Response Surface Optimization of Extraction Conditions for the Active Components with High Acetylcholinesterase Inhibitory Activity and Identification of Key Metabolites from Acer truncatum Seed Oil Residue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Reagents
2.2. Ultrasound-Assisted Extraction Process
2.3. In Vitro AChE Inhibitory Activity Assays
2.4. Optimization of Extraction Process of Active Components on AChE Inhibition from ASR
2.4.1. Ultrasound-Assisted Extraction Independent Variables Assay
2.4.2. Response Surface Methodology Design
2.5. UPLC-QTOF-MS Analysis of ASR Compositions
2.6. Identification of Compounds and Statistical Analysis
3. Results and Discussion
3.1. Determination of the Range of Independent Variables
3.2. RSM Optimization of Extraction Condition for the Active Components
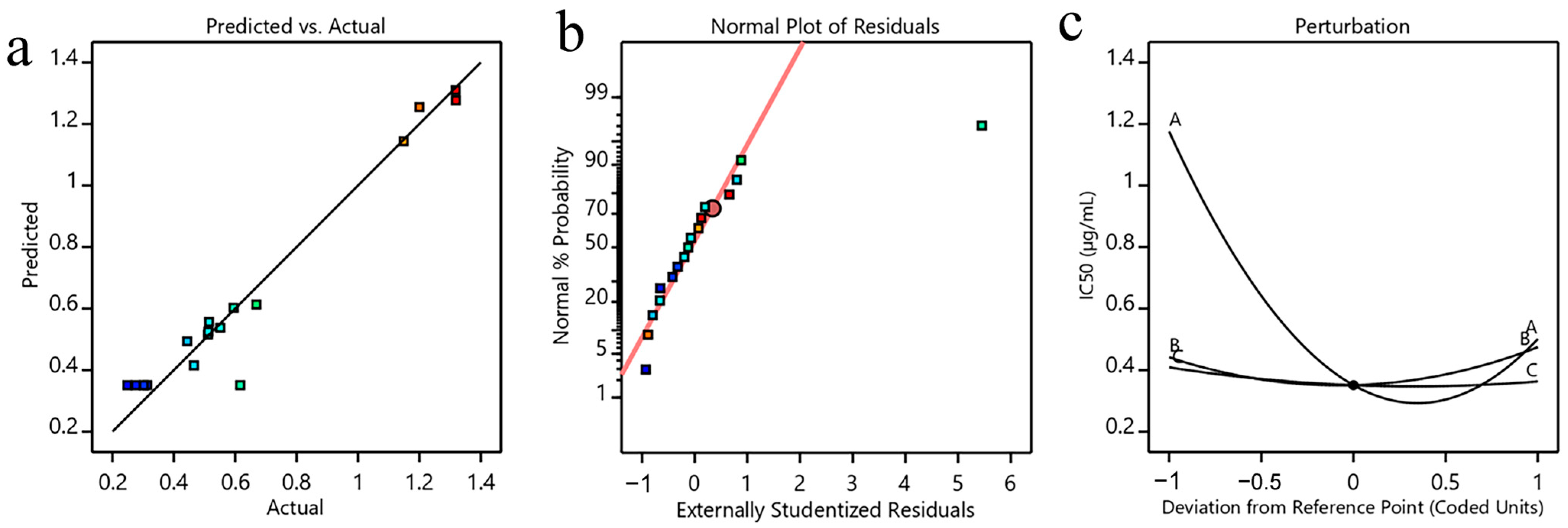
3.2.1. Box–Behnken Design and Model Fitting
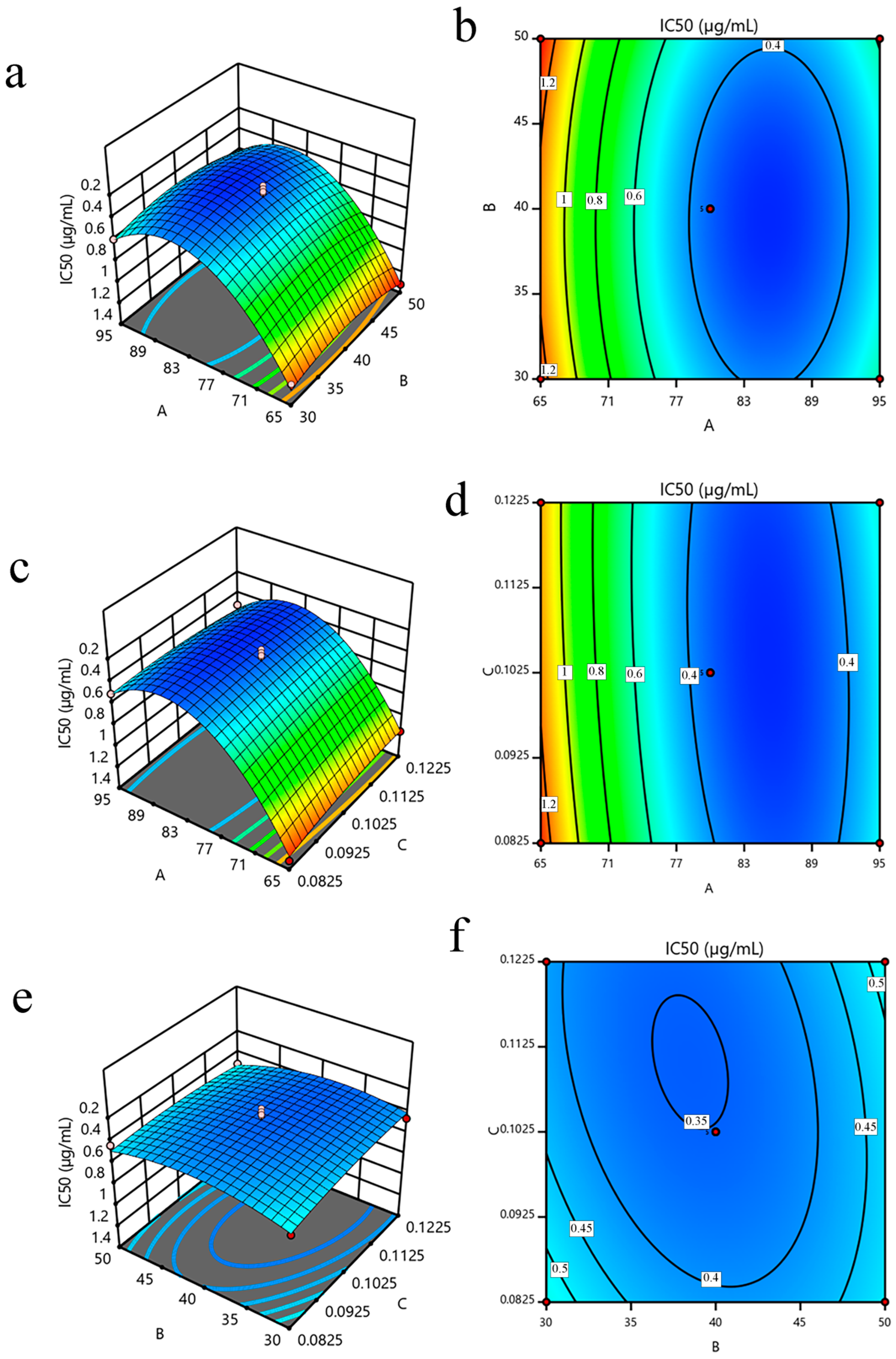
3.2.2. Analysis of the Variable Interaction
3.2.3. Optimization and Validation of the Model
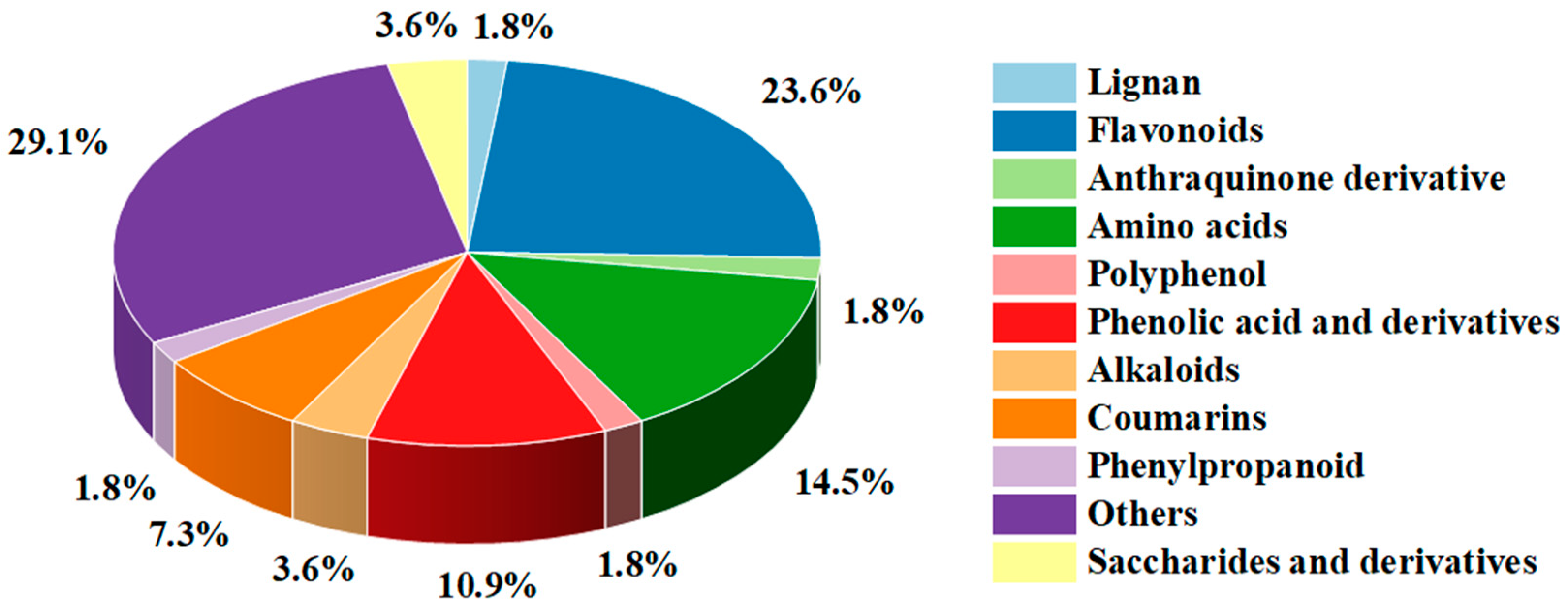
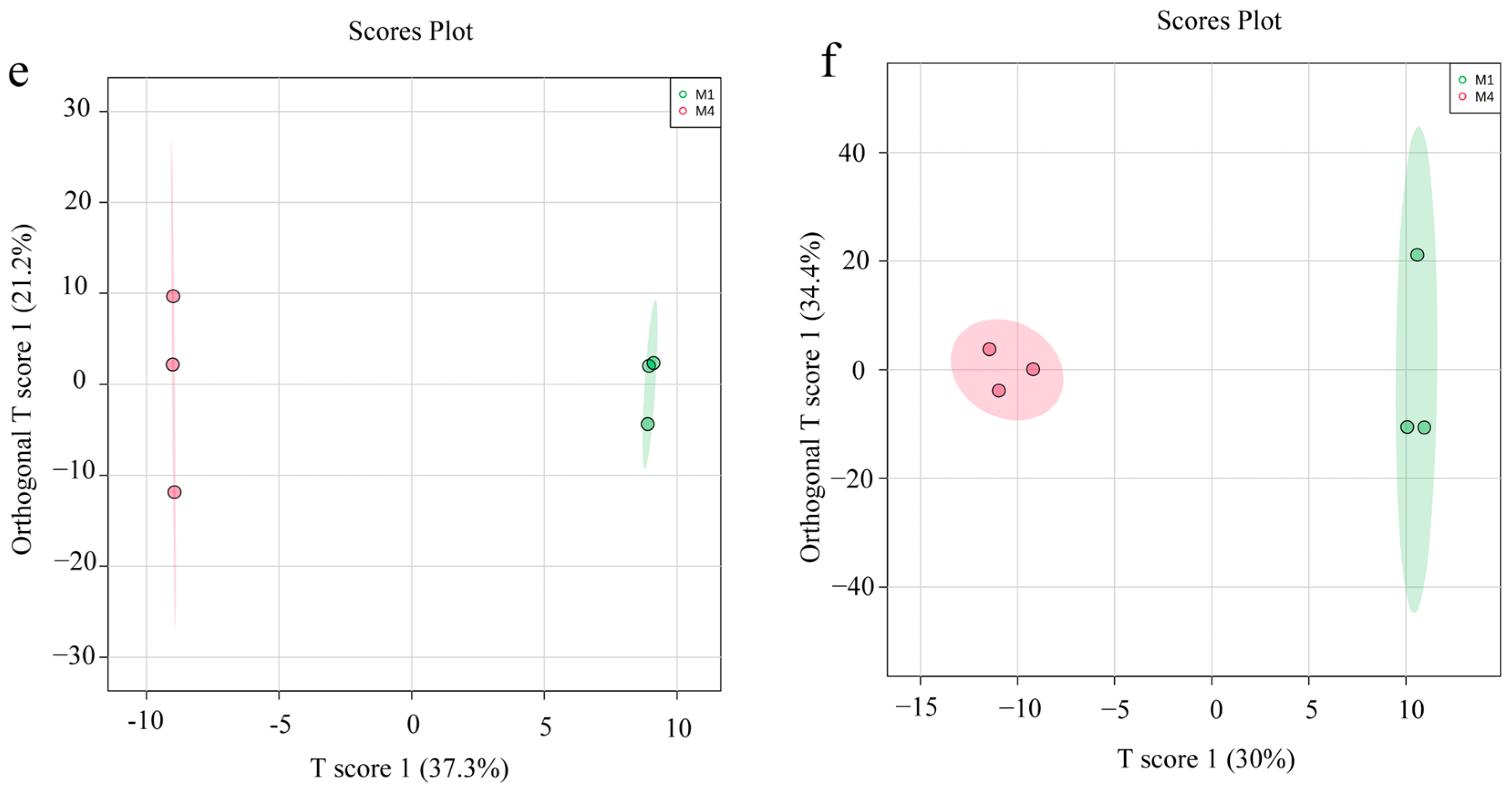
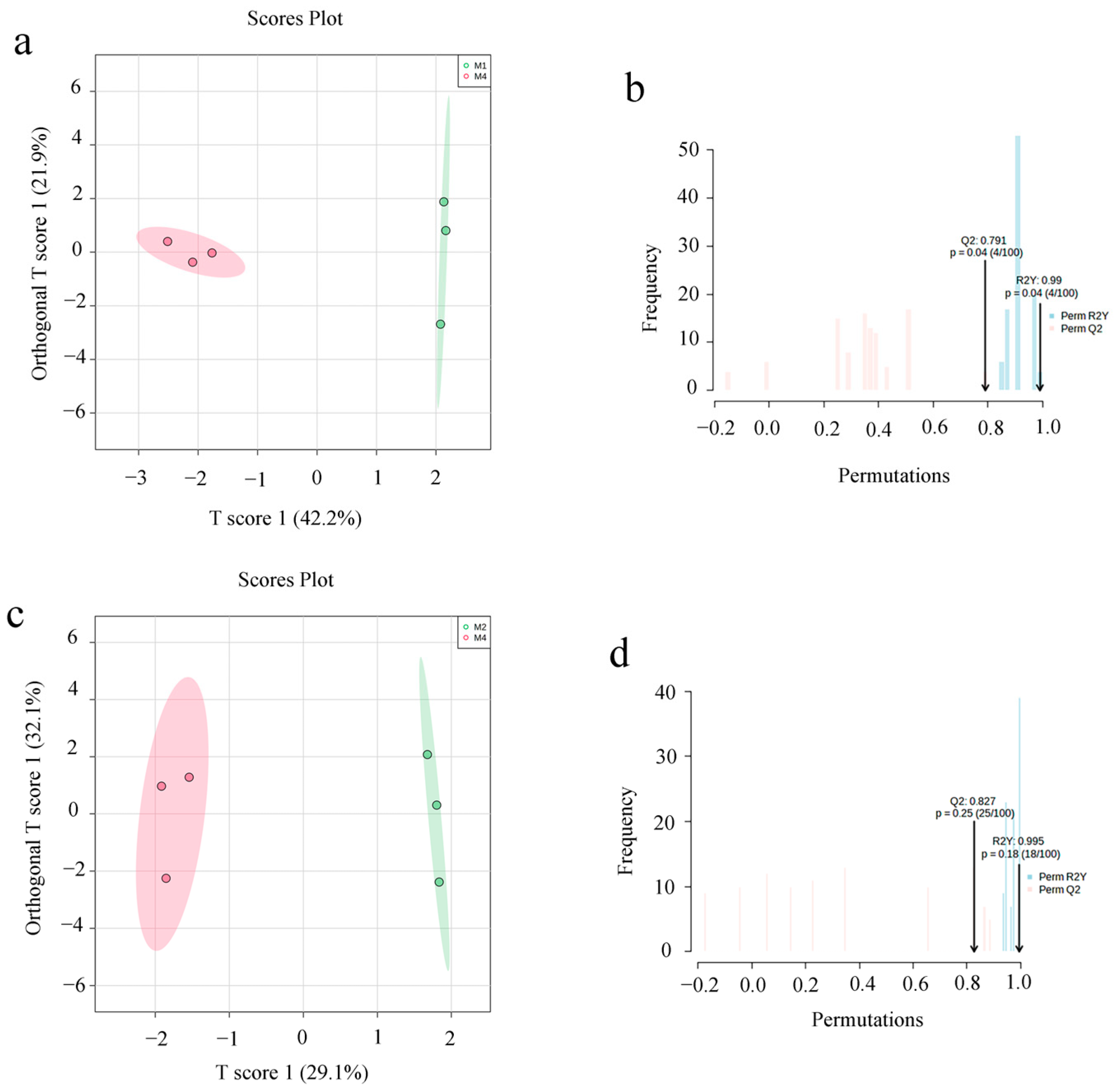
3.3. Non-Targeted Metabolite Analysis of ASR
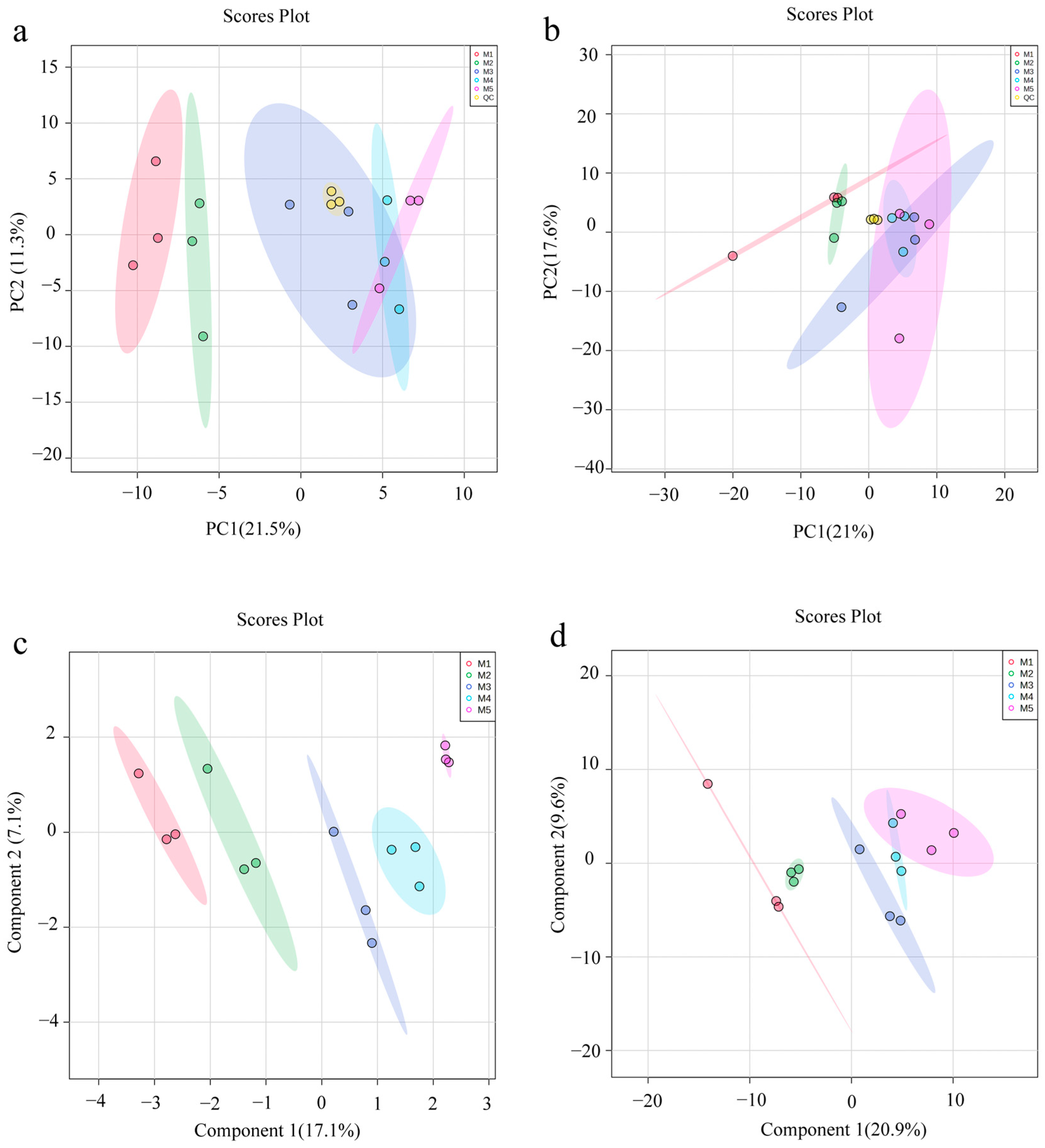
3.4. Multivariate Statistical Analysis
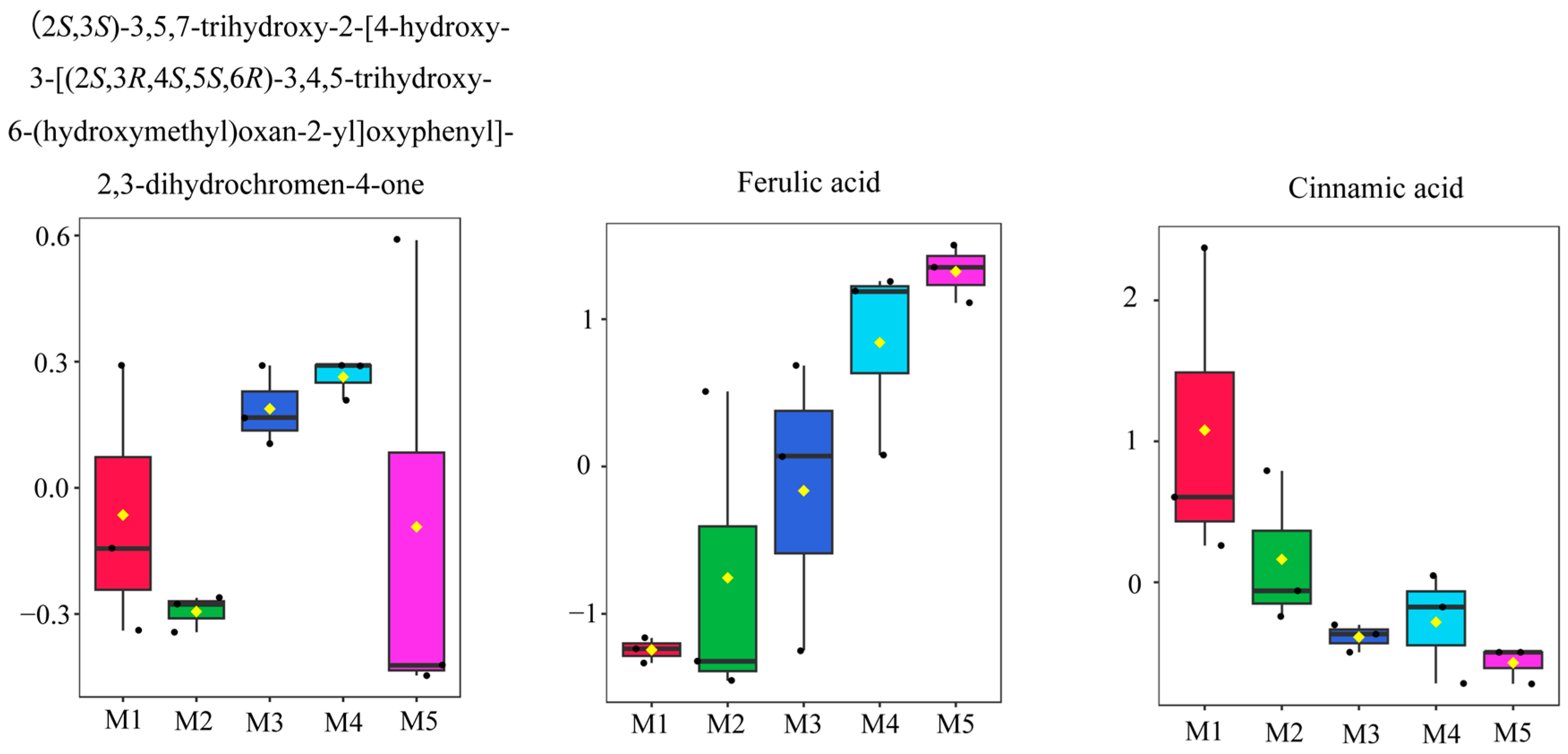
3.5. Screening of Differential Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, Y.; Kong, F.; Ma, X.F.; Shu, Q.Y. Inhibitory effect of Acer truncatum Bunge seed coat extract on fatty acid synthase, differentiation and lipid accumulation in 3T3-L1 adipocytes. Molecules 2022, 27, 1324. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, S.Q. New resource food Acer truncatum seed oil. China Oils Fats 2011, 36, 56–59. [Google Scholar]
- Wang, X.Y.; Fan, J.S.; Wang, S.Y.; Sun, R.C. A new resource of nervonic acid from purpleblow maple (Acer truncatum) seed oil. For. Prod. J. 2006, 56, 147–150. [Google Scholar] [CrossRef]
- Yang, R.N.; Zhang, L.X.; Li, P.W.; Yu, L.; Mao, J.; Wang, X.P.; Zhang, Q. A review of chemical composition and nutritional properties of minor vegetable oils in China. Trends Food Sci. Technol. 2018, 74, 26–32. [Google Scholar] [CrossRef]
- Jin, J.Q.; Ding, D.N.; He, L.C.; Lv, J.X.; Zhao, H. Preliminary study on the polysaccharides of Acer truncatum Leaves. Northwest J. Pharm. 1998, 13, 62–63. [Google Scholar]
- Zhang, F.; Luo, S.Y.; Ye, Y.B.; Zhao, W.H.; Sun, X.G.; Wang, Z.Q.; Li, R.; Sun, Y.H.; Tian, W.X.; Zhang, Y.X. The antibacterial efficacy of an aceraceous plant Shantung maple (Acer truncatum Bunge) may be related to inhibition of bacterial beta-oxoacyl-acyl carrier protein reductase (FabG). Biotechnol. Appl. Biochem. 2008, 51, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.X.; Lin, F.K.; Zhang, R.F.; Wang, M.M.; Gu, R.H.; Long, C.L. Acer truncatum Bunge: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 282, 114572. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, S.Q. New resource of nervonic acid Acer truncatum oil. China Oils Fats 2005, 30, 62–64. [Google Scholar]
- Yue, Y. Mechanisms of Polyunsaturated Fatty Acid-Mediated Development in Spatial Memory of Developing Rats. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2015. [Google Scholar]
