Silver Nanoparticle Synthesis by Rumex vesicarius Extract and Its Applicability against Foodborne Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ARLE
2.3. Analysis of the ARLE
2.4. AgNPs’ Biosynthesis
2.5. Characterization of ARLE-AgNPs
2.6. Antibacterial Activity of ARLE-AgNPs
2.6.1. Bacterial Inoculums Preparation
2.6.2. Antimicrobial Activity Measurement
2.7. Synergistic Activity of ARLE-AgNPs
2.8. Antioxidant Activity of ARLE-AgNPs
2.8.1. DPPH Scavenging Activity
2.8.2. Nitric Oxide (NO) Radical Scavenging Activity
2.8.3. Hydrogen Peroxide Scavenging Assay
2.9. In Vitro Biocompatibility Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Identification of ARLE Polyphenolic Compounds
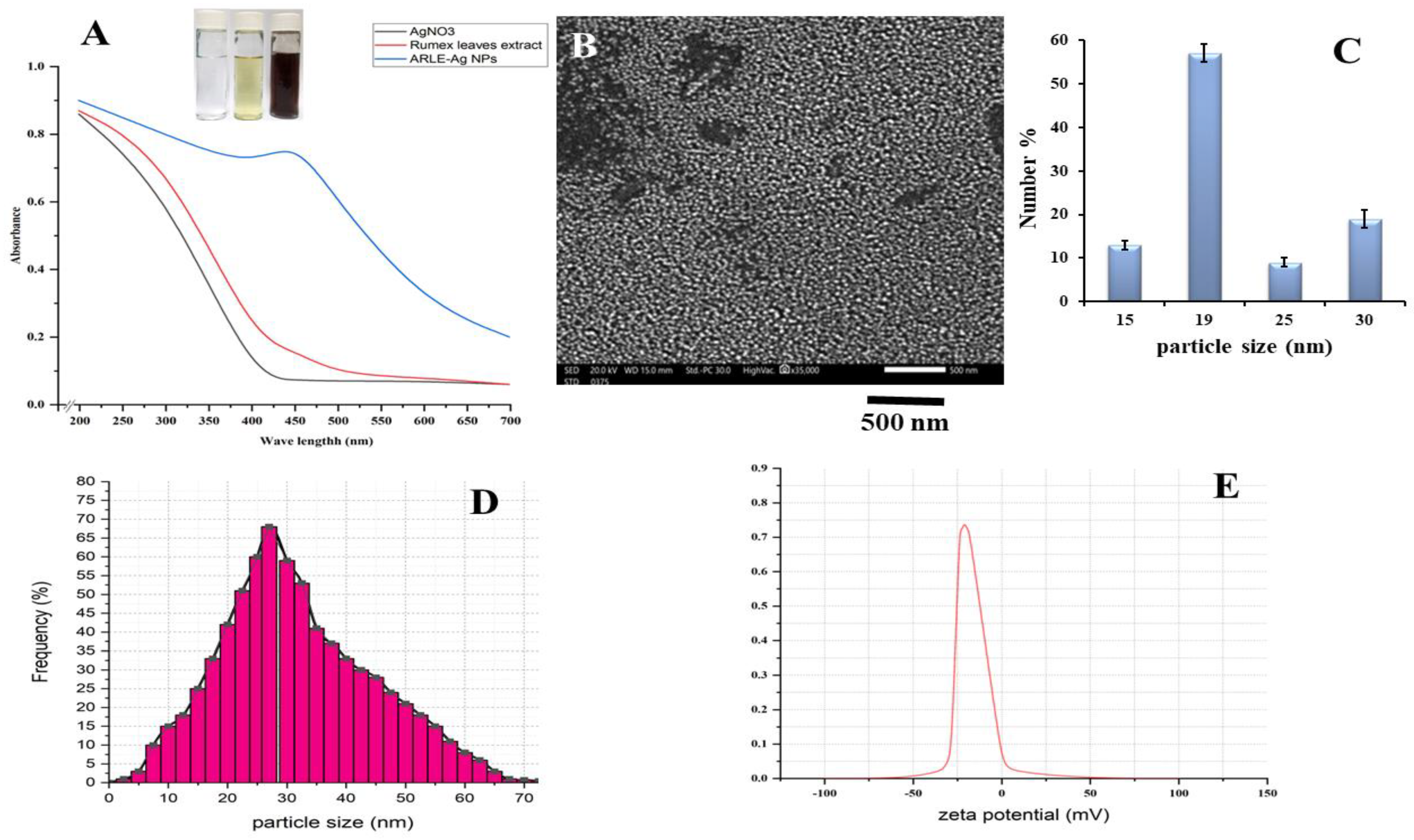
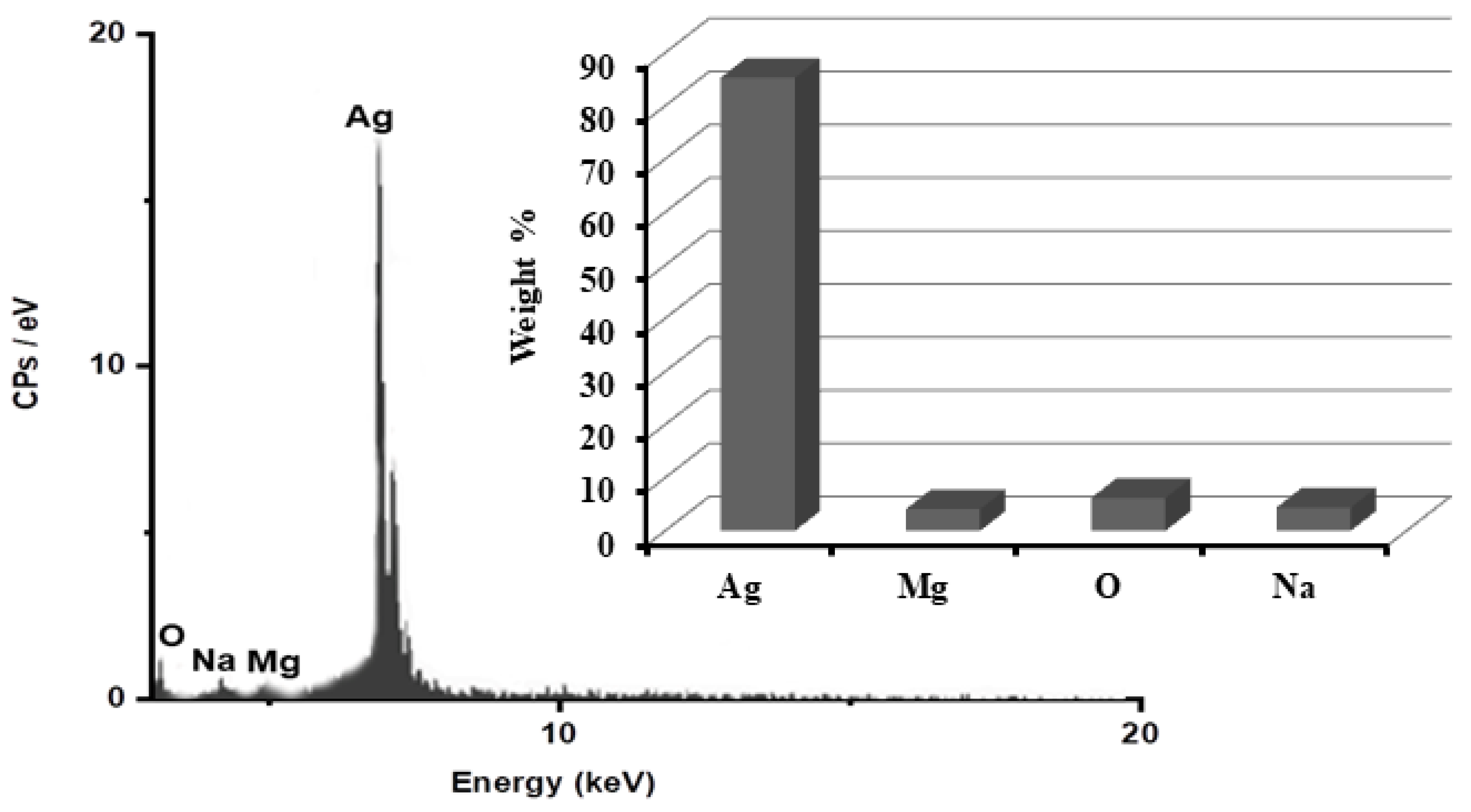
3.2. Characterization of ARLE-AgNPs
3.3. ARLE-AgNPs’ Antimicrobial Activity
3.4. Synergistic Activity of ARLE-AgNPs
