Effect of Apple Polyphenols on the Antioxidant Activity and Structure of Three-Dimensional Printed Processed Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation
2.3. Determination of Antioxidant Activity
2.3.1. Preparation of Sample Solution
2.3.2. 2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH) Assay
2.3.3. 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Assay
2.4. Determination of Rheological Characteristics
2.5. Determination of Structural Properties
2.5.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5.2. Endogenous Tryptophan Fluorescence
2.5.3. Scanning Electron Microscopy
2.6. Measurement of 3D Printing Molding Effects
2.7. Color Analysis
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
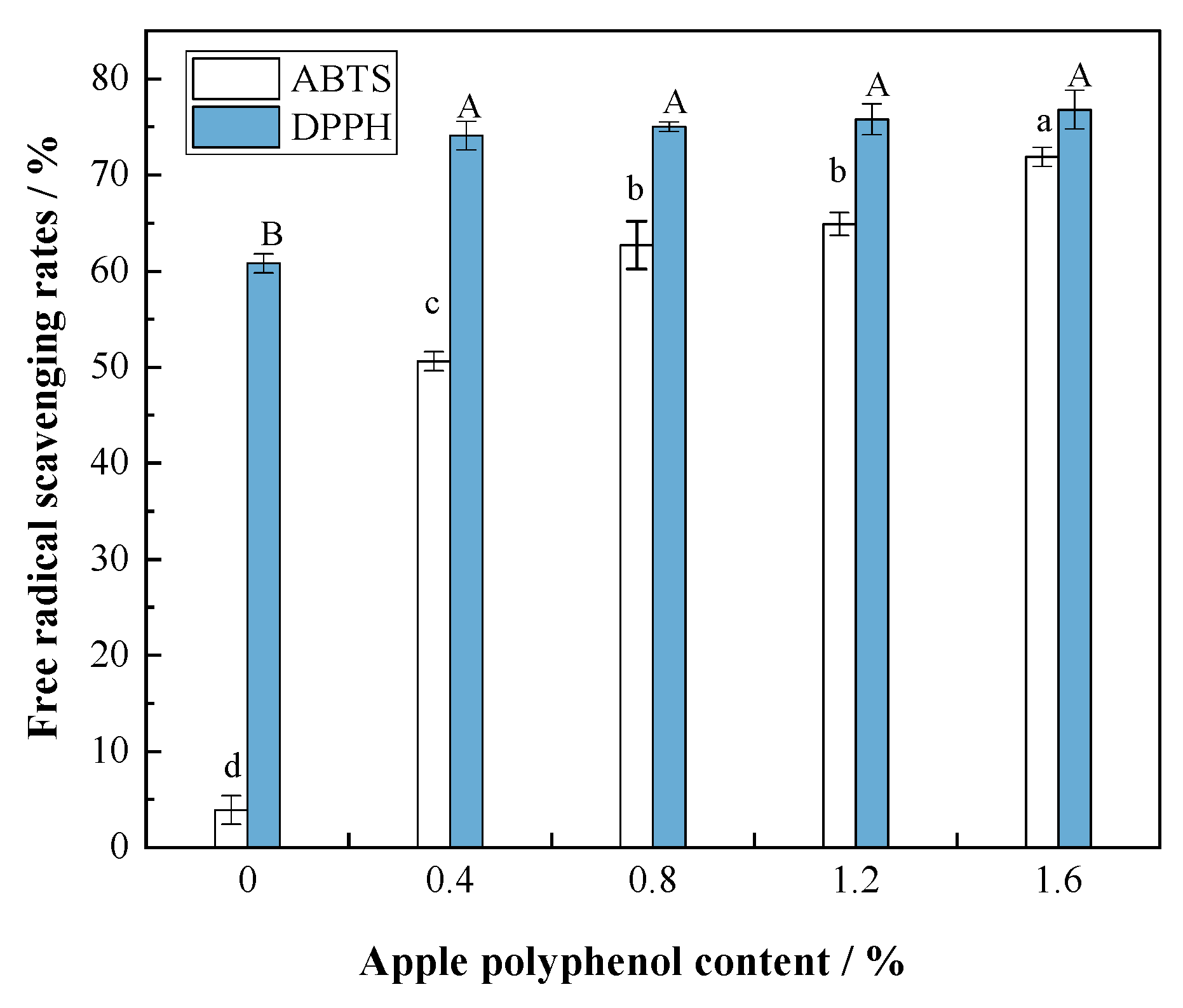
3.1. DPPH and ABTS Free Radical Scavenging Rates
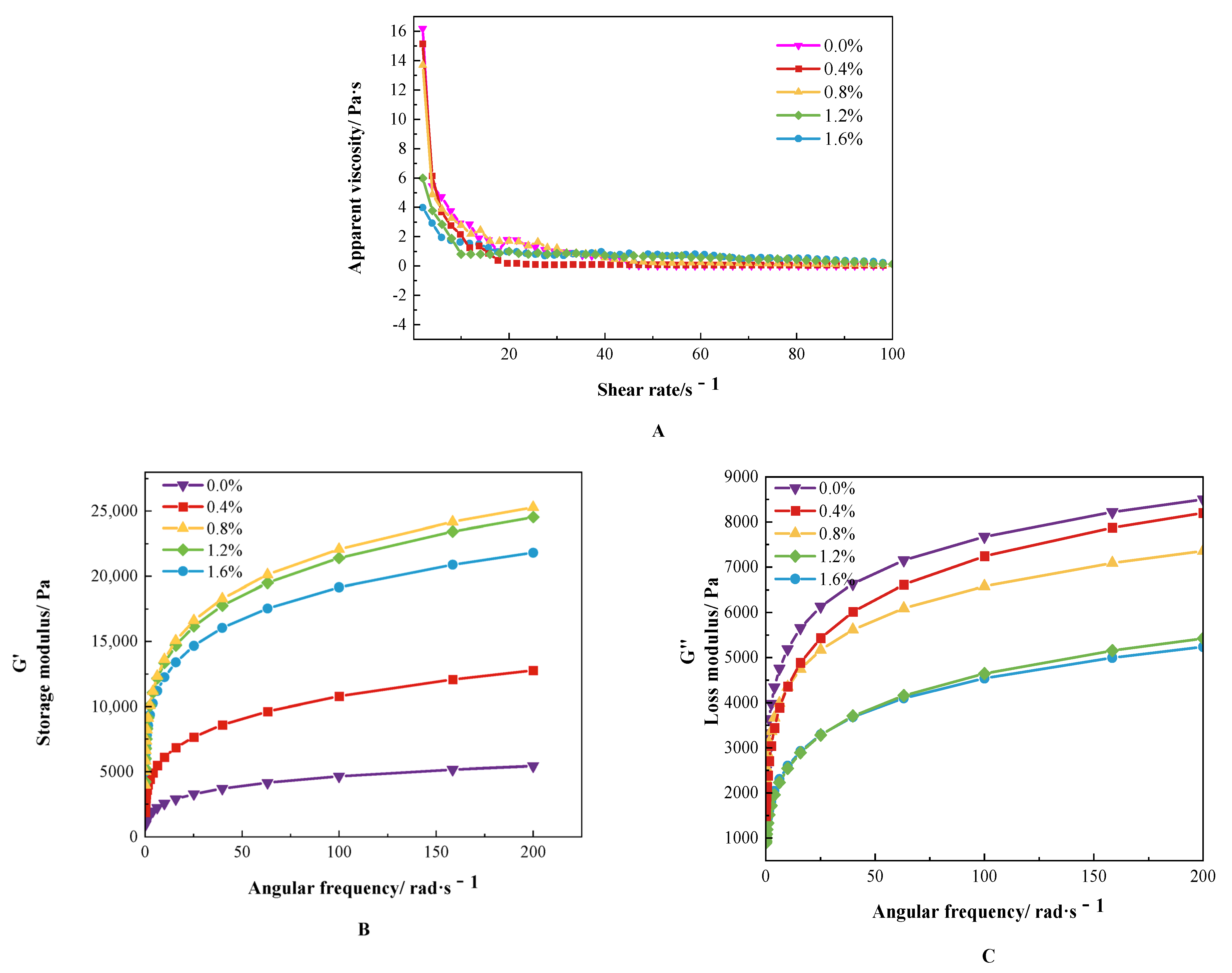
3.2. Rheological Analysis
3.3. Structural Analysis
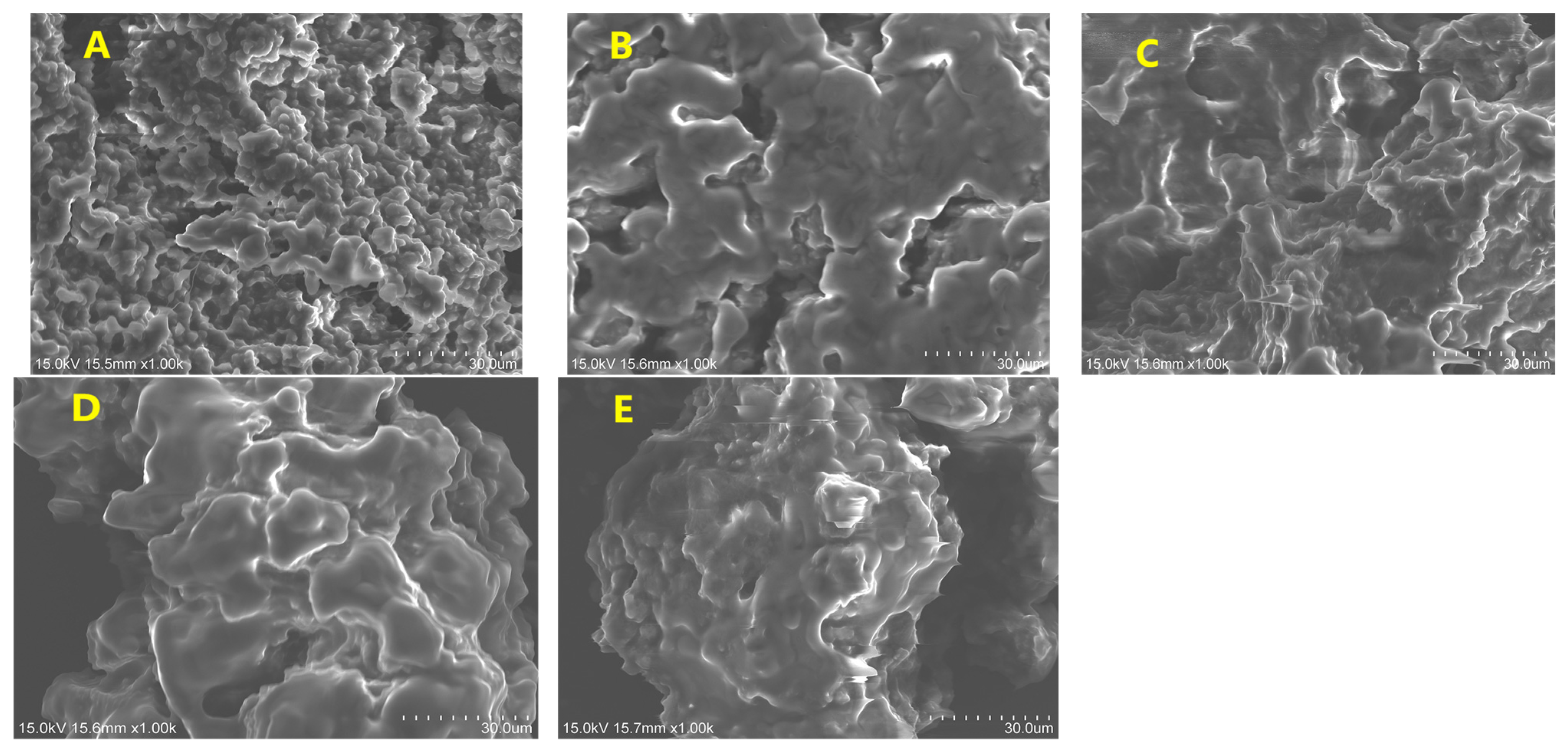
3.3.1. Scanning Electron Microscopy
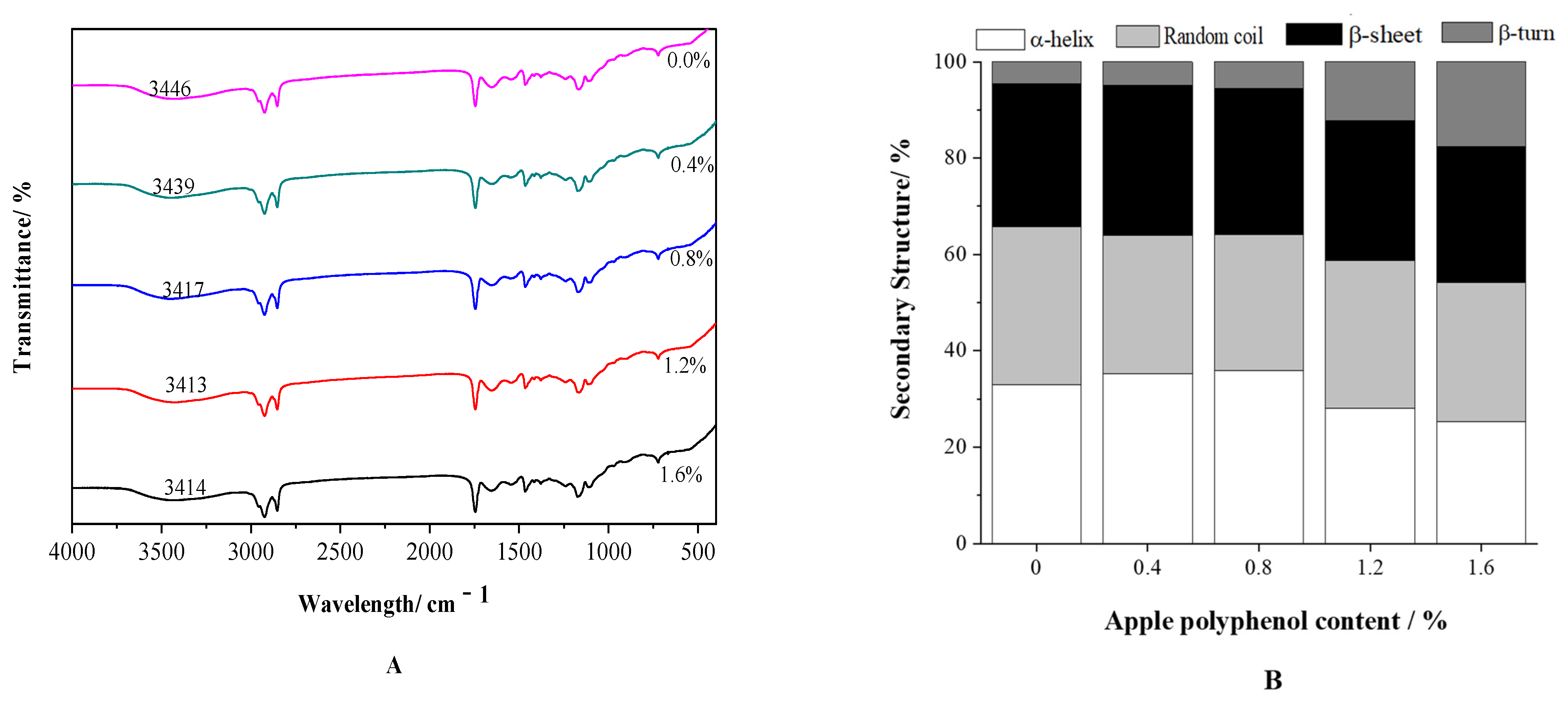
3.3.2. FT-IR Spectroscopy
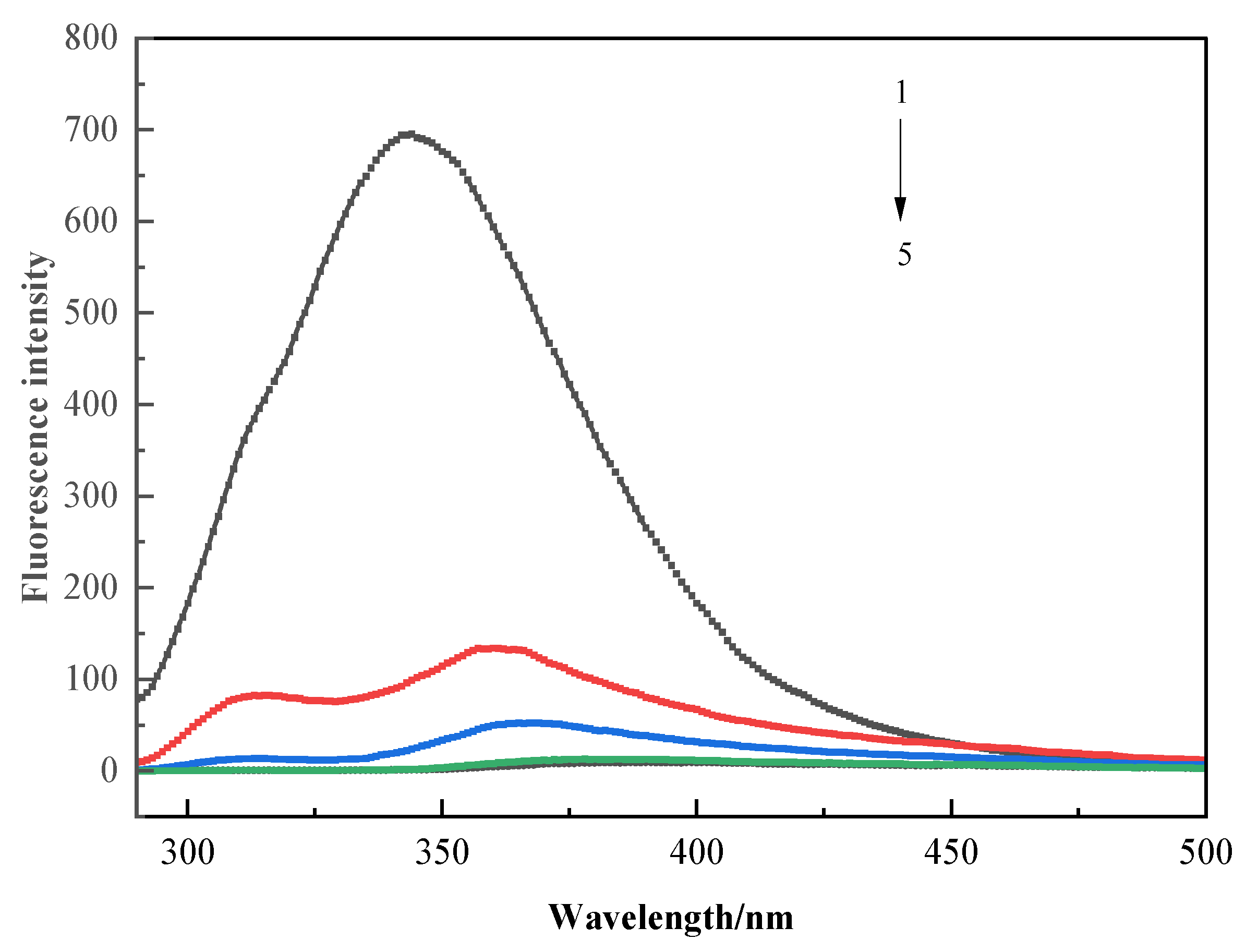
3.3.3. Fluorescence Spectroscopy
3.4. D Printing Performance and Colour Analysis
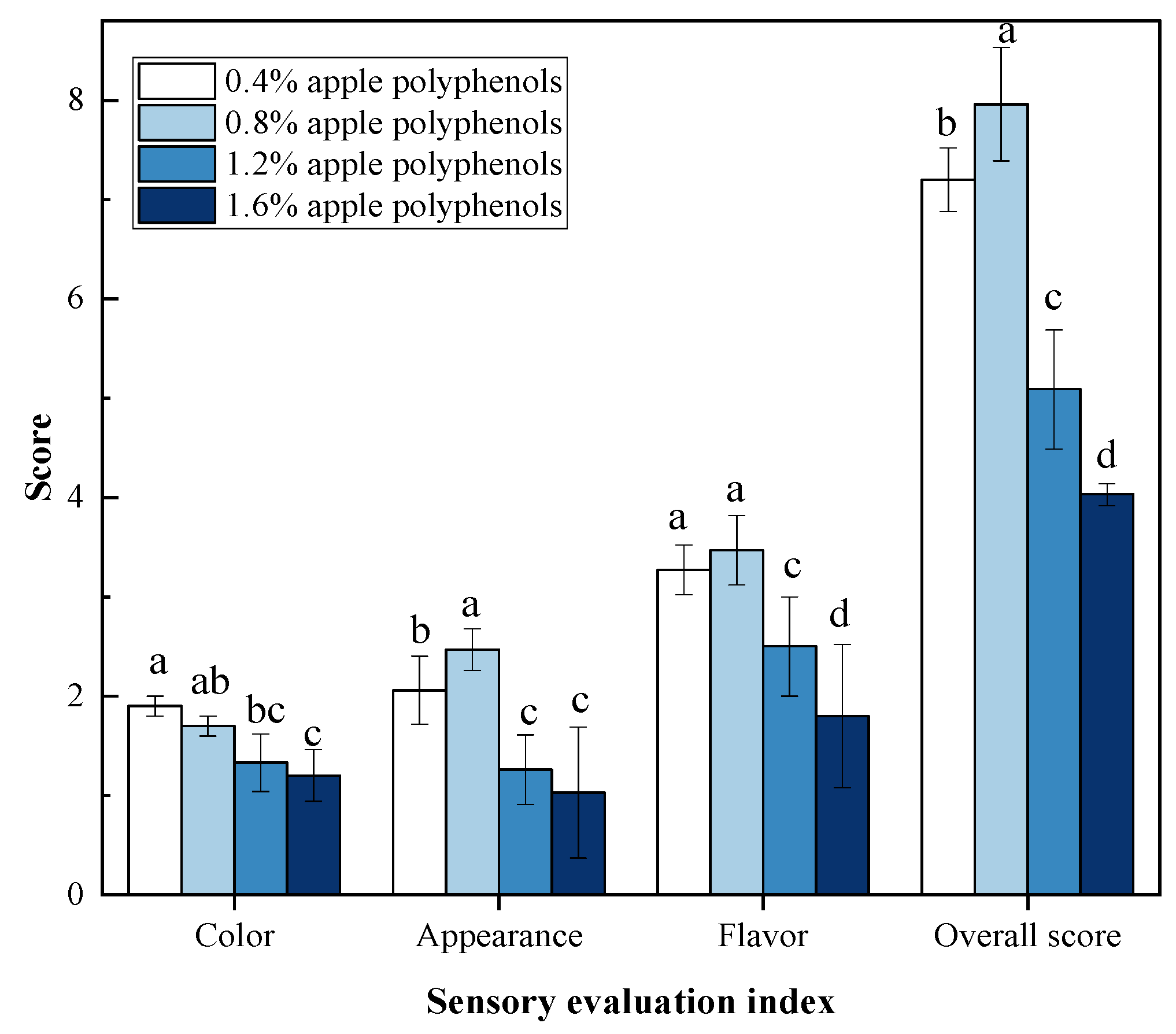