- Bi, X.J.; Wei, L.; Chai, Y.M.; Yang, H.Y.; Yu, X.X.; Pu, D.W.; Huang, D.C.; Lu, Q. Study on component analysis and activity of ethanol extract from processing residues of new resource food peony. J. Food Nutr. Sci. 2021, 10, 133–147. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Gao, J.Y.; Du, M.Z.; Zhang, K.; Zhang, J.L.; Xue, N.C.; Yan, M.; Qu, C.X.; Deng, R.X. HPLC-DAD analysis of 15 monoterpene glycosides in oil peony seed cakes sourced from different cultivation areas in China. Ind. Crops Prod. 2018, 118, 259–270. [Google Scholar] [CrossRef]
- Chen, F.L.; Zhang, X.L.; Zhang, Q.; Du, X.Q.; Lei, Y.; Zu, Y.G.; Yang, F.J. Simultaneous synergistic microwave-ultrasonic extraction and hydrolysis for preparation of trans-resveratrol in tree peony seed oil-extracted residues using imidazolium-based ionic liquid. Ind. Crops Prod. 2016, 94, 266–280. [Google Scholar] [CrossRef]
- Hong, C.C.; Chang, C.; Zhang, H.; Jin, Q.Z.; Wu, G.C.; Wang, X.G. Identification and characterization of polyphenols in different varieties of Camellia oleifera seed cakes by UPLC-QTOF-MS. Food Res. Int. 2019, 126, 108614. [Google Scholar] [CrossRef]
- Li, T.T.; Wu, C.E.; Meng, X.Y.; Fan, G.J.; Tang, Y. Structural characterization and antioxidant activity of a glycoprotein isolated from Camellia oleifera Abel seeds against D-galactose-induced oxidative stress in mice. J. Funct. Foods 2020, 64, 103594. [Google Scholar] [CrossRef]
- Milinčića, D.D.; Stanisavljević, S.N.; Kostića, A.Z.; Bajićb, S.S.; Kojićb, M.O. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. Food Sci. Tech.-Braz. 2021, 138, 110739. [Google Scholar] [CrossRef]
- Costa, G.N.S.; Tonon, R.V.; Mellinger-silva, C.; Galdeano, M.C.; Lacomini, M.; Santiago, M.C. Grape seed pomace as a valuable source of antioxidant fibers. J. Sci. Food Agric. 2019, 99, 4593–4601. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Ahmed, A.; Fusi, F.; Sciubba, F.; Aiello, F. Vasorelaxant effects induced by red wine and pomace extracts of Magliocco dolce Cv. Pharmaceuticals 2020, 13, 87. [Google Scholar] [CrossRef]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between total antioxidant capacity, polyphenol and fatty acid content of native grape seed and pomace of four different grape varieties in hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Li, J.M.; Wang, K.H.; Wang, X.J.; Xu, H.D. Nutritional and physicochemical properties of Acer truncatum seed protein. Food Sci. 2021, 42, 271–277. [Google Scholar]
- Bi, X.J.; Wei, L.; Yang, H.Y.; Yu, X.X.; Pu, D.W.; Lu, Q.; Zu, Y.G. Optimization of the enzymatic hydrolysis process of Acer truncatum seed meal by response surface methodology and the functional characteristics of the polypeptide obtained. Sci. Technol. Food Ind. 2022, 43, 204–214. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Li, S.S.; Song, M.J.; Chen, J.B.; Liu, C.; Zhang, L.; Li, D.L.; Sun, Y.S. Chemical constituents of 70% EtOH extract from the kernel of Acer truncatum Bunge. Food Sci. 2017, 38, 137–143. [Google Scholar]