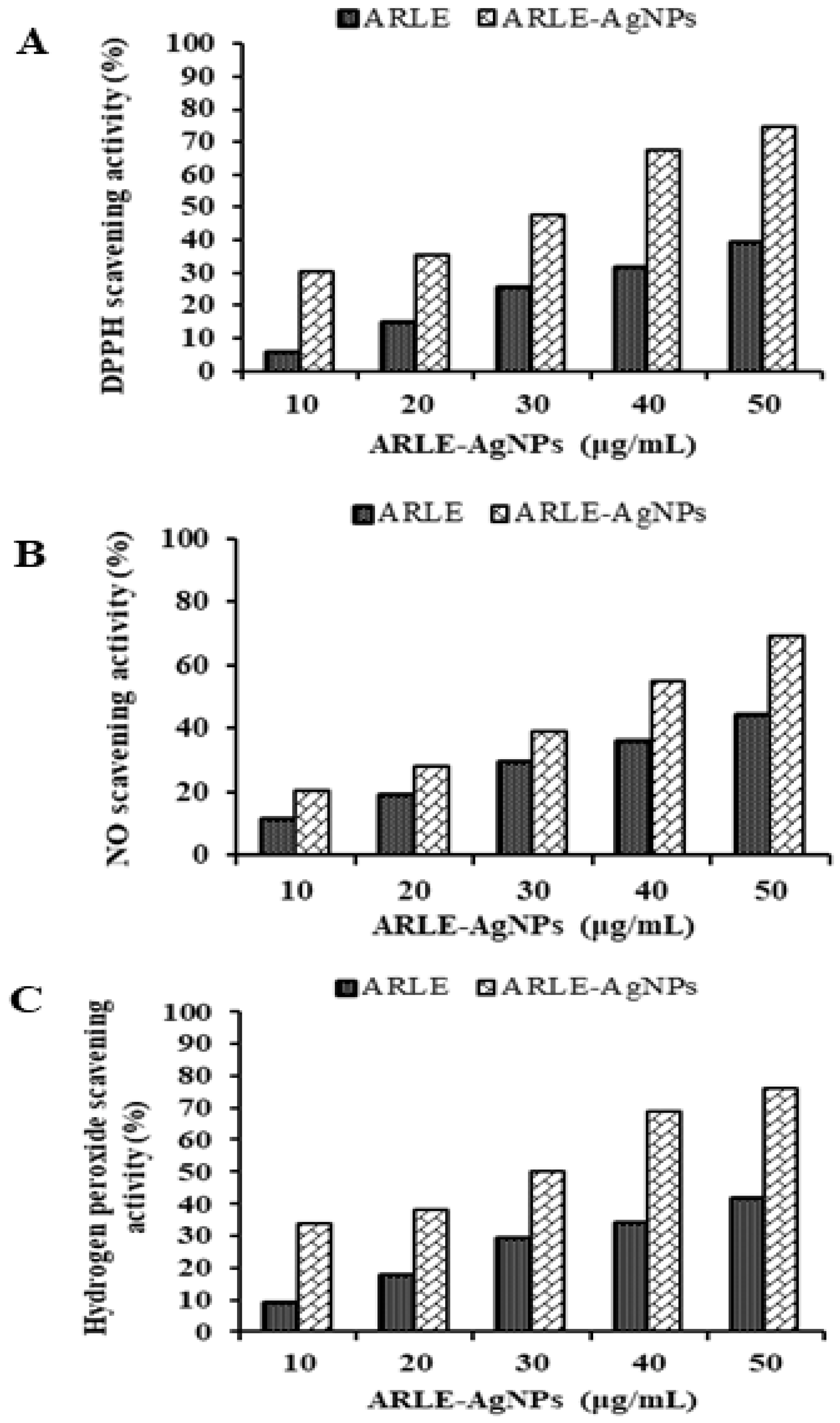
3.5. Antioxidant Activity of ARLE-AgNPs
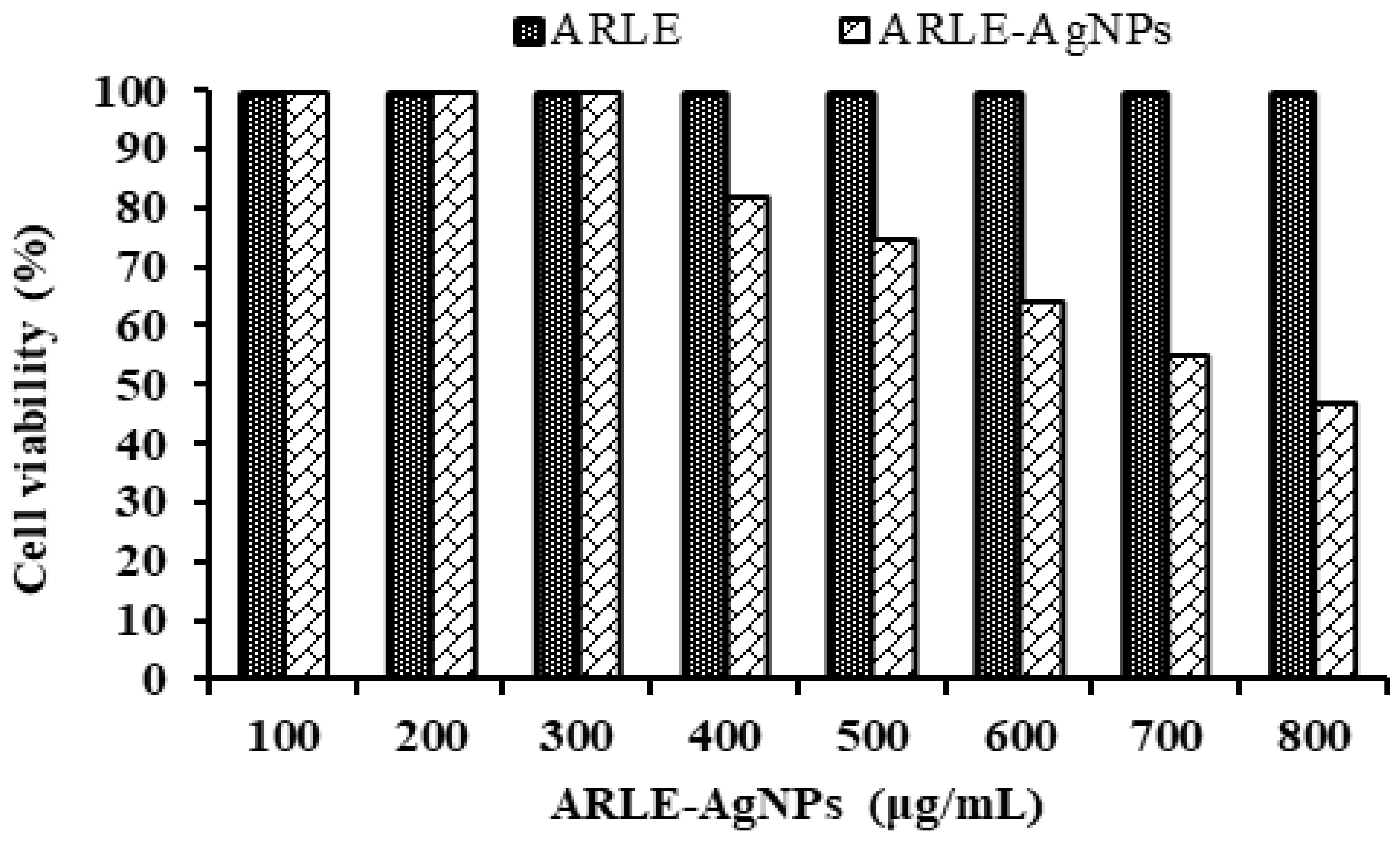
3.6. Biocompatibility Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tajkarimi, M.; Ibrahim, S.A.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Gabriël, S.; Dorny, P.; Saelens, G.; Dermauw, V. Foodborne Parasites and Their Complex Life Cycles Challenging Food Safety in Different Food Chains. Foods 2023, 12, 142. [Google Scholar] [CrossRef]
- WHO. World Health Organization’s First ever Global Estimates of Foodborne Diseases Find Children under 5 Account for Almost One Third of Deaths; WHO: Geneva, Switzerland, 2015; Available online: https://www.who.int/news-room/detail/03-12-2015-who-s-first-ever-global-estimates-of-foodborne-diseases-find-children-under-5-account-for-almost-one-third-of-deaths (accessed on 13 February 2021).
