaborated in accordance with the Clinical & Laboratory Standards Institute (CLSI) breakpoints determined as relevant for humans [24]. Bacterial isolates that resulted resistant to three or more different antibiotics were classified as multidrug resistant (MDR), as previously reported by Magiorakos et al. [25].
MRS and MSS Staphylococcus spp.
Before PCR assays, all identified Staphylococci were also tested using the CHROMID® MRSA—bioMérieux—Culture Media (Paris, France) as possible MRS strains. After this first analysis and ASTs, all of them were also bimolecularly screened for detection of the so-called mecA gene, which is responsible for methicillin and oxacillin resistance patterns. The phenotypic and the genotypic confirmation permitted classifying Staphylococci as methicillin-resistant (MRS) or methicillin-susceptible (MSS). Specific primers, designed by McClure et al. [26], and their respective PCR setting parameters were performed.
2.4. Biomolecular Assays
Bacterial DNA Extraction and ARG Screening
A culture-dependent approach was performed both for food matrices and process samples. The DNA extracts were obtained using the High Pure PCR Template Preparation Kit (Roche®, Indianapolis, IN, USA), obtaining final volumes of 100 μL. These aliquots were stored at −20 °C until their biomolecular screenings. The PCR assays, performed as uniplex or multiplex reactions, were realized introducing specific primers in the reaction volumes, as reported in Table 2. The screened ARGs included the veterinary legally permitted molecules as beta-lactams (blaTEM, blaCTX-M, blaCIT,) and tetracycline (tetA, tetC, tetM, tetK, tetL), and the CIAs as vancomycin (vanA, vanB, vanC1, vanC2, vanD, vanM, vanN), carbapenems (blaIMP, blaNDM, blaOXA-48-like, blaKPC), etc. [2]. A list of all tested genes is included in Table 2. The thermocycler and annealing settings were in accordance with their respective reference indications.

Table 2.
Target genes used for ARG screening in the present study.
The PCR reactions were performed in a total volume of 25 μL/reaction using the Green Master Mix Promega® (Milano, Italy) and then 1 μL of the extracted DNA was added to the respective tube. The amplification conditions were performed following the procedures indicated in the respective references, which are listed in Table 1. This step was followed by electrophoresis, which was realized by loading the obtained amplicons on the agarose gels (1.0–1.5% depending on band sizes, setting at 80 Volt for 30 min). The nitid bands were compared with specific DNA ladders (Genetics, FastGene 100 bp or 100–1000 bp DNA Marker, Düren, Germany). The suspected positive samples were purified using the Qiagen QIAquick® PCR Purification Kit (Hilden, Germany). The obtained oligonucleotide specimens were successively sequenced by BioFab Research (Rome, Italy) through the Sanger method. The nucleotide similarities analyses were performed using the BLASTN system (http://www.ncbi.nlm.nih.gov/genbank/index.html) (accessed on 23 July 2022).
2.5. Statistical Analysis
The statistical analysis was performed using the SPSS 20.0 (SPSS, Chicago, IL, USA). The t test was applied to compare differences between resistant isolated bacteria both from food matrices and the environment. The same test was also used to analyze ARG frequencies. Results were considered statistically significant in the case of a p value < 0.05. For all prevalence percentage values, confidence intervals (CI 95%) were calculated when applicable.
3. Results
3.1. Bacterial Identification
In this biomolecular study, a total of 684 bacterial strains were isolated from 450 fish food matrices and 100 process samples (including food operator hands and industrial surfaces). Four hundred and twenty-five Gram-positive (62.13%) and 259 Gram-negative (37.87%) strains were identified from all specimens, as illustrated in Table 3.

Table 3.
Identified bacterial strains in this research study.
Staphylococcus genus was the most identified in both specimens. More specifically, 73.17% (95% CI: 68.96–77.38%) or 311/425 Gram-positive isolates belonged to the mentioned genus. Two-hundred forty-seven out of 311 (79.42% 95% CI: 74.93–83.91%) were isolated from food matrices, while 64/311 (20.57% 95% CI: 16.08–25.06%) were isolated from process specimens (See Table 4).

Table 4.
Identified strains belonging to the Staphylococcus genus.
Enterococcus spp. was also widely identified, representing 16.0% or 68/425 (95% CI: 12.52–19.48%) of Gram-positive bacteria. Forty-four out of 68 isolates were Enterococcus faecalis, representing 64.7% (95% CI: 53.35–76.05%), followed by Enterococcus durans 17/68 or 25.0% (95% CI: 14.71–35.29%), and, finally, 7/68 Enterococcus faecium, representing 10.29% (95% CI: 3.07–17.51%). Among the Gram-positive bacteria, 35/425 (8.23% 95% CI: 5.62–10.84%) were Kocuria spp., and specifically, 24/35 were classified as Kocuria kristinae (68.57% 95% CI: 53.19–83.95%), and 11/35 as Kocuria varians (31.42% 95% CI: 16.05–46.79%). The least-identified genus was Micrococcus spp., with 11/425 (2.58% 95% CI: 1.08–4.08%). Most of these microorganisms were isolated from SD and SP products. Among the isolated Gram-negative bacteria, two bacterial species (reported in the EU Reg. No. 2073/2005) were identified, 18 Escherichia coli and two Cronobacter sakazakii, and one Salmonella enterica serovar Enteritidis from food matrices. Further detailed information is reported in Table 5.

Table 5.
Identified Gram-negative strains in this research study.
The most representative Gram-negative microorganisms were Acinetobacter lwofii 62/259 (23.93% 95% CI: 18.74–29.12%), S. paucimobilis 50/259 (19.30% 95% CI: 14.50–24.10%), Pseudomonas luteola 41/259 (15.83% 95% CI: 20.27–11.39%), S. fonticola 37/259 (14.28% 95% CI: 10.02–18.54%), and P. fluorescens 27/259 (10.42% 95% CI: 6.70–14.14%).
3.2. AST Results
Regarding the AST results, wide phenotypic resistances were observed in the Gram-positive isolates against tetracycline, 312/425 or 73.41% (95% CI: 69.21–77.61%), and lincomycin (clindamycin), 201/425, of which 47.29% (95% CI: 42.55–52.03%) were resistant against other antibiotic classes, as shown in Table 6 and Table 7. One-hundred ninety-nine out of 311 bacteria (63.98% 95% CI: 58.65–69.31%), belonging to the Staphylococcus genus, were also resistant to oxacillin. Additionally, the negative coagulase Staphylococcus spp. resulted in being resistant to linezolid (13.18% 95% CI: 9.42–16.94%), oxacillin (63.98% 95% CI: 58.65–69.31%), and vancomycin (14.79% 95% CI: 10.85–18.73%). The resistance patterns against vancomycin and linezolid were widely observed in Enterococcus spp., at the rate of 22/68 32.35% (95% CI: 21.23–43.47%) and 33/68 48.52% (95% CI: 37.34–59.70%), respectively. Further detailed information regarding AST results is reported in Table 6.