- Fan, H.; Sun, L.W.; Yang, L.G.; Zhou, J.C.; Yin, P.P.; Li, K.; Xue, Q.; Li, X.; Liu, Y.J. Assessment of the bioactive phenolic composition of Acer truncatum seed coat as a byproduct of seed oil. Ind. Crops Prod. 2018, 118, 11–19. [Google Scholar] [CrossRef]
- Gu, R.H.; Morcol, T.; Liu, B.; Shi, M.J.; Long, C.L. GC–MS, UPLC-QTOF-MS, and bioactivity characterization of Acer truncatum seeds. Ind. Crops Prod. 2019, 138, 111480. [Google Scholar] [CrossRef]
- Li, X.Y.; Bao, X.J.; Wang, R.Z. Neurogenesis-based epigenetic therapeutics for Alzheimer’s disease. Mol. Med. Rep. 2016, 14, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Zheng, X.W.; Chen, S.; Shan, C.S.; Xu, Q.Q.; Zhu, J.Z.; Bao, X.Y.; Lin, Y.; Zheng, G.Q.; Wang, Y. Chinese herbal medicine for Alzheimer’s disease: Clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 2017, 141, 143–155. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Akhtar, S.; Jamal, Q.M.; Rizvki, S.M.D. Multiple targets for the management of Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2016, 15, 1279–1289. [Google Scholar] [CrossRef]
- Xue, X.F.; Zhao, A.L.; Wang, Y.K.; Ren, H.Y.; Li, Y.; Li, D.K.; Du, J.J. Metabolomics-based analysis of flavonoid metabolites in Chinese jujube and sour jujube fruits from different harvest periods. J. Food Sci. 2022, 87, 3752–3765. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.N.; Wang, H.C.; Guo, C.C.; Zhang, W.E.; Luo, P.S.; Zhou, J.; Hao, W.W.; Yang, G.Y.; Huang, J.J. Identification of key taste components in Baccaurea ramiflora Lour. fruit using non-targeted metabolomics. Food Sci. Hum. Wellness 2023, 12, 94–101. [Google Scholar] [CrossRef]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csóka, L. Ultrasound-assisted extraction of cannabinoids from Cannabis sativa L. optimized by response surface methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef]
- Yang, Y.C.; Wei, M.C.; Chiu, H.F.; Huang, T.C. Development and validation of a modified ultrasound-assisted extraction method and a HPLC method for the quantitative determination of two triterpenic acids in Hedyotis diffusa. Nat. Prod. Commun. 2013, 8, 1683–1686. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Cui, L.; Ma, Z.; Wang, D.; Niu, Y. Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from astragalus membranaceus stems and leaves. Ultrason. Sonochem. 2022, 90, 106190. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, H.; Nakabayashi, R.; Mori, T.; Yamada, Y.; Takahashi, M.; Rai, A.; Sugiyama, R.; Yamamoto, H.; Nakaya, T.; Yamazaki, M.; et al. A cheminformatics approach to characterize metabolomes in stable-isotope-labeled organisms. Nat. Methods 2019, 16, 295–298. [Google Scholar] [CrossRef]
- Zong, Y.; Xiong, D.L.; Li, Y.; Wang, L.Q.; Cao, X.W.; Ouyang, S.B.; Yang, J.B. Research on extracting technology of tea saponin by ultrasonic assisted methanol. J. Jiangxi Univ. Sci. Technol. 2019, 40, 35–39. [Google Scholar] [CrossRef]
- Shirzad, H.; Niknam, V.; Taheri, M.; Ebrahimzadeh, H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: A nutraceutical study using RSM and LC–ESI–DAD–MS. J. Food Sci. Technol. 2017, 54, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.L.; Liang, C.G.; Huang, P.; Liu, Q.; Xu, Y.J.; Ding, C.B.; Li, T. Optimization of rice lipid production from ultrasound-assisted extraction by response surface methodology. J. Cereal Sci. 2016, 70, 23–28. [Google Scholar] [CrossRef]