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Enfield, R.; Pandya, J.; Lu, J.; McClements, D.; Kinchla, A. The Future of 3D Food Printing: Opportunities for Space Applications. Crit. Rev. Food Sci. Nutr. 2022, 20, 7299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bhandari, B.; Zhang, M. Incorporation of probiotics (Bifidobacterium animalis subsp. Lactis) into 3D printed mashed potatoes: Effects of variables on the viability. Food Res. Int. 2020, 128, 108795. [Google Scholar] [CrossRef]
- An, Y.; Guo, C.; Zhang, M.; Zhong, Z. Investigation on characteristics of 3D printing using Nostoc sphaeroides biomass. J. Sci. Food Agric. 2019, 99, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigation on fish surimi gel as promising food material for 3D printing. J. Food Eng. 2018, 220, 101–108. [Google Scholar] [CrossRef]
- Johnson, M.E.; Kapoor, R.; McMahon, D.J.; McCoy, D.R.; Narasimmon, R.G. Reduction of Sodium and Fat Levels in Natural and Processed Cheeses: Scientific and Technological Aspects. Compr. Rev. Food Sci. Food Saf. 2009, 8, 252–268. [Google Scholar] [CrossRef]
- Talbot-Walsh, G.; Kannar, D.; Selomulya, C. A review on technological parameters and recent advances in the fortification of processed cheese. Trends Food Sci. Tech. 2018, 81, 193–202. [Google Scholar] [CrossRef]