Table 6.
Gram-positive bacterial strains: the phenotypic resistance patterns.

Table 7.
Gram-negative bacterial strains: the phenotypic resistance patterns.
Among the 425 Gram-positive strains, 230 bacteria, mostly isolated from SD and SP products before HPP treatment, resulted resistant to at least three antibiotic molecules belonging to different classes (beta-lactams, glycopeptide, lincomycin, oxazolidinone, and tetracycline). For this reason, these strains were classified as multidrug resistant (MDR). Specifically, 192/311 Staphylococci isolates (61.73% 95% CI: 56.33–67.13%) and 38/68 Enterococci isolates (55.88% 95% CI: 44.08–67.68%) resulted MDR, as indicated in Table 6 and Table 7. Six out of 46 isolated Kocuria spp. strains (13.04% 95% CI: 3.31–22.77%) were resistant only to tetracyclines.
More specifically, tetracyclines showed higher phenotypic resistance patterns than the other tested molecules among the Staphylococcus genus: 76.47% (95% CI: 46.64–92.88%) of S. aureus, 82.95% (95% CI: 75.09–90.81%) of S. sciuri, 87.50% (95% CI: 80.60–87.57%) of S. lentus, and 89.33% (95% CI: 82.35–96.31%) of S. saprophyticus strains. A similar result was observed among the Enterococcus faecalis genus [79.54% (95% CI: 67.62–91.46%)] (MIC values are illustrated in Table 6).
The 259 Gram-negative bacteria isolated from food matrices and process specimens mostly showed resistance against the cefotaxime molecule (beta-lactam class): 31/259 isolates corresponding to 11.96% (95% CI: 8.01–15.91%), as illustrated in Table 7.
Four out of 41 P. luteola (9.75% 95% CI: 0.75–18.75%) were classified as MDR due to their phenotypic resistance patterns against beta-lactams, carbapenem, and nitrofuran. Finally, only 2/18 E. coli strains (11.11% 95% CI: 0.37–15.89%) resulted MDR, showing similar resistance results to the above-mentioned antibiotic classes. Ten out of 50 Sphingomonas paucimobilis isolates (20.00% 95% CI: 8.92–31.08%) were not susceptible to the cefotaxime molecule. Gram-negative MDR bacteria were mostly isolated from salted and seasoned products.
3.3. ARG Detection
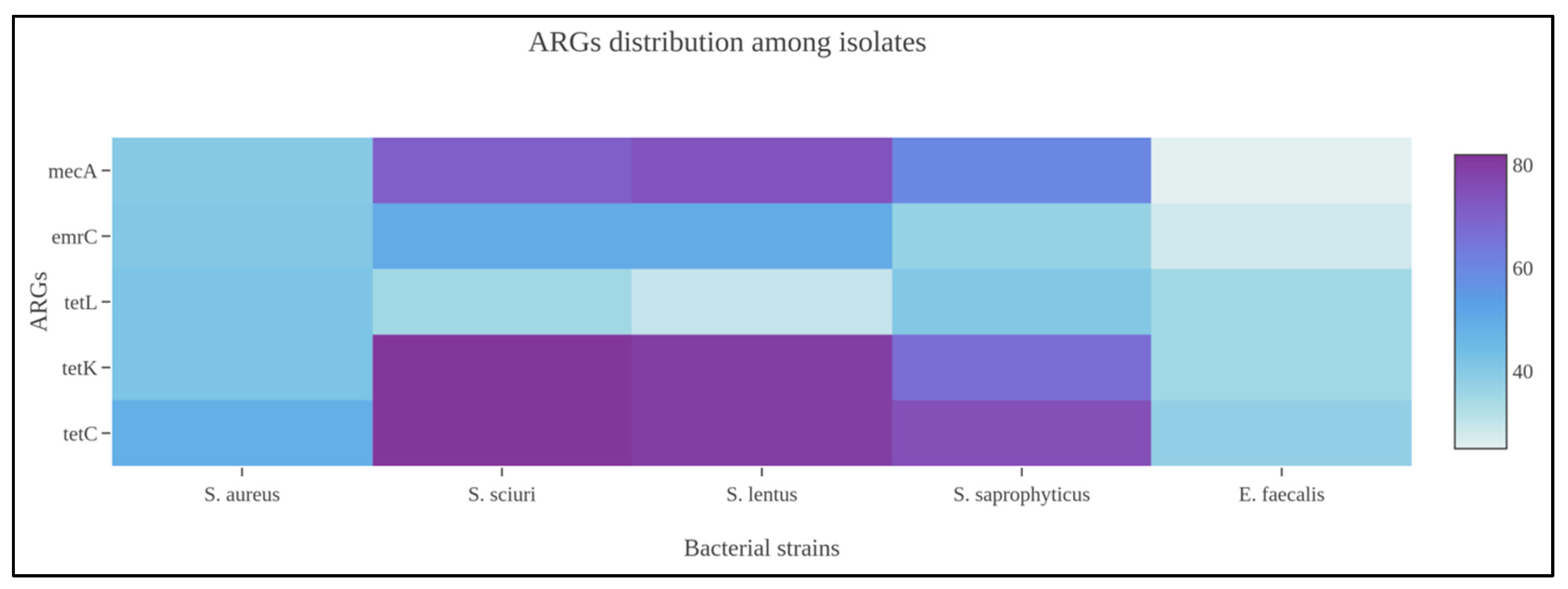
Among Gram-positive strains, and more specifically the Staphylococcus spp., tetracycline ARGs (tetC, tetK, tetL), clindamycin (ermC), and oxacillin (mecA) were widely amplified by the biomolecular assays from food and process specimens. At same time, the genotypic screening identified numerous unencoded ARGs to other antibiotic classes, i.e., aminoglycosides, beta-lactams, carbapenems, and sulfonamides. Concerning MRS or MSS strains, 11/51 or 21.56% (95% CI: 10.28–32.84%) bacteria, which phenotypically resulted susceptible to oxacillin, also harbored the mecA gene. Comparing the mecA gene amplification, discovered from Staphylococci isolated in food matrices and environmental specimens, there was a statistical difference with a p-value < 0.001. As previously observed, Enterococcus spp. also showed ARGs against tetracycline, clindamycin, and linezolid antibiotic molecules. From a statistical perspective, the Staphylococcus genus harbored numerous ARGs presenting a statistical difference if compared with Enterococcus spp. (p-value: 0.001), as illustrated in Figure 1.