- Yue, T.L.; Shao, D.Y.; Yuan, Y.H.; Wang, Z.L.; Qiang, C.Y. Ultrasound-assisted extraction, HPLC analysis, and antioxidant activity of polyphenols from unripe apple. J. Sep. Sci. 2012, 35, 2138–2145. [Google Scholar] [CrossRef]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, H.; Sadramell, S.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crops Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Zhang, E.H.; Wang, Y.; Nie, Z.H.; Ma, T.J. On the ultrasoni cextraction of flavonoids in tartary buckwheat sprouts. J. Beijing Univ. Agric. 2022, 37, 104–108. [Google Scholar] [CrossRef]
- Thu, H.N.; Huyen, T.T.; Van, P.N. Application of multivariate linear regression models for selection of deep eutectic solvent for extraction of apigenin and luteolin from Chrysanthemum indicum L. Phytochem. Anal. 2022, 33, 427–440. [Google Scholar] [CrossRef]
- Mollica, J.N.; Franca, M.M. Gas chromatographic determination of nonvolatile organic acids in sap of sugar maple (Acer saccharum Marsh.). J. Assoc. Off. Anal. Chem. 1984, 67, 1125–1129. [Google Scholar] [CrossRef]
- Ashihara, H.; Nobusawa, E. Metabolic fate of adenine and hypoxanthine in higher plants. Z. Fuer Pflanzenphysiol. 1981, 104, 443–458. [Google Scholar] [CrossRef]
- Pukacka, S.; Pukacki, P.M. Changes in soluble sugars in relation to desiccation tolerance and effects of dehydration on freezing characteristics of Acer platanoides and Acer pseudoplatanus seeds. Acta Physiol. Plant. 1997, 19, 147–154. [Google Scholar] [CrossRef]
- Garcia, E.J.; McDowell, T.; Ketola, C.; Jennings, M.; Miller, J.D.; Renaud, J.B. Metabolomics reveals chemical changes in Acer saccharumsap over a maple syrup production season. PLoS ONE 2020, 15, e0235787. [Google Scholar] [CrossRef] [PubMed]
- Barrales-Cureo, H.J.; Salgado-Garciglia, R.; López-Valdez, L.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Lucho-Constantino, G.G.; Zaragoza-Martínez, F.; Herrera-Cabrera, B.E.; Reyes, C. Metabolomic data of phenolic compounds from Acer negundo extracts. Data Brief 2020, 30, 105569. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, F.; Achim, A.; Stevanovic, T. Composition of ethanolic extracts of wood and bark from Acer saccharum and Betula alleghaniensis trees of different vigor classes. Ind. Crops Prod. 2013, 41, 179–187. [Google Scholar] [CrossRef]
- Ma, X.; Wu, L.; Ito, Y.; Tian, W. Application of preparative high-speed counter-current chromatography for separation of methyl gallate from Acer truncatum Bunge. J. Chromatogr. A 2005, 1076, 212–215. [Google Scholar] [CrossRef]
- Park, K.M.; Yang, M.C.; Lee, K.H.; Kim, K.R.; Choi, S.U.; Lee, K.R. Cytotoxic phenolic constituents of Acer tegmentosum maxim. Arch. Pharm. Res. 2006, 29, 1086–1090. [Google Scholar] [CrossRef]
- Wan, C.; Yuan, T.; Xie, M.; Seeram, N.P. Acer rubrum phenolics include A-type procyanidins and a chalcone. Biochem. Syst. Ecol. 2012, 44, 1–3. [Google Scholar] [CrossRef]