- Lu, Y.; Du, Y.; Qin, X.; Wu, H.; Huang, Y.; Cheng, Y. Comprehensive evaluation of effective polyphenols in apple leaves and their combinatory antioxidant and neuroprotective activities. Ind. Crops Prod. 2019, 129, 242–252. [Google Scholar] [CrossRef]
- Huang, T.; Che, Q.; Chen, X.; Chen, D.; Yu, B.; He, J.; Chen, H.; Yan, H.; Zheng, P.; Luo, Y. Apple Polyphenols Improve Intestinal Antioxidant Capacity and Barrier Function by Activating the Nrf2/Keap1 Signaling Pathway in a Pig Model. J. Agric. Food Chem. 2022, 70, 7576–7585. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.; Lan, Y.; Yang, Z.; Rao, J.; Chen, B. Molecular interaction mechanism and structure–activity relationships of protein–polyphenol complexes revealed by side-directed spin labeling-electron paramagnetic resonance (SDSL-EPR) spectroscopy. Food Chem. 2023, 402, 134354. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, H.; Zhao, Y.; Zhao, M.; Wang, S. Effect of apple polyphenols on the gel properties of whey protein isolate. Food Hydrocol. 2019, 90, 433–440. [Google Scholar] [CrossRef]
- Gülçin, I.; Huyut, Z.; Elmastas, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, X.; Liu, L.; Wang, G.; Song, H.; Geng, F.; Luo, P. Effect of nano eggshell calcium on the structure, physicochemical, and gel properties of threadfin bream (Nemipterus virgatus) actomyosin. LWT Food Sci. Technol. 2021, 150, 112047. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Arcoraci, T.; Martorana, M.; Turco, V.; Cacciola, F.; Rich, G.T.; Bisignano, C.; Saija, A.; Dugo, P.; et al. Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). J. Food Compos. Anal. 2010, 23, 166–174. [Google Scholar] [CrossRef]
- Geng, F.; Xie, Y.; Wang, Y.; Wang, J. Depolymerization of chicken egg yolk granules induced by high-intensity ultrasound. Food Chem. 2021, 354, 129580. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, S.; Ye, H.; Luo, W.; Huang, Q.; Geng, F. Ovomucin may be the key protein involved in the early formation of egg-white thermal gel. Food Chem. 2022, 366, 130596. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Liu, Y. Effect of Post-Treatment Microwave Vacuum Drying on the Quality of 3D-Printed Mango Juice Gel. Dry Technol. 2019, 37, 1757–1765. [Google Scholar] [CrossRef]