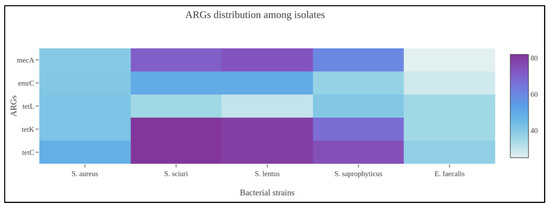
Figure 1.
Heatmap representing ARG distribution among Staphylococcus spp. identified in the present scientific investigation.
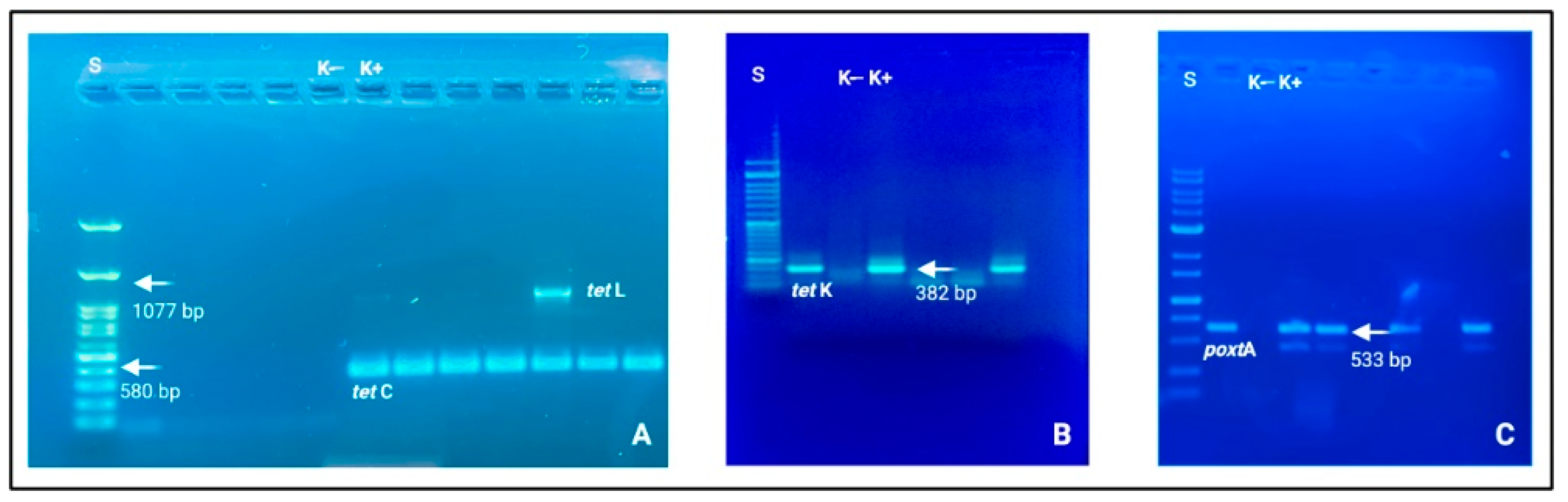
The corresponding genetic determinants were identified in all phenotypically resistant isolates. More specifically, the obtained AST data can be considered the translation results of specific ARGs. Among Gram-positive strains, Staphylococcus and Enterococcus genera were the prevalent microbial populations, as reported above, and tetracycline ARGs were largely amplified. These patterns were supported by the biomolecular amplification of different gene determinants, i.e., tetC, tetK, and tetL (See Figure 2).
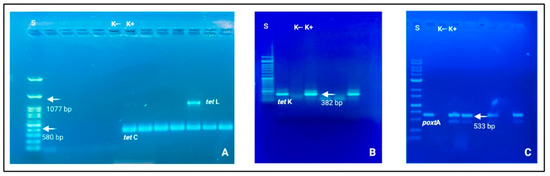
Figure 2.
Electrophoresis gels (agarose 1.0 and 1.5%) in which it is possible to observe nitid positive amplicons and amplification results. (A) tetC (580 bp) and tetL (1077 bp); (B) tetK (382 bp); (C) poxtA (533 bp). Loading wells: S: DNA ladder 1 Kb (Genetic® FastGene 100–10,000 bp DNA Marker, Düren, Germany); K−: negative control and K+: positive control.