- Park, S.; Lee, H.Y.; Nhiem, N.X.; Lee, T.H.; Kim, N.; Cho, S.H.; Kim, S.H. A new phenyl ethyl glycoside from the twigs of Acer tegmentosum. Nat. Prod. Commun. 2015, 10, 1251–1252. [Google Scholar] [CrossRef]
- Thakur, M.L. Phenolic growth inhibitors isolated from dormant buds of sugar maple (Acer saccharum Marsh). J. Exp. Bot. 1977, 28, 795–803. [Google Scholar] [CrossRef]
- Yang, H.Y.; Sung, S.H.; Kim, Y.C. Two new hepatoprotective stilbene glycosides from Acer mono leaves. J. Nat. Prod. 2005, 68, 101–103. [Google Scholar] [CrossRef]
- Singh, Y.P.; Rai, H.; Singh, G.; Singh, G.K.; Mishra, S.; Kumar, S.; Srikrishna, S.; Modi, G. A review on ferulic acid and analogs based scaffolds for the management of Alzheimer’s disease. Eur. J. Med. Chem. 2021, 215, 113278. [Google Scholar] [CrossRef] [PubMed]
- Rollinger, J.M.; Hornick, A.; Langer, T.; Stuppner, H.; Prast, H. Acetylcholinesterase inhibitory activity of scopolin and scopoletin discovered by virtual screening of natural products. J. Med. Chem. 2004, 47, 6248–6254. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E.; Kucukboyaci, N.; Calis, I.; Ceron-Carrasco, J.P.; den-Haan, H.; Pena-Garcia, J.; Perez-Sanchez, H. Acetylcholinesterase inhibitory assessment of isolated constituents from Salsola grandis Freitag, Vural & Adiguzel and molecular modeling studies on N-acetyltryptophan. Phytochem. Lett. 2017, 20, 373–378. [Google Scholar] [CrossRef]
- Gacar, N.; Mutlu, O.; Utkan, T.; Komsuoglu Celikyurt, I.; Gocmez, S.S.; Ulak, G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol. Biochem. Behav. 2011, 99, 316–323. [Google Scholar] [CrossRef]
- Okello, E.J.; Mather, J. Comparative kinetics of acetyl- and butyryl-cholinesterase inhibition by green tea catechins relevance to the symptomatic treatment of Alzheimer’s disease. Nutrients 2020, 12, 1090. [Google Scholar] [CrossRef]
- Lan, J.S.; Hou, J.W.; Liu, Y.; Ding, Y.; Zhang, Y.; Li, L.; Zhang, T. Design, synthesis and evaluation of novel cinnamic acid derivatives bearing N-benzyl pyridinium moiety as multifunctional cholinesterase inhibitors for Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2017, 32, 776–788. [Google Scholar] [CrossRef]
- He, P.; Yan, S.; Zheng, J.; Gao, Y.; Zhang, S.; Liu, Z.; Liu, X.; Xiao, C. Eriodictyol attenuates LPS-induced neuroinflammation, amyloidogenesis, and cognitive impairments via the inhibition of NF-KB in male C57BL/6J mice and BV2 microglial cells. J. Agric. Food Chem. 2018, 66, 10205–10214. [Google Scholar] [CrossRef]



| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1: methanol concentration (%) | 65 | 80 | 95 |
| X2: ultrasonic time (min) | 30 | 40 | 50 |
| X3: material-to-liquid ratio (g/mL) | 0.825:10 | 1.025:10 | 1.225:10 |
| Run | Methanol Concentration (X1, %) | Ultrasonic Time (X2, min) | Material-to-Liquid Ratio (X3, g/mL) | IC50 (Y, µg/mL) |
|---|---|---|---|---|
| 1 | 80 | 30 | 0.825:10 | 0.5510 ± 0.0560 |
| 2 | 80 | 40 | 1.025:10 | 0.3130 ± 0.0810 |
| 3 | 95 | 40 | 0.825:10 | 0.5105 ± 0.0535 |
| 4 | 80 | 40 | 1.025:10 | 0.2480 ± 0.0210 |
| 5 | 95 | 50 | 1.025:10 | 0.6685 ± 0.0595 |
| 6 | 80 | 50 | 0.825:10 | 0.4435 ± 0.0055 |
| 7 | 65 | 40 | 1.225:10 | 1.1490 ± 0.1530 |
| 8 | 65 | 50 | 1.025:10 | 1.3185 ± 0.0035 |
| 9 | 80 | 40 | 1.025:10 | 0.3020 ± 0.0500 |