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. [Google Scholar] [CrossRef]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Nayak, D.; Pradhan, S.; Ashe, S.; Rauta, P.R.; Nayak, B. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 2015, 457, 329–338. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef]
- Abbasi, A.; Sarker, S.; Chiang, R.H. Big data research in information systems: Toward an inclusive research agenda. J. Assoc. Inf. Syst. 2016, 17, 3. [Google Scholar] [CrossRef]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Salam, H.A.; Rajiv, P.; Kamaraj, M.; Jagadeeswaran, P.; Gunalan, S.; Sivaraj, R. Plants: Green route for nanoparticle synthesis. Int. Res. J. Biol. Sci. 2012, 1, 85–90. [Google Scholar]
- Bindhu, M.; Umadevi, M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015, 135, 373–378. [Google Scholar] [CrossRef]
- Lakshmeesha, T.; Kumar, N.H.; Singh, A.; Udayashankar, A.; Jogaiah, S. Phytofabrication of nanoparticles through plant as nanofactories. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Elsevier: Amsterdam, The Netherlands, 2021; pp. 153–169. [Google Scholar]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A review of silver nanoparticles: Synthesis methods, properties and applications. Int. J. Mater. Sci. Appl. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Yadav, R.K.; Singh, N.; Singh, A.; Yadav, V.; Niharika, K.; Khare, S. Bio-based synthesis of nano silver using Tridax procumbens leaf extract and its impacts on germination and metabolic activity of Solanum lycopersicum L. J. Plant Biochem. Biotechnol. 2021, 30, 602–607. [Google Scholar] [CrossRef]
- Saad, A.M.; El-Saadony, M.T.; El-Tahan, A.M.; Sayed, S.; Moustafa, M.A.; Taha, A.E.; Taha, T.F.; Ramadan, M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021, 28, 5674–5683. [Google Scholar] [CrossRef] [PubMed]
- Al-Quran, S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009, 123, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.A.M.; El-Bakry, A.A.; Alam, E.A. Evaluation of antibacterial activity of different plant parts of Rumex vesicarius L. at early and late vegetative stages of growth. Int. J. Pharm. Pharm. Sci. 2012, 4, 426–435. [Google Scholar]
- Liang, H.-X.; Dai, H.-Q.; Fu, H.-A.; Dong, X.-P.; Adebayo, A.H.; Zhang, L.-X.; Cheng, Y.-X. Bioactive compounds from Rumex plants. Phytochem. Lett. 2010, 3, 181–184. [Google Scholar] [CrossRef]
- El-Hawary, S.A.; Sokkar, N.M.; Ali, Z.Y.; Yehia, M.M. A profile of bioactive compounds of Rumex vesicarius L. J. Food Sci. 2011, 76, C1195–C1202. [Google Scholar] [CrossRef]
- Gescher, K.; Hensel, A.; Hafezi, W.; Derksen, A.; Kühn, J. Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antivir. Res. 2011, 89, 9–18. [Google Scholar] [CrossRef]
- Ameta, G.; Punjabi, P.B. Biocidal Activity of Ag-Peg-Chitosan Nanocomposite Film Prepared Using Cannabis sativa Aqueous Leaf Extract by Sonication. Eur. Chem. Bull. 2018, 7, 233–238. [Google Scholar] [CrossRef]
- Barakat, O.; Elsebaie, E.; Ammar, A.; Elnemr, K. Utilization of Faba bean hulls (seeds coat) as a source to produce antioxidants. J. Food Dairy Sci. 2017, 8, 275–278. [Google Scholar] [CrossRef]
- Elsebaie, E.M.; Essa, R.Y. Microencapsulation of red onion peel polyphenols fractions by freeze drying technicality and its application in cake. J. Food Process. Preserv. 2018, 42, e13654. [Google Scholar] [CrossRef]
- Essa, R.; Elsebaie, E.M. Immobilization of synthesized silver nanoparticles using mango peel extract on low density polyethylene surface and its application as biologically active packages. Alex. J. Food Sci. Technol. 2016, 13, 31–38. [Google Scholar] [CrossRef]