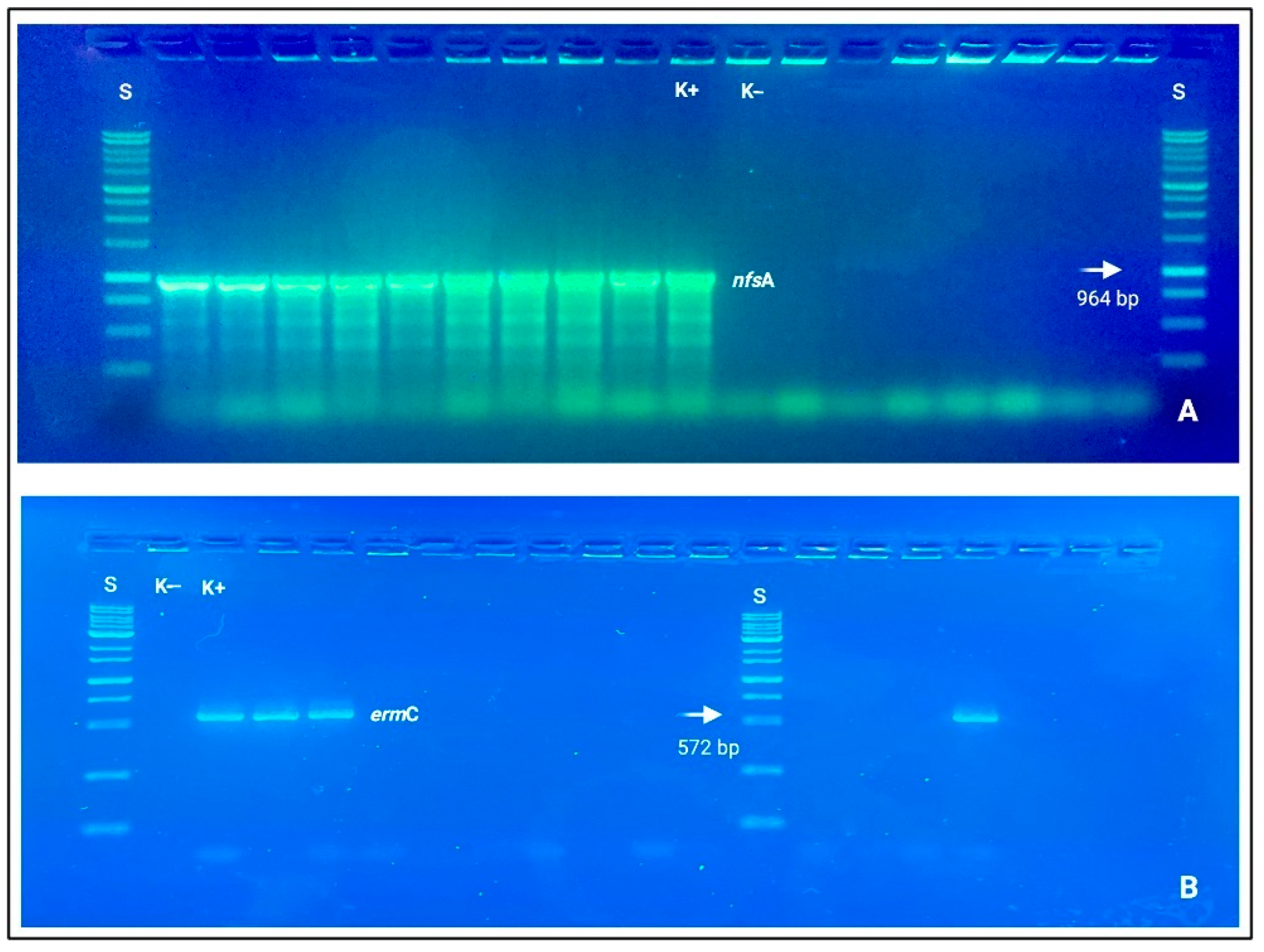
Clindamycin resistance was also genotypically based on ermB and ermC detections. Concerning Gram-negative resistant bacteria, Pseudomonas spp. and E. coli strains mostly covered a driver role for beta-lactams (blaTEM, blaCIT, and blaCTX-M), carbapenems (blaIMP, blaKPC, blaOXA-48-like), and nitrofurantoin (nfsA and nfsB), which resulted statistically different from the other bacterial species Acinetobacter spp., Sphingomonas spp., and Serratia spp. with a p-value < 0.001 (See Figure 3).
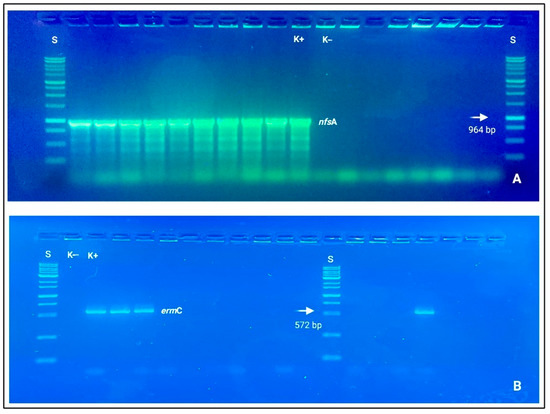
Figure 3.
Electrophoresis gels (agarose 1.0%), in which it is possible to observe nitid positive amplicons and amplification results. (A) nfsA (964 bp); (B) ermC (572 bp). Loading wells: S: DNA ladder 1 Kb (Genetic® FastGene 100–10,000 bp DNA Marker, Düren, Germany); K−: negative control and K+: positive control.
Among Gram-negative DNA, specific ARGs, belonging to the beta-lactam antibiotic class, were mainly amplified: blaTEM (4.25% 95% CI: 3.20–5.30%), blaCIT (1.84% 95% CI: 1.13–2.55), blaCTX-M (2.05% 95% CI: 1.31–2.79%), blaIMP (1.49% 95% CI: 0.86–2.12%), blaKPC (1.49% 95% CI: 0.86–2.12%), and blaOXA-48-like (0.42% 95% CI: 0.09–0.75%). A complete scheme representing ARG distributions are reported in Table 8.

Table 8.
ARGs detection from resistant and susceptible bacterial strains.
3.4. Statistical Analysis
From a macro perspective, the studied identified bacteria were mostly isolated from SD and SP products from both fish species. Significant differences were observed between SD and SP bacterial amounts (p-values < 0.0001) and between SP and HPP-treated products (p-value: 0.002). At the same time, there was also a significant difference comparing the number of microorganisms isolated from food operator hands and surfaces (p-value < 0.001). Concerning the AST results, the difference between Gram-positive and negative bacteria (isolated from foodstuffs) was statistically significant (p-value: 0.002).
From a biomolecular point of view, the statistical analysis also showed a significant difference in the ARG distributions between food matrices and process specimens (p-value < 0.001). Focusing on the food-isolated-resistant strains, the gene amplification amounts between SD, SP, and HPP-treated samples (both screened fish species) were statistically significant with p-values < 0.0001. More specifically, tetracycline genes (tetC and tetK), amplified from SD and SP in Staphylococcus spp., resulted statistically different (p-value < 0.0001). A similar result was observed for clindamycin ARGs [ermB (p-value: 0.001) and ermC (p-value < 0.001) genes] and oxacillin [mecA (p-value < 0.001)] between SD and SP specimens. BlaTEM (beta-lactams), blaIMP, and blaOXA-48-like (carbapenems) were differently amplified between SD and SP samples in both studied fish species (p-values: 0.001). More specifically, blaTEM and blaIMP presented statistically significant differences with p-values < 0.001 between SD and SP specimens. Based on the same comparison, blaOXA-48-like showed a p-value of 0.015.
4. Discussion
This study performed a culture-dependent and biomolecular investigation focused on the AMR phenomenon and, more specifically, on the circulation of ARGs at the fish food industry level. These screenings involved the Gadidae Family, specifically, the G. morhua and G. macrocephalus finfish species, which are normally used for salted and seasoned codfish production [17]. All analytical steps were also organized and performed following the industrial processes, starting from raw and processed fish food matrices, including operator hands and industrial surfaces (the environmental variable). The ASTs were designed adapting to the screened industrial productive system or line peculiarities. The screened gene targets, for molecular biology assays, included legally permitted antibiotics for veterinary medicine (especially in the aquaculture zootechnic sector) and the so-called Critical Important Antimicrobials (CIA), the usage of which has been strongly recommended for humans only [2].