- Lao, Y.; Zhang, M.; Devahastin, S.; Ye, Y. Effect of Combined Infrared Freeze Drying and Microwave Vacuum Drying on Quality of Kale Yoghurt Melts. Drying Technol. 2020, 38, 621–633. [Google Scholar] [CrossRef]
- Staroszczyk, H.; Kusznierewicz, B.; Malinowska-Pańczyk, E.; Sinkiewicz, I.; Gottfried, K.; Kołodziejska, I. Fish gelatin films containing aqueous extracts from phenolic-rich fruit pomace. LWT Food Sci. Technol. 2020, 117, 108613. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Chen, H.; Xie, F.; Chen, L.; Zheng, B. Effect of rheological properties of potato, rice and corn starches on their hot-extrusion 3D printing behaviors. J. Food Eng. 2019, 244, 150–158. [Google Scholar] [CrossRef]
- Millet, M.; Poupard, P.; Guilois-Dubois, S.; Poiraud, A.; Fanuel, M.; Rogniaux, H.; Guyot, S. Heat-unstable apple pathogenesis-related proteins alone or interacting with polyphenols contribute to haze formation in clear apple juice. Food Chem. 2020, 309, 125636. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, M.; Yong, H.; Jang, H.; Jung, S.; Choi, Y. Impacts of fat types and myofibrillar protein on the rheological properties and thermal stability of meat emulsion systems. Food Chem. 2021, 346, 128930. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Janaswamy, S.; Chen, L.; Chi, C. Further insights into the evolution of starch assembly during retrogradation using SAXS. Int. J. Biol. Macromol. 2020, 154, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Han, M.; Huang, M.; Xu, X. Enhanced heat stability and antioxidant activity of myofibrillar protein-dextran conjugate by the covalent adduction of polyphenols. Food Chem. 2021, 352, 129376. [Google Scholar] [CrossRef]
- Anvari, M.; Chung, D. Dynamic rheological and structural characterization of fish gelatin–gum Arabic coacervate gels cross-linked by tannic acid. Food Hydrocoll. 2016, 60, 516–524. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of binding interaction between β-lactoglobulin and three common polyphenols using multi-spectroscopy and modeling methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- You, G.; Niu, G.; Long, H.; Zhang, C.; Liu, X. Elucidation of interactions between gelatin aggregates and hsian-tsao gum in aqueous solutions. Food Chem. 2020, 319, 126532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, G.; Wang, H. Investigation of the mechanism of conformational alteration in ovalbumin as induced by glycation with different monoses through conventional spectrometry and liquid chromatography high-resolution mass spectrometry. J. Agric. Food Chem. 2019, 67, 3096–3105. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, L.; Wen, Y.; Qin, W.; Wu, D.; Chen, H.; Zhang, Q.; Zhang, Q. Comparison of apple polyphenol-gelatin binary complex and apple polyphenol-gelatin-pectin ternary complex: Antioxidant and structural characterization. LWT Food Sci. Technol. 2021, 148, 111740. [Google Scholar] [CrossRef]
- Gan, X.; Li, H.; Wang, Z.; Emara, A.; Zhang, D.; He, Z. Does protein oxidation affect proteolysis in low sodium Chinese traditional bacon processing. Meat Sci. 2019, 150, 14–22. [Google Scholar] [CrossRef]
| Addition (%) | Image | Length (mm) | Width (mm) | Height (mm) | Porosity (%) |
|---|---|---|---|---|---|
| 0.0 |  | 48.13 ± 0.01 c | 27.96 ± 0.05 b | 2.95 ± 0.01 a | 4.16 ± 0.06 b |
| 0.4 |  | 48.22 ± 0.03 a | 28.33 ± 0.21 b | 2.95 ± 0.02 a | 4.23 ± 0.08 b |
| 0.8 |  | 48.12 ± 0.02 c | 27.93 ± 0.06 b | 2.95 ± 0.03 a | 4.1 ± 0.10 b |
| 1.2 |  | 48.15 ± 0.05 b | 29.67 ± 0.13 a | 2.92 ± 0.03 a | 10.6 ± 0.46 a |
| 1.6 |  | 48.17 ± 0.08 b | 30.33 ± 0.58 a | 2.93 ± 0.02 a | 11.1 ± 0.77 a |
| Addition (%) | L* | a* | b* |
|---|---|---|---|
| 0.0 | 84.69 ± 0.03 a | −1.12 ± 0.01 e | 9.56 ± 0.01 b |