| 10 | 80 | 40 | 1.025:10 | 0.2765 ± 0.0545 |
| 11 | 95 | 30 | 1.025:10 | 0.5945 ± 0.0255 |
| 12 | 65 | 40 | 0.825:10 | 1.3190 ± 0.0035 |
| 13 | 95 | 40 | 1.225:10 | 0.5145 ± 0.0115 |
| 14 | 80 | 40 | 1.025:10 | 0.6160 ± 0.0560 |
| 15 | 65 | 30 | 1.025:10 | 1.2000 ± 0.0230 |
| 16 | 80 | 50 | 1.225:10 | 0.5120 ± 0.0340 |
| 17 | 80 | 30 | 1.225:10 | 0.4655 ± 0.0525 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2.02 | 9 | 0.2249 | 14.93 | 0.0009 | Significant |
| X1-Methanol concentration | 0.9102 | 1 | 0.9102 | 60.43 | 0.0001 | |
| X2-Ultrasonic time | 0.0022 | 1 | 0.0022 | 0.1435 | 0.7160 | |
| X3-Material-to-liquid ratio | 0.0042 | 1 | 0.0042 | 0.2779 | 0.6143 | |
| X1X2 | 0.0005 | 1 | 0.0005 | 0.0329 | 0.8613 | |
| X1X3 | 0.0076 | 1 | 0.0076 | 0.5025 | 0.5013 | |
| X2X3 | 0.0059 | 1 | 0.0059 | 0.3936 | 0.5503 | |
| X12 | 0.9997 | 1 | 0.9997 | 66.37 | <0.0001 | |
| X22 | 0.0482 | 1 | 0.0482 | 3.20 | 0.1167 | |
| X32 | 0.0051 | 1 | 0.0051 | 0.3403 | 0.5780 | |
| Residual | 0.1054 | 7 | 0.0151 | |||
| Lack of fit | 0.0152 | 3 | 0.0051 | 0.2246 | 0.8750 | Not significant |
| Pure error | 0.0902 | 4 | 0.0226 | |||
| Cor total | 2.13 | 16 | ||||
| R2 = 0.9505 | R2Adj = 0.8868 | R2pred = 0.8196 |
| NO. | Rt (min) | Parent Ions | Error (ppm) | Chemical Formula | MS Fragments | Identifications | References |
|---|---|---|---|---|---|---|---|
| 1 | 0.532 | 191.0199 [M−H]− | 3 | C6H8O7 | — | Citric acid | [42] |
| 2 | 0.681 | 341.1088 [M−H]− | −1.3 | C12H22O11 | 323.0971 (C12H19O10) [M−H−H2O]−, 305.0873 (C12H17O9) [M−H−2H2O]−, 179.0553 (C6H11O6) [M−C6H11O5]− | Trehalose | |
| 3 | 0.693 | 119.0353 [M−H]− | −6 | C5H4N4 | — | Purine | [43] |
| 4 | 0.694 | 181.0706 [M−H]− | 3 | C6H14O6 | 163.0609 (C6H11O5) [M−H−H2O]−, 119.0346 (C4H7O4) [M−2H−C2H5O2]− | Sorbitol | |
| 5 | 0.696 | 341.1088 [M−H]− | −3.9 | C12H22O11 | — | Sucrose | [44] |
| 6 | 0.822 | 343.1228 [M+H]+ | −2 | C12H22O11 | 163.0604 (C6H11O5) [M−C6H11O6]+ | 2-O-alpha-D-Mannopyranosyl-D-mannopyranose | |
| 7 | 1.052 | 146.0921 [M+H]+ | 0.7 | C5H11N3O2 | 129.0654 (C5H9N2O2) [M−NH2]+, 128.0818 (C5H10N3O) [M−OH]+ | 4-Guanidinobutyric acid | |
| 8 | 1.067 | 124.0391 [M+H]+ | −4.9 | C6H5NO2 | 106.0292 (C6H4NO) [M+H−H2O]+ | Nicotinic acid | |
| 9 | 1.114 | 206.0813 [M−H]− | −3.2 | C11H13NO3 | 164.0716 (C9H10NO2) [M−CO−CH3]− | N-acetyl-L-phenylalanine | |
| 10 | 1.159 | 130.08 82 [M−H]− | 8.1 | C6H13NO2 | — | Leucine | [45] |
| 11 | 1.161 | 147.0446 [M−H]− | 3 | C9H8O2 | — | Cinnamic acid | [46] |
| 12 | 1.518 | 136.0619 [M+H]+ | 7.6 | C5H5N5 | 119.0358 (C5H3N4) [M−NH2]+ | Adenine | |
| 13 | 1.607 | 245.0933 [M−H]− | 3.4 | C13H14N2O3 | 116.0504 (C8H6N) [M−C5H8NO3]−, 203.0828 (C11H11N2O2) [M−CO−CH3]− | N-acetyltryptophan | |
| 14 | 1.655 | 289.0700 [M−H]− | −5.2 | C14H14N2O5 | 245.0930 (C13H13N2O3) [M−COOH]−, 203.0822 (C11H11N2O2) [M−C3H3O3]− | N-Malonyltryptophan | |
| 15 | 1.85 | 101.0599 [M+H]+ | 9.8 | C5H8O2 | 59.0492 (C3H7O) [M+H−C2H2O]+, 73.0654 (C4H9O) [M+H−CO]+ | 5-Valerolactone | |
| 16 | 2.333 | 164.0717 [M−H]− | 6.7 | C9H11NO2 | 147.0449 (C9H7O2) [M−H−NH3]− | Phenylalanine | |
| 17 | 5.066 | 205.0968 [M+H]+ | −4.2 | C11H12N2O2 | 118.0655 (C8H8N) [M+2H−C3H6NO2]+, 132.0804 (C9H10N) [M+2H-C2H4NO2]+ | D-Tryptophan | |