Gram-positive strains were mostly identified both from food matrices and process specimens, i.e., food operator hands. Among the halotolerant bacteria, the Staphylococcus spp., positive and negative coagulase species, was predominant, with 73.17% (95% CI: 68.96–77.38%) of the identified bacteria. These data were in line with previous studies on microbial communities (pathogens and spoilage bacterial species) that studied salted and seasoned products (belonging to the Gadidae Family) [14,40,41]. Among the food hygiene criteria (reported in the EU Reg. No. 2073/2005), 51 S. aureus, 18 E. coli, two C. sakazakii, and one Salmonella serovar. Enteritidis isolates were also identified. Their detection can be considered as evidence of human contamination that may possibly be explained by the improper application of the so-called “Good Hygiene Practices” (GHP) (reported in the EU Reg. No. 852/2004) [5]. Commensal Gram-negative bacterial species were widely discovered (as illustrated in Table 5), in line with previous microflora investigations [14,42]. These findings are explained by the food microenvironmental conditions (aw and pH values) that select and improve commensal bacteria multiplication; indeed, the described and discovered microbiota results are invariable and in line with previous studies, as reported by Rodrigues et al. [14] and Helsens et al. [43].
The AST results, involving all isolated bacteria, produced original preliminary data from differently processed fish products and environmental specimens, as schematically reported in Table 6 and Table 7. The most frequently discovered resistances, in Gram-positive bacteria (Staphylococci), resulted against the following antibiotic molecules: tetracycline and clindamycin, both from food matrices and food operator hands (as previously described in the Results section). Similar patterns were also observed by other authors who conducted microbiological surveys on salted or salted and fermented fish products [14,43,44]. The resistance data, observed in negative coagulase Staphylococci reported in the AST Results section, have also been largely observed in nosocomial isolates, especially in MRS strains [45]. Their potential role as drivers, due to their harboring of ARGs from food matrices to the final consumers’ microbiome has been largely confirmed, as recently reported by Timmermans et al. [46]. Gene cassettes, responsible for the genetic transmission of genetic determinants, were demonstrated to be involved in the spreading of genotypic resistance to tetracyclines, clindamycin, and oxacillin [44,45].
Enterococcus spp. also presented wide resistance to the linezolid molecule, which was added by the EMA in the CIA lists, and for this reason, it is not indicated for therapeutic purposes in veterinary medicine. Similar AST results were also observed in other Enterococci strains isolated from wild mammalian feces [47]. These findings can be explained by the high impacts of anthropic activities and their ecological repercussions on humans and wildlife. These observations have been explained by the European Agencies, focusing attention on the chemical pressures caused by improper antibiotic administration or misuse [1].
Among Gram-negative strains, E. coli resulted significantly resistant to the beta-lactam antibiotic class (especially against carbapenems and cefotaxime molecules) and nitrofurans (nitrofurantoin). Similar phenotypic patterns were also described in other salted finfish matrices, where the phylogenetic analysis of amplified ARGs (described in the following paragraph) confirmed the evolutionary human origins [5,40,41]. The obtained AST data were mostly discovered from food matrix specimens. Indeed, a significant difference was observed (p-value: 0.001) between product and food operator hand isolates.
Regarding ARG circulation, the biomolecular screenings involved all isolated bacterial strains. All PCR reactions were performed following the flow chart production, following previous affirmations mentioned in the Results section.
The ARG screenings revealed a high circulation, amplifying a total of 1.410 oligonucleotide determinants; more specifically: 11,015/1410 (78.37% 95% CI: 76.22–80.52%) were detected from phenotypically resistant strains supporting the genetic basis of the AST results and the remaining 395/1410 (28.01% 95% CI: 25.67–30.35%) from susceptible strains. This evidence enforced the scientific hypothesis concerning the critical role of many bacteria (including pathogen and commensal ones) as environmental reservoirs, confirming the scientific alert announced by the European Food Safety Agency. The scientific concern was the critical role of the susceptible bacterial reservoirs indicated as “genetic environmental resistance forms”. This last-mentioned role was largely documented from commensal strains [1,2].
Concerning ARG distributions, tetC (25.17% 95% CI: 22.91–27.43%), tetK (21.06% 95% CI: 18.93–23.19%), and tetL (11.70% 95% CI: 10.03–13.37%) for tetracycline and ermC (17.23% 95% CI: 15.26–19.20%) and ermB (7.60% 95% CI: 6.22–8.98%) for clindamycin were amplified, both from products and food operator hands (mainly from Gram-positive DNA). Similar detections were also observed in previous studies conducted on salted fish products, demonstrating that simultaneous resistance to tetracyclines and clindamycin may possibly be associated with gene cassettes [48,49].
These data agreed with the results shown by the isolated strains from salted and fermented fish products by Majumdaret and Gupta [50]. These findings can be explained by different macro- and microenvironmental conditions, which provided fundamental aspects facilitating so-called horizontal gene transmission. The first consideration is linked to the food specimen characteristics; salted and seasoned products are usually handled by different food operators from fishing (as primary products) to their processing at the industrial level. From a technological perspective, the salting process generally induces bacterial lysis and nucleotide structural alterations. However, halotolerant species (i.e., Staphylococcus spp., Vibrio spp.) can survive by adapting their osmotic equilibrium to the low aw values. This extracellular stimulus acts as an inductor for ARG trades [6]. Their detections cannot exclusively represent an improper GHP application. Indeed, Staphylococcus spp., which have frequently harbored these tet and erm genes, are part of the normal microbiome identified from salted and seasoned codfish or other salted or fermented fish species [14,29,43,51]. Secondly, horizontal ARG transmission has a key role in their spreading among organic substrates [1]. The genomic combination of resistance against tetracycline and clindamycin, among Staphylococcus spp., is usually related to possible gene cassettes, as first observed by Strommenger et al. [52].