- Valsalam, S.; Agastian, P.; Arasu, M.V.; Al-Dhabi, N.A.; Ghilan, A.-K.M.; Kaviyarasu, K.; Ravindran, B.; Chang, S.W.; Arokiyaraj, S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B Biol. 2019, 191, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.M.; Salem, N.M. A green and facile approach for synthesis of magnetite nanoparticles. Nanosci. Nanotechnol. 2012, 2, 208–213. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Jabeen, M.; Chauhan, A.; Ansari, M.A. Therapeutic potential of functionalized siRNA nanoparticles on regression of liver cancer in experimental mice. Sci. Rep. 2019, 9, 15825. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, C.; Padma, M.; Mareeswaran, R.; Suyavaran, A.; Kumar, M.S.; Premkumar, K.; Thirunavukkarasu, C. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf. B Biointerfaces 2013, 102, 808–815. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Zhang, X.; Esmail, G.A.; Alzeer, A.F.; Arasu, M.V.; Vijayaraghavan, P.; Choi, K.C.; Al-Dhabi, N.A. Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saudi J. Biol. Sci. 2020, 27, 3505–3513. [Google Scholar] [CrossRef]
- Fang, Y.; Hong, C.-Q.; Chen, F.-R.; Gui, F.-Z.; You, Y.-X.; Guan, X.; Pan, X.-H. Green synthesis of nano silver by tea extract with high antimicrobial activity. Inorg. Chem. Commun. 2021, 132, 108808. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Fujita, K.-I.; Kubo, A.; Nihei, K.-I.; Ogura, T. Antibacterial activity of coriander volatile compounds against Salmonella choleraesuis. J. Agric. Food Chem. 2004, 52, 3329–3332. [Google Scholar] [CrossRef] [PubMed]
- Rahim, K.A.A.A.; Mohamed, A.M.A. Bactericidal and antibiotic synergistic effect of nanosilver against methicillin-resistant Staphylococcus aureus. Jundishapur J. Microbiol. 2015, 8, e25867. [Google Scholar]
- Moteriya, P.; Chanda, S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Ferreira, I.C.; Barros, L.; Bento, A.; Pereira, J.A. Effect of solvent and extraction temperatures on the antioxidant potential of traditional stoned table olives “alcaparras”. LWT-Food Sci. Technol. 2008, 41, 739–745. [Google Scholar] [CrossRef]
- Rao, M. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Laouini, S.E.; Ouahrani, M.R. Phytochemical screening, in vitro antioxidant and antibacterial activity of Rumex vesicarius L. extract. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2017, 18, 367–376. [Google Scholar]
- Mohammed, S.A.; Panda, R.C.; Madhan, B.; Demessie, B.A. Extraction of bio-active compounds from Ethiopian plant material Rumex abyssinicus (mekmeko) root—A study on kinetics, optimization, antioxidant and antibacterial activity. J. Taiwan Inst. Chem. Eng. 2017, 75, 228–239. [Google Scholar] [CrossRef]
- Workineh, Y.T. Phytochemical Analysis and Determination of Antioxidant and Antibacterial Activities of Leaf Extracts of Rumex nervosus (Embacho). Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2021. [Google Scholar]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Xie, A.; Yu, X.; Qiu, L.; Zhang, L.; Zhang, Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007, 9, 852–858. [Google Scholar] [CrossRef]
- El Sheikha, A.F. Rumex nervosus: An overview. Int. J. Innov. Hortic. 2015, 4, 87–95. [Google Scholar]
- Habeeb Rahuman, H.B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechn. 2022, 16, 115–144. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.C.; Rusch, P.; Scheper, T.; Walter, J.-G. Hypericum perforatum L.-mediated green synthesis of silver nanoparticles exhibiting antioxidant and anticancer activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef]
- Tippayawat, P.; Phromviyo, N.; Boueroy, P.; Chompoosor, A. Green synthesis of silver nanoparticles in aloe vera plant extract prepared by a hydrothermal method and their synergistic antibacterial activity. PeerJ 2016, 4, e2589. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Qais, F.A.; Shafiq, A.; Khan, H.M.; Husain, F.M.; Khan, R.A.; Alenazi, B.; Alsalme, A.; Ahmad, I. Antibacterial effect of silver nanoparticles synthesized using Murraya koenigii (L.) against multidrug-resistant pathogens. Bioinorg. Chem. Appl. 2019, 2019, 4649506. [Google Scholar] [CrossRef]