| 0.4 | 62.03 ± 0.02 b | −0.08 ± 0.02 d | 12.89 ± 0.01 a |
| 0.8 | 55.03 ± 0.01 c | 0.21 ± 0.01 c | 8.71 ± 0.01 d |
| 1.2 | 52.02 ± 0.01 d | 0.51 ± 0.01 b | 8.52 ± 0.02 e |
| 1.6 | 50.81 ± 0.01 e | 1.35 ± 0.01 a | 8.84 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Zhao, G.; Cheng, K.; Shi, C.; Xiao, G. Effect of Apple Polyphenols on the Antioxidant Activity and Structure of Three-Dimensional Printed Processed Cheese. Foods 2023, 12, 1731. https://doi.org/10.3390/foods12081731
Deng Y, Zhao G, Cheng K, Shi C, Xiao G. Effect of Apple Polyphenols on the Antioxidant Activity and Structure of Three-Dimensional Printed Processed Cheese. Foods. 2023; 12(8):1731. https://doi.org/10.3390/foods12081731
Chicago/Turabian StyleDeng, Yiqiu, Guangsheng Zhao, Kewei Cheng, Chuanchuan Shi, and Gongnian Xiao. 2023. "Effect of Apple Polyphenols on the Antioxidant Activity and Structure of Three-Dimensional Printed Processed Cheese" Foods 12, no. 8: 1731. https://doi.org/10.3390/foods12081731
APA StyleDeng, Y., Zhao, G., Cheng, K., Shi, C., & Xiao, G. (2023). Effect of Apple Polyphenols on the Antioxidant Activity and Structure of Three-Dimensional Printed Processed Cheese. Foods, 12(8), 1731. https://doi.org/10.3390/foods12081731