In this study, 392/684, or 57.30%, (95% CI: 53.60–61.00%) bacterial strains harbored numerous ARGs (blaTEM, blaCIT, blaCTX-M, blaKPC, blaIMP, blaOXA-48-like, tetC, tetK, tetL, ermB, ermC, sul2, and sul3) against specific antibiotic classes that were not phenotypically expressed (See Table 8). Indeed, the wide detection of ARGs is not always associated with their encoding and its consequential transcriptions [29]. The suggested scientific hypothesis was that these commensal or pathogenic strains were involved as microenvironmental reservoir forms of resistance, in agreement with previous studies [29,51,53]. The genetic expressions depend on multiple factors, and results are strongly influenced by environmental stressors, which have resulted to be inductors of specific forms of resistance. An emblematic example was represented by the sodium-chloride efflux pumps encoding (i.e., salted foodstuffs, high-content human diets, etc.), which was demonstrated to be altered, as reported by genomic studies. This condition has led to the evidence that high salt contents can modify bacterial susceptibility to antibiotic molecules. This process was observed in the bacterial cross-protection against antibiotics determined by the increased AcrAB-TolC efflux pump expression levels. This physio-pathological pathway has conferred resistance to those molecules, whose pharma-dynamical action involves ion transmembrane protein structures. This aspect was experimentally observed by Zhu and Dai [6] in E. coli strains, which presented low stress-induced tetracycline and chloramphenicol susceptibility after exposure to high salt contents. The strong connections that emerged between cellular physiology (both intra and extra) and the environment further clarified consistent repercussions on the AMR phenomenon influencing antibiotic therapeutic efficacies [2]. The cytological interactions between human and food microbiomes may also be involved in the horizontal transmission of resistant forms involving pathogenic or commensal strains [53,54,55]. This condition represents one of the crucial aspects of the real complexity and pleomorphism of AMR concern.
Parallelly to the proper GHP applications, food industries have introduced new food technologies, i.e., the HPP for seafood products’ shelf-life prolongation. This last method (HPP) has determined consistent CFU/g reductions in processed fish products [17]. In this study, a significant difference between HPP-untreated and HPP-treated products was observed (p-values: <0.001). This technology demonstrated high efficacy against both Gram-negative and Gram-positive bacteria. Indeed, the highly selective culturing procedures, involved in this qualitative study did not identify pathogens or commensal strains. The obtained results were in line with the scientific findings reported by Arnaud et al. [16]. Furthermore, the DNA biomolecular screenings, performed on isolates identified from HPP products, did not amplify ARGs. A possible explanation was first proposed by Oliveira et al. [17] and successively confirmed by Rode and Rotabaak [15], regarding the HPP denaturation effects on the hydrogen bonds between DNA strands and on covalent strands between nucleotides.
This research, in consideration of EFSA report [1], highlighted as spoilage or pathogenic bacteria, isolated from food matrices or the environment, can harbor different ARGs, reinforcing the AMR phenomenon. Legally permitted GHP measures (EU Reg. No. 852/2004) in combination with innovative technologies (i.e., HPP) could provide safe foods. However, it is necessary that a significant reduction in antibiotic usage as the main selective pressure be considered [2]. Due to the complexity of the AMR phenomenon, this study wants to provide a preliminary investigation combining industrial food processes with ARG diffusion, proposing an environmental perspective from the macro- to the microworlds.
5. Conclusions
All performed PCR assays amplified ARGs from both food matrices and environmental cultured bacteria. Of the 425 Gram-positive strains (with special regard to the Staphylococcus genus), tetracycline (tetC; tetK; tetL), clindamycin (ermB and ermC), and oxacillin (mecA) resistance genes were largely discovered. Beta-lactams (blaTEM, blaCIT, blaCTX-M, blaIMP, blaKPC, blaOXA-48-like) and nitrofurantoin (nfsA and nfsB) genes were detected from 259 Gram-negative ones. Phenotypically resistant strains were mostly isolated from salted and soaked products, while the HPP food technology confirmed its sanitary application, highlighting its bactericidal effect and the ability to modify nucleotide macromolecular structures. The amplification of any genetic determinants, i.e., cfr, optrA, poxtA (carbapenem molecule), and vanD (vancomycin), belonging to the so-called CIAs, provides an ecological perspective concerning ARG diffusion in the screened seafood industry. The obtained results further highlight the complexity of the AMR phenomenon and the importance of biomolecular surveillance in anthropized environments. For these reasons, deep attention to Good Hygiene Practices and high-sanitary levels applied during food technological processes will uncover fundamental roles in reducing possible cross-contaminations, in agreement with the new concepts introduced by the Hazard Analysis and Risk-Based Preventive Controls (FDA, 2018). Combining the approaches of food industries and the applied food microbiology sciences will provide more detailed explanations concerning cytological interactions and the inductive pathways involved in chemical signaling on extra- and intracellular structures.
Author Contributions
Conceptualization, G.F. and A.V.; methodology, G.F.; software, G.F.; validation, G.F., C.L. and A.V.; formal analysis, G.F.; investigation, G.F.; resources, A.V.; data curation, G.F.; writing—original draft preparation, G.F.; writing—review and editing, G.F., C.L., M.S. and A.V.; visualization, G.F.; supervision, A.V.; project administration, A.V.; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Acknowledgments
Authors want to express their gratitude to the Post-Graduate Specialization School in Food Inspection “G. Tiecco” which enabled this manuscript to be prepared. Furthermore, we want to mention appreciation to the EFS-MUR (European Social Fund-Italian University Ministry)-PON (Innovation and Research 2014–2020) CUP: C44I19001650006; Ph.D. program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- European Food Safety Authority (EFSA); Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021, 19, e06651. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Advice on the Designation of Antimicrobials or Groups of Antimicrobials Reserved for Treatment of Certain Infections in Humans—In relation to Implementing Measures under Article 37(5) of Regulation (EU) 2019/6 on Veterinary MEDICINAL Products. 2022. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/advice-designation-antimicrobials-groups-antimicrobials-reserved-treatment-certain-infections-humans/6-veterinary-medicinal-products_en.pdf (accessed on 4 August 2022).
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Approved Drugs for Use in Seafoods; FAO: Rome, Italy, 2020; Available online: http://www.fao.org/3/ca9229en/ca9229en.pdf (accessed on 4 August 2022).