- Kadam, J.; Dhawal, P.; Barve, S.; Kakodkar, S. Green synthesis of silver nanoparticles using cauliflower waste and their multifaceted applications in photocatalytic degradation of methylene blue dye and Hg2+ biosensing. SN Appl. Sci. 2020, 2, 738. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Alsaif, N.; Wani, T.A.; Bhat, M.A. Pomegranate peel induced biogenic synthesis of silver nanoparticles and their multifaceted potential against intracellular pathogen and cancer. Saudi J. Biol. Sci. 2021, 28, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef]
- Jinu, U.; Rajakumaran, S.; Senthil-Nathan, S.; Geetha, N.; Venkatachalam, P. Potential larvicidal activity of silver nanohybrids synthesized using leaf extracts of Cleistanthus collinus (Roxb.) Benth. ex Hook. f. and Strychnos nux-vomica L. nux-vomica against dengue, Chikungunya and Zika vectors. Physiol. Mol. Plant Pathol. 2018, 101, 163–171. [Google Scholar]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.-i.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Dunlap, P.V.; Clark, D.P. Brock biology of microorganisms 12th edn. Int. Microbiol. 2008, 11, 65–73. [Google Scholar]
- Sui, Z.; Chen, X.; Wang, L.; Xu, L.; Zhuang, W.; Chai, Y.; Yang, C. Capping effect of CTAB on positively charged Ag nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 33, 308–314. [Google Scholar] [CrossRef]
- Amro, N.A.; Kotra, L.P.; Wadu-Mesthrige, K.; Bulychev, A.; Mobashery, S.; Liu, G.-Y. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: Structural basis for permeability. Langmuir 2000, 16, 2789–2796. [Google Scholar] [CrossRef]
- Murray, P.R.; Rosenthal, K.S.; Pfaller, M.A. Medical Microbiology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Mani, M.; Okla, M.K.; Selvaraj, S.; Kumar, A.R.; Kumaresan, S.; Muthukumaran, A.; Kaviyarasu, K.; El-Tayeb, M.A.; Elbadawi, Y.B.; Almaary, K.S. A novel biogenic Allium cepa leaf mediated silver nanoparticles for antimicrobial, antioxidant, and anticancer effects on MCF-7 cell line. Environ. Res. 2021, 198, 111199. [Google Scholar] [CrossRef] [PubMed]
- Arokiyaraj, S.; Dinesh Kumar, V.; Elakya, V.; Kamala, T.; Park, S.K.; Ragam, M.; Saravanan, M.; Bououdina, M.; Arasu, M.V.; Kovendan, K. Biosynthesized silver nanoparticles using floral extract of Chrysanthemum indicum L.—Potential for malaria vector control. Environ. Sci. Pollut. Res. 2015, 22, 9759–9765. [Google Scholar] [CrossRef]
- Venkatadri, B.; Shanparvish, E.; Rameshkumar, M.; Arasu, M.V.; Al-Dhabi, N.A.; Ponnusamy, V.K.; Agastian, P. Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: In-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J. Biol. Sci. 2020, 27, 2980–2986. [Google Scholar] [CrossRef]
- Bindhu, M.; Umadevi, M.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111836. [Google Scholar] [CrossRef]
- Batarseh, K.I. Anomaly and correlation of killing in the therapeutic properties of silver (I) chelation with glutamic and tartaric acids. J. Antimicrob. Chemother. 2004, 54, 546–548. [Google Scholar] [CrossRef]
- MubarakAli, D.; LewisOscar, F.; Gopinath, V.; Alharbi, N.S.; Alharbi, S.A.; Thajuddin, N. An inhibitory action of chitosan nanoparticles against pathogenic bacteria and fungi and their potential applications as biocompatible antioxidants. Microb. Pathog. 2018, 114, 323–327. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.D.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008, 106, 888–895. [Google Scholar] [CrossRef]
- Sangaonkar, G.M.; Pawar, K.D. Garcinia indica mediated biogenic synthesis of silver nanoparticles with antibacterial and antioxidant activities. Colloids Surf. B Biointerfaces 2018, 164, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Muniyappan, N.; Nagarajan, N. Green synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activities. Process Biochem. 2014, 49, 1054–1061. [Google Scholar] [CrossRef]