| 18 | 5.132 | 205.0963 [M+H]+ | −5.6 | C11H12N2O2 | 118.0650 (C8H8N) [M+2H−C3H6NO2]+, 132.0814 (C9H10N) [M+2H−C2H4NO2]+ | L-Tryptophan | |
| 19 | 5.184 | 141.0551 [M+H]+ | −3.7 | C7H8O3 | 109.0294 (C6H5O2) [M−CH2OH]+, 123.0451(C7H7O2) [M+H−H2O]+ | Gentisyl alcohol | |
| 20 | 5.202 | 205.0966 [M+H]+ | −1.7 | C11H12N2O2 | 118.0652 (C8H8N) [M+2H−C3H6NO2]+, 132.0806 (C9H10N) [M+2H-C2H4NO2]+ | DL-Tryptophan | |
| 21 | 5.834 | 146.0583 [M+H]+ | −1 | C9H7NO | 118.0662 (C8H8N) [M+H−CO]+ | 4-Hydroxyquinoline | |
| 22 | 6.403 | 291.0874 [M+H]+ | 2 | C15H14O6 | 39.0378 (C7H7O3) [M+H−C8H8O3]+, 123.0475 (C7H7O2) [M+H−C8H8O4]+ | Catechin | |
| 23 | 6.486 | 199.0591 [M+H]+ | 0.5 | C9H10O5 | — | Syringic acid | [47] |
| 24 | 6.713 | 247.1437 [M+H]+ | −4.1 | C14H18N2O2 | 188.0706 (C11H10NO2) [M+H−C3H9N]+, 118.0656 (C8H8N) [M+2H−C6H12NO2]+ | Hypaphorine | |
| 25 | 6.733 | 183.0302 [M−H]− | 2.7 | C8H8O5 | — | Methyl gallate | [48] |
| 26 | 7.026 | 169.0490 [M+H]+ | 0.4 | C8H8O4 | — | Vanillic acid | [46] |
| 27 | 7.215 | 369.0826 [M−H]- | −1.1 | C16H18O10 | — | Fraxin | [49] |
| 28 | 7.221 | 193.0504 [M+H]+ | 7.1 | C10H8O4 | 65.0550 (C9H9O3) [M+H−CO]+, 133.0292 (C8H5O2) [M−CH3−CO2]+ | 5,7-Dihydroxy-4-methylcoumarin | |
| 29 | 7.305 | 209.0435 [M+H]+ | 1.2 | C10H8O5 | — | Fraxetin | [50] |
| 30 | 7.405 | 261.1337 [M−H]− | 4.7 | C12H22O6 | 187.0972 (C9H15O4) [M−C3H7O2]−, 125.0971 (C8H13O) [M−H−OH−C4H7O4]− | 9-(2,3-dihydroxypropoxy)-9-oxononanoic acid | |
| 31 | 7.561 | 165.0524 [M+H]+ | −1.3 | C9H8O3 | — | 4-Hydroxycinnamic acid | [46] |
| 32 | 7.563 | 123.0445 [M+H]+ | 2 | C7H6O2 | 105.0341 (C7H5O) [M+H−H2O]+ | Benzoic acid | |
| 33 | 7.615 | 289.0708 [M−H]− | 1.9 | C15H14O6 | — | L-Epicatechin | [22] |
| 34 | 7.812 | 357.1329 [M−H]− | −6.9 | C20H22O6 | — | Pinoresinol | [51] |
| 35 | 7.98 | 177.0546 [M−H2O+H]+ | 0.4 | C10H10O4 | — | Ferulic acid | [52] |
| 36 | 8.039 | 215.1289 [M−H]− | −2.7 | C11H20O4 | 153.1277 (C10H17O) [M−H2O−COOH]−, 197.1178 (C11H17O3) [M−2H−OH]− | Undecanedioic acid | |
| 37 | 8.18 | 465.1009 [M−H]− | −2 | C21H22O12 | 285.0407 (C15H9O6) [M−2H−C6H11O6]−, 303.0518 (C25H11O7) [M−C6H11O5]− | (2S,3S)-3,5,7-trihydroxy-2-[4-hydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-2,3-dihydrochromen-4-one | |
| 38 | 8.322 | 273.0756 [M+H]+ | 1.6 | C15H12O5 | — | Naringenin | [22] |
| 39 | 8.345 | 435.1264 [M+H]+ | −5.5 | C21H22O10 | — | Naringenin-7-O-glucoside | [53] |
| 40 | 8.38 | 609.1411 [M−2H]− | −2.6 | C27H30O16 | 301.0355 (C15H9O7) [M−H−C12H21O9]− | Rutin | |
| 41 | 8.482 | 229.0872 [M+H]+ | 1.7 | C14H12O3 | — | Resveratrol | [46] |
| 42 | 8.669 | 191.0326 [M−H]− | −1.5 | C10H8O4 | — | Scopoletin | [46] |
| 43 | 8.688 | 303.0503 [M−H]− | −4.7 | C15H12O7 | 285.0400 (C15H9O6) [M−H−H2O]−, 177.0183 (C9H5O4) [M−H−OH−C6H5O2]− | Dihydroquercetin | |
| 44 | 8.706 | 447.0946 [M−H]− | −0.2 | C21H20O11 | — | Quercitrin | [46] |
| 45 | 8.716 | 447.0925 [M−H]− | −3.8 | C21H20O11 | 285.0396 (C15H9O6) [M−C6H11O5]− | 4-(3,4-Dihydroxyphenyl)-5-β-D-glucopyranosyloxy-7-hydroxycoumarin | |
| 46 | 9.269 | 271.0592 [M−H]− | 0 | C15H12O5 | 119.0506 (C8H7O) [M−C7H5O4]-, 151.0041 (C7H3O4) [M−2H−C8H7O]− | Naringenin chalcone | |