- Loayza, F.; Graham, J.P.; Trueba, G. Factors Obscuring the Role of E. coli from Domestic Animals in the Global Antimicrobial Resistance Crisis: An Evidence-Based Review. Int. J. Environ. Res. Public Health 2020, 17, 3061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Dai, X. High Salt Cross-Protects Escherichia coli from Antibiotic Treatment through Increasing Efflux Pump Expression. mSphere 2018, 3, e00095-18. [Google Scholar] [CrossRef]
- Higuera-Llantén, S.; Vásquez-Ponce, F.; Barrientos-Espinoza, B.; Mardones, F.O.; Marshall, S.H.; Olivares-Pacheco, J. Extended antibiotic treatment in salmon farms select multiresistant gut bacteria with a high prevalence of antibiotic resistance genes. PLoS ONE 2018, 13, e0203641. [Google Scholar] [CrossRef] [PubMed]
- Devirgiliis, C.; Zinno, P.; Perozzi, G. Update on antibiotic resistance in foodborne Lactobacillus and Lactococcus species. Front. Microbiol. 2013, 4, 301. [Google Scholar] [CrossRef] [PubMed]
- Dafale, N.A.; Srivastava, S.; Purohit, H.J. Zoonosis: An Emerging Link to Antibiotic Resistance Under “One Health Approach”. Indian J. Microbiol. 2020, 60, 139–152. [Google Scholar] [CrossRef]
- Hassan, M.M.; El Zowalaty, M.E.; Lundkvist, Å.; Järhult, J.D.; Khan Nayem, M.R.; Tanzin, A.Z. Residual antimicrobial agents in food originating from animals. Trends Food Sci. Technol. 2021, 111, 141–150. [Google Scholar] [CrossRef]
- Delannoy, S.; Hoffer, C.; Youf, R.; Dauvergne, E.; Webb, H.E.; Brauge, T. High Throughput Screening of Antimicrobial Resistance Genes in Gram-Negative Seafood Bacteria. Microorganisms 2022, 10, 1225. [Google Scholar] [CrossRef]
- Hossain, A.; Habibullah-Al-Mamun, M.; Nagano, I.; Masunaga, S.; Kitazawa, D.; Matsuda, H. Antibiotics, antibiotic-resistant bacteria, and resistance genes in aquaculture: Risks, current concern, and future thinking. Environ. Sci. Pollut. Res. Int. 2022, 29, 11054–11075. [Google Scholar] [CrossRef]
- Thiang, L.E.; Chai, C.Y.S.; Lee, C.W.; Takada, H.; Wang, A.J.; Chai, L.C.; Bong, C.W. Tetracycline Resistance and Prevalence of Tetracycline Resistance Genes in Bacteria from Marine Aquaculture Farms in Peninsular Malaysia. Sains Malays. 2022, 51, 345–357. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Ho, P.; López-Caballero, M.E.; Vaz-Pires, P.; Nunes, M.L. Characterization and identification of microflora from soaked cod and respective salted raw materials. Food Microbiol. 2003, 20, 471–481. [Google Scholar] [CrossRef]
- Rode, T.M.; Rotabakk, B.T. Extending shelf life of desalted cod by high pressure processing. Inn. Food Sci. Emerg. Dis. 2021, 69, 102476. [Google Scholar] [CrossRef]
- Arnaud, C.; de Lamballerie, M.; Pottier, L. Effect of high-pressure processing on the preservation of frozen and re-thawed sliced cod (Gadus morhua) and salmon (Salmo salar) fillets. High Press. Proces. 2018, 38, 62–79. [Google Scholar] [CrossRef]
- Oliveira, F.A.d.; Neto, O.C.; Santos, L.M.R.d.; Ferreira, E.H.R.; Rosenthal, A. Effect of high pressure on the fish meat quality—A review. Trends Food Sci. Technol. 2017, 66, 1–19. [Google Scholar] [CrossRef]
- ISO 11290-1,2:2017; Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method; Part 2: Enumeration Method. ISO: London, UK, 2017. Available online: https://www.iso.org/standard/60313.html (accessed on 2 August 2022).
- ISO 21872-1:2017; Microbiology of the Food Chain—Horizontal Method for the Determination of Vibrio spp.—Part 1: Detection of Potentially Enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. ISO: London, UK, 2017. Available online: https://www.iso.org/standard/74112.html (accessed on 5 September 2022).
- ISO 6888-1:2018; Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Parker Agar Medium. ISO: London, UK, 2018. Available online: https://www.iso.org/standard/64947.html (accessed on 15 September 2022).
- ISO 7898-2:2000; Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 2: Membrane Filtration Method. ISO: London, UK, 2000. Available online: https://www.iso.org/standard/14854.html (accessed on 10 October 2022).
- ISO 16654-1:2017; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection of Escherichia coli O157—Amendment 1: Annex B: Result of Interlaboratory Studies. ISO: London, UK, 2017. Available online: https://www.iso.org/standard/64704.html (accessed on 15 October 2022).
- ISO 16266:2008; Water Quality—Detection and Enumeration of Pseudomonas aeruginosa—Method by Membrane Filtration. ISO: London, UK, 2008. Available online: https://www.iso.org/standard/39272.html (accessed on 15 October 2022).
- Clinical & Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 33rd Edition. Available online: https://clsi.org/ (accessed on 10 January 2023).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- McClure, J.A.; Conly, J.M.; Lau, V.; Elsayed, S.; Louie, T.; Hutchins, W. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J. Clin. Microbiol. 2006, 44, 1141–1144. [Google Scholar] [CrossRef]
- Kozak, G.K.; Boerlin, P.; Janecko, N.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009, 75, 559–566. [Google Scholar] [CrossRef]
- Monstein, H.J.; Ostholm-Balkhed, A.; Nilsson, M.V.; Nilsson, M.; Dornbusch, K.; Nilsson, L.E. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 2007, 115, 1400–1408. [Google Scholar] [CrossRef]
- Liao, C.Y.; Balasubramanian, B.; Peng, J.J.; Tao, S.R.; Liu, W.C.; Ma, Y. Antimicrobial Resistance of Escherichia coli From Aquaculture Farms and Their Environment in Zhanjiang, China. Front. Vet. Sci. 2021, 8, 806653. [Google Scholar] [CrossRef]
- Helmy, M.M.; Wasfi, R. Phenotypic and molecular characterization of plasmid mediated AmpC β-lactamases among Escherichia coli, Klebsiella spp., and Proteus mirabilis isolated from urinary tract infections in Egyptian hospitals. BioMed Res. Int. 2014, 2014, 171548. [Google Scholar] [CrossRef] [PubMed]
- Hatrongjit, R.; Kerdsin, A.; Akeda, Y.; Hamada, S. Detection of plasmid-mediated colistin-resistant and carbapenem-resistant genes by multiplex PCR. MethodsX 2018, 5, 532–536. [Google Scholar] [CrossRef]