- Lin, J.-J.; Lin, W.-C.; Li, S.-D.; Lin, C.-Y.; Hsu, S.-H. Evaluation of the antibacterial activity and biocompatibility for silver nanoparticles immobilized on nano silicate platelets. ACS Appl. Mater. Interfaces 2013, 5, 433–443. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Saeri, M.R.; Azizi, M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicol. Environ. Saf. 2015, 120, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Saptami, K.; Venkatesan, J.; Rekha, P. Microwave-assisted rapid synthesis of silver nanoparticles using fucoidan: Characterization with assessment of biocompatibility and antimicrobial activity. Int. J. Biol. Macromol. 2020, 163, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Tejashree, S.; Thanuja, V.; Hemashekhar, B.; Srinivas, C.; Nasif, O.; Pugazhendhi, A.; Raghavendra, V.B. Pomegranate fruit fleshy pericarp mediated silver nanoparticles possessing antimicrobial, antibiofilm formation, antioxidant, biocompatibility and anticancer activity. J. Drug Deliv. Sci. Technol. 2021, 61, 102289. [Google Scholar] [CrossRef]
| Phenolic Compound | % of the Total | Phenolic Compound | % of the Total |
|---|---|---|---|
| Gallic | 2.80 | caffeic | 1.84 |
| Protocatechuic | 5.19 | Vanillic | 3.93 |
| Pyrogallol | 23.32 | Caffeine | 2.60 |
| Epicatechin gallate | 27.42 | Ferulic | 1.61 |
| Chlorogenic | 4.09 | Β-OH Benzoic | 10.80 |
| Catechin | 11.45 | Epi-catechin | 4.95 |
| Bacteria | Inhibition Zones (mm) | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|
| Gram-negative bacteria | |||
| Escherichia coli ATCC25721 | 26 ± 3 a | 5.19 e | 46 e |
| Pseudomonas aeruginosa ATCC27843 | 22 ± 2 b | 14.07 d | 61 d |
| Gram-positive bacteria | |||
| Streptococcus gordonii ATCC49716 | 18 ± 3 d | 46.41 b | 92 c |
| Enterococcus faecalis ATCC700813 | 16 ± 2 e | 61.00 a | 101 b |
| Staphylococcus aureus ATCC4342 | 20 ± 2 c | 40.13 c | 119 a |
| Bacteria | Inhibition Zones (mm) | Fold Increase % = [(b − a)/a] × 100 | ||
|---|---|---|---|---|
| Ampicillin (a) | ARLE | Ampicillin + ARLE-AgNPs (b) | ||
| Escherichia coli ATCC25721 | 19 | 10 | 32 | 68.42 |
| Pseudomonas aeruginosa ATCC27843 | 15 | 5 | 26 | 73.33 |
| Streptococcus gordonii ATCC49716 | 16 | 7 | 21 | 31.25 |
| Enterococcus faecalis ATCC700813 | 14 | 5 | 18 | 28.57 |
| Staphylococcus aureus ATCC4342 | 18 | 8 | 25 | 38.89 |
| Overall synergistic antibacterial effect (%) = 48.09 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsebaie, E.M.; El-Wakeil, N.H.M.; Khalil, A.M.M.; Bahnasy, R.M.; Asker, G.A.; El-Hassnin, M.F.; Ibraheim, S.S.; El-Farsy, M.F.A.; Faramawy, A.A.; Essa, R.Y.; et al. Silver Nanoparticle Synthesis by Rumex vesicarius Extract and Its Applicability against Foodborne Pathogens. Foods 2023, 12, 1746. https://doi.org/10.3390/foods12091746
Elsebaie EM, El-Wakeil NHM, Khalil AMM, Bahnasy RM, Asker GA, El-Hassnin MF, Ibraheim SS, El-Farsy MFA, Faramawy AA, Essa RY, et al. Silver Nanoparticle Synthesis by Rumex vesicarius Extract and Its Applicability against Foodborne Pathogens. Foods. 2023; 12(9):1746. https://doi.org/10.3390/foods12091746
Chicago/Turabian StyleElsebaie, Essam Mohamed, Nora Hamdy Mouhamed El-Wakeil, Azhar Mostafa Mohamed Khalil, Rasha M. Bahnasy, Galila Ali Asker, Marwa Fawzy El-Hassnin, Suzan S. Ibraheim, Marwa Fawzi Ahmed El-Farsy, Asmaa Antar Faramawy, Rowida Younis Essa, and et al. 2023. "Silver Nanoparticle Synthesis by Rumex vesicarius Extract and Its Applicability against Foodborne Pathogens" Foods 12, no. 9: 1746. https://doi.org/10.3390/foods12091746
APA StyleElsebaie, E. M., El-Wakeil, N. H. M., Khalil, A. M. M., Bahnasy, R. M., Asker, G. A., El-Hassnin, M. F., Ibraheim, S. S., El-Farsy, M. F. A., Faramawy, A. A., Essa, R. Y., & Badr, M. R. (2023). Silver Nanoparticle Synthesis by Rumex vesicarius Extract and Its Applicability against Foodborne Pathogens. Foods, 12(9), 1746. https://doi.org/10.3390/foods12091746