| 47 | 9.490 | 435.1275 [M−H]− | −6.4 | C21H24O10 | 273.0763 (C15H13O5) [M−C6H11O5]− | Phlorizin | |
| 48 | 9.518 | 275.0917 [M+H]+ | 1.8 | C15H14O5 | 107.0505 (C7H7O) [M−C8H7O4]+, 169.0496 (C8H9O4) [M−C7H5O]+ | Phloretin | |
| 49 | 9.633 | 151.0402 [M−H]− | 3.5 | C8H8O3 | — | Vanillin | [46] |
| 50 | 9.991 | 243.1587 [M−H]− | −0.8 | C13H24O4 | 225.1487 (C13H21O3) [M−H−H2O]−, 181.1586 (C12H21O) [M−H−H2O−COOH]− | Tridecanedioic acid | |
| 51 | 10.192 | 271.0614 [M+H]+ | −2.6 | C15H10O5 | 253.0496 (C25H9O4) [M+H−H2O]+, 243.0650 (C14H11O4) [M−H−CO]+ | Emodin | |
| 52 | 10.368 | 287.0554 [M−H]− | 2.4 | C15H12O6 | — | Eriodictyol | [53] |
| 53 | 10.507 | 329.2341 [M−H]− | −4.7 | C18H34O5 | 183.1383 (C11H19O2) [M−2H−C7H13O3]− | (Z)-5,8,11,-trihydroxyoctadec-9-enoic acid | |
| 54 | 10.544 | 285.0405 [M−H]− | −0.2 | C15H10O6 | — | Luteolin | [46] |
| 55 | 10.558 | 301.0360 [M−H]− | −1.3 | C15H10O7 | — | Quercetin | [22] |
| No. | Metabolites Name | VIP | log2 FC | OPLS-DA Model |
|---|---|---|---|---|
| 1 | (2S,3S)-3,5,7-trihydroxy-2-[4-hydroxy-3-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-2,3-dihydrochromen-4-one | 1.5322 | 2.2256 | M4 vs. M1 |
| 1.7243 | 2.3676 | M4 vs. M2 | ||
| 2 | Ferulic acid | 1.4681 | 5.8030 | M4 vs. M1 |
| 1.2704 | 1.8072 | M4 vs. M2 | ||
| 3 | Cinnamic acid | 1.3155 | −1.2676 | M4 vs. M1 |
| 4 | Eriodictyol | 1.0573 | −2.0386 | M4 vs. M1 |
| 1.2531 | −1.6422 | M4 vs. M2 | ||
| 5 | Scopoletin | 1.2856 | −1.2213 | M4 vs. M3 |
| 6 | N-acetyltryptophan | 1.6156 | −1.0432 | M4 vs. M3 |
| 1.1541 | 1.1553 | M4 vs. M5 | ||
| 7 | L-Epicatechin | 1.4797 | −1.3857 | M4 vs. M5 |
| 8 | D-Tryptophan | 1.5888 | −1.1378 | M4 vs. M5 |
| 9 | Resveratrol | 1.0606 | −1.083 | M4 vs. M5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, R.; Ou, K.; Chen, L.; Jiao, Y.; Jiang, F.; Gu, R. Response Surface Optimization of Extraction Conditions for the Active Components with High Acetylcholinesterase Inhibitory Activity and Identification of Key Metabolites from Acer truncatum Seed Oil Residue. Foods 2023, 12, 1751. https://doi.org/10.3390/foods12091751
Meng R, Ou K, Chen L, Jiao Y, Jiang F, Gu R. Response Surface Optimization of Extraction Conditions for the Active Components with High Acetylcholinesterase Inhibitory Activity and Identification of Key Metabolites from Acer truncatum Seed Oil Residue. Foods. 2023; 12(9):1751. https://doi.org/10.3390/foods12091751
Chicago/Turabian StyleMeng, Ruonan, Kaixiang Ou, Ling Chen, Yu Jiao, Fangjie Jiang, and Ronghui Gu. 2023. "Response Surface Optimization of Extraction Conditions for the Active Components with High Acetylcholinesterase Inhibitory Activity and Identification of Key Metabolites from Acer truncatum Seed Oil Residue" Foods 12, no. 9: 1751. https://doi.org/10.3390/foods12091751
APA StyleMeng, R., Ou, K., Chen, L., Jiao, Y., Jiang, F., & Gu, R. (2023). Response Surface Optimization of Extraction Conditions for the Active Components with High Acetylcholinesterase Inhibitory Activity and Identification of Key Metabolites from Acer truncatum Seed Oil Residue. Foods, 12(9), 1751. https://doi.org/10.3390/foods12091751