- Kishk, R.M.; Anani, M.M.; Nemr, N.A.; Soliman, N.M.; Fouad, M.M. Inducible clindamycin resistance in clinical isolates of Staphylococcus aureus in Suez Canal University Hospital, Ismailia, Egypt. J. Infect. Dev. Ctries. 2020, 14, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.K.; Fleige, C.; Klare, I.; Werner, G. Development of a multiplex-PCR to simultaneously detect acquired linezolid resistance genes cfr, optrA and poxtA in enterococci of clinical origin. J. Microbiol. Methods 2019, 160, 101–103. [Google Scholar] [CrossRef] [PubMed]
- García, V.; Montero, I.; Bances, M.; Rodicio, R.; Rodicio, M.R. Incidence and Genetic Bases of Nitrofurantoin Resistance in Clinical Isolates of Two Successful Multidrug-Resistant Clones of Salmonella enterica Serovar Typhimurium: Pandemic “DT 104” and pUO-StVR2. Microb. Drug Resist. 2017, 23, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Poeta, P.; Sáenz, Y.; Vinué, L.; Coelho, A.C.; Matos, M. Mechanisms of antibiotic resistance in Escherichia coli isolates recovered from wild animals. Microb. Drug Res. 2008, 14, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.Q.; Wang, H.N.; Zhang, A.Y. Detection of tetracycline resistant genes in bacterial by using a triple-PCR method. J. Sichuan Univ. 2013, 50, 171–176. [Google Scholar] [CrossRef]
- Gevers, D.; Danielsen, M.; Huys, G.; Swings, J. Molecular characterization of tet(M) genes in Lactobacillus isolates from different types of fermented dry sausage. J. Appl. Environ. Microbiol. 2003, 69, 1270–1275. [Google Scholar] [CrossRef]
- Trzcinski, K.; Cooper, B.S.; Hryniewicz, W.; Dowson, C.G. Expression of resistance to tetracyclines in strains of methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 45, 763–770. [Google Scholar] [CrossRef]
- Nomura, T.; Hashimoto, Y.; Kurushima, J.; Hirakawa, H.; Tanimoto, K.; Zheng, B. New colony multiplex PCR assays for the detection and discrimination of vancomycin-resistant enterococcal species. J. Microbiol. Methods 2018, 145, 69–72. [Google Scholar] [CrossRef]
- Kuuliala, L.; Al Hage, Y.; Ioannidis, A.G.; Sader, M.; Kerckhof, F.M.; Vanderroost, M. Microbiological, chemical and sensory spoilage analysis of raw Atlantic cod (Gadus morhua) stored under modified atmospheres. Food Microbiol. 2018, 70, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Abdou, M.S.; Ebied, N.A. Isolation of highly antibiotic-resistant Staph. aureus from salted fish sold in markets. Alex. J. Vet. Sci. 2021, 71, 30–45. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, X.; Lei, Y.; Regenstein, J.M.; Luo, Y. Characterization of the microbial composition and quality of lightly salted grass carp (Ctenopharyngodon idellus) fillets with vacuum or modified atmosphere packaging. Int. J. Food Microbiol. 2019, 293, 87–93. [Google Scholar] [CrossRef]
- Helsens, N.; Calvez, S.; Prevost, H.; Bouju-Albert, A.; Maillet, A.; Rossero, A. Antibiotic Resistance Genes and Bacterial Communities of Farmed Rainbow Trout Fillets (Oncorhynchus mykiss). Front. Microbiol. 2020, 11, 590902. [Google Scholar] [CrossRef]
- Biswas, K.; Sharma, P.; Joshi, S.R. Co-occurrence of antimicrobial resistance and virulence determinants in enterococci isolated from traditionally fermented fish products. J. Glob. Antimicrob. Resist. 2019, 17, 79–83. [Google Scholar] [CrossRef]
- Shariati, A.; Dadashi, M.; Chegini, Z.; van Belkum, A.; Mirzaii, M.; Khoramrooz, S.S. The global prevalence of Daptomycin, Tigecycline, Quinupristin/Dalfopristin, and Linezolid-resistant Staphylococcus aureus and coagulase-negative staphylococci strains: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, M.; Bogaerts, B.; Vanneste, K.; De Keersmaecker, S.; Roosens, N.; Kowalewicz, C. Large diversity of linezolid-resistant isolates discovered in food-producing animals through linezolid selective monitoring in Belgium in 2019. J. Antimicrob. Chemother. 2021, 77, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Smoglica, C.; Vergara, A.; Angelucci, S.; Festino, A.R.; Antonucci, A.; Marsilio, F.; Di Francesco, C.E. Evidence of Linezolid Resistance and Virulence Factors in Enterococcus spp. Isolates from Wild and Domestic Ruminants, Italy. Antibiotics 2022, 11, 223. [Google Scholar] [CrossRef]
- Li, H.; Stegger, M.; Dalsgaard, A.; Leisner, J.J. Bacterial content and characterization of antibiotic resistant Staphylococcus aureus in Danish sushi products and association with food inspector rankings. Int. J. Food Microbiol. 2019, 305, 108244. [Google Scholar] [CrossRef]
- Yuan, J.; Ni, M.; Liu, M.; Zheng, Y.; Gu, Z. Occurrence of antibiotics and antibiotic resistance genes in a typical estuary aquaculture region of Hangzhou Bay, China. Mar. Pollut. Bullet. 2019, 138, 376–384. [Google Scholar] [CrossRef]
- Majumdar, R.K.; Gupta, S. Isolation, identification and characterization of Staphylococcus sp. from Indian ethnic fermented fish product. Lett. Appl. Microbiol. 2020, 71, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Muziasari, W.I.; Pitkänen, L.K.; Sørum, H.; Stedtfeld, R.D.; Tiedje, J.M.; Virta, M. The Resistome of Farmed Fish Feces Contributes to the Enrichment of Antibiotic Resistance Genes in Sediments below Baltic Sea Fish Farms. Front. Microbiol. 2017, 7, 2137. [Google Scholar] [CrossRef]
- Strommenger, B.; Kettlitz, C.; Werner, G.; Witte, W. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J. Clin. Microbiol. 2003, 41, 4089–4094. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, E.; Van Coillie, E.; Van Meervenne, E.; Boon, N.; Heyndrickx, M.; Van de Wiele, T. Commensal E. coli rapidly transfer antibiotic resistance genes to human intestinal microbiota in the Mucosal Simulator of the Human Intestinal Microbial Ecosystem (M-SHIME). Int. J. Food Microbiol. 2019, 311, 108357. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Manuzon, M.; Lehman, M.; Wan, K.; Luo, H.; Wittum, T.E. Food commensal microbes as a potentially important avenue in transmitting antibiotic resistance genes. FEMS Microbiol. Lett. 2006, 254, 226–231. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Draft Guidance for Industry: Hazard Analysis and Risk-Based Preventive Controls for Human Food. 2018. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-hazard-analysis-and-risk-based-preventive-controls-human-food (accessed on 11 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).