Molecular Characterization and Bioactivities of a Novel Polysaccharide from Phyllostachys pracecox Bamboo Shoot Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction and Fractionation of PBSR
2.3. Physicochemical Analysis
2.3.1. Monosaccharide Composition
2.3.2. Homogeneity and Molecular Weight
2.3.3. UV and FT-IR Analysis
2.3.4. Methylation Analysis
2.3.5. 2D NMR Measurement
2.4. Advanced Structure and Physical Property of PBSR1
2.4.1. Chain Conformation Determination
2.4.2. Atomic Force Microscopy (AFM)
2.4.3. Congo Red Analysis
2.4.4. Thermal Analysis
2.5. Antioxidant Activities
2.5.1. DPPH Scavenging Activity
2.5.2. Oxygen Radical Absorbing Capacity (ORAC)
2.6. Immunomodulatory Activity
2.6.1. Cell Viability of RAW264.7
2.6.2. Pinocytic Assay
2.6.3. ROS Generation Measurement
2.6.4. Cytokines Production
2.7. Statistical Analysis
3. Results
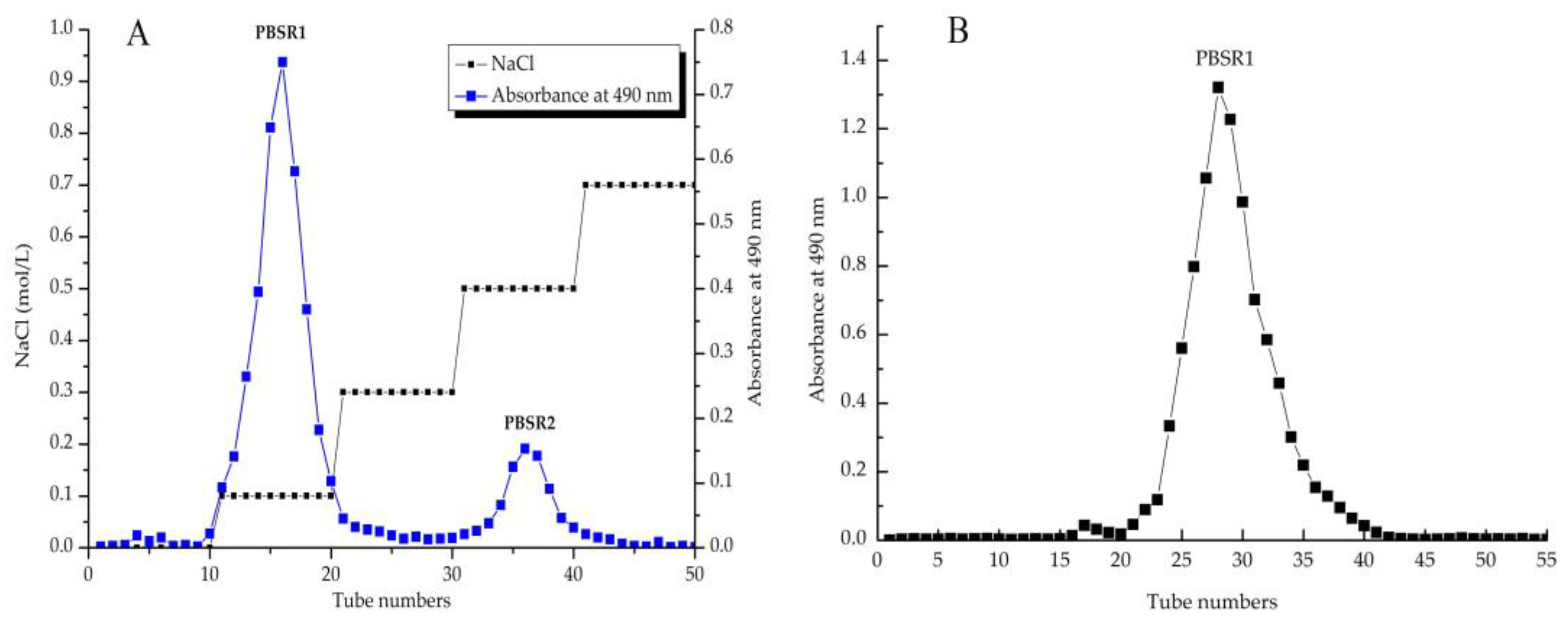
3.1. Extraction and Fractionation of PBSR
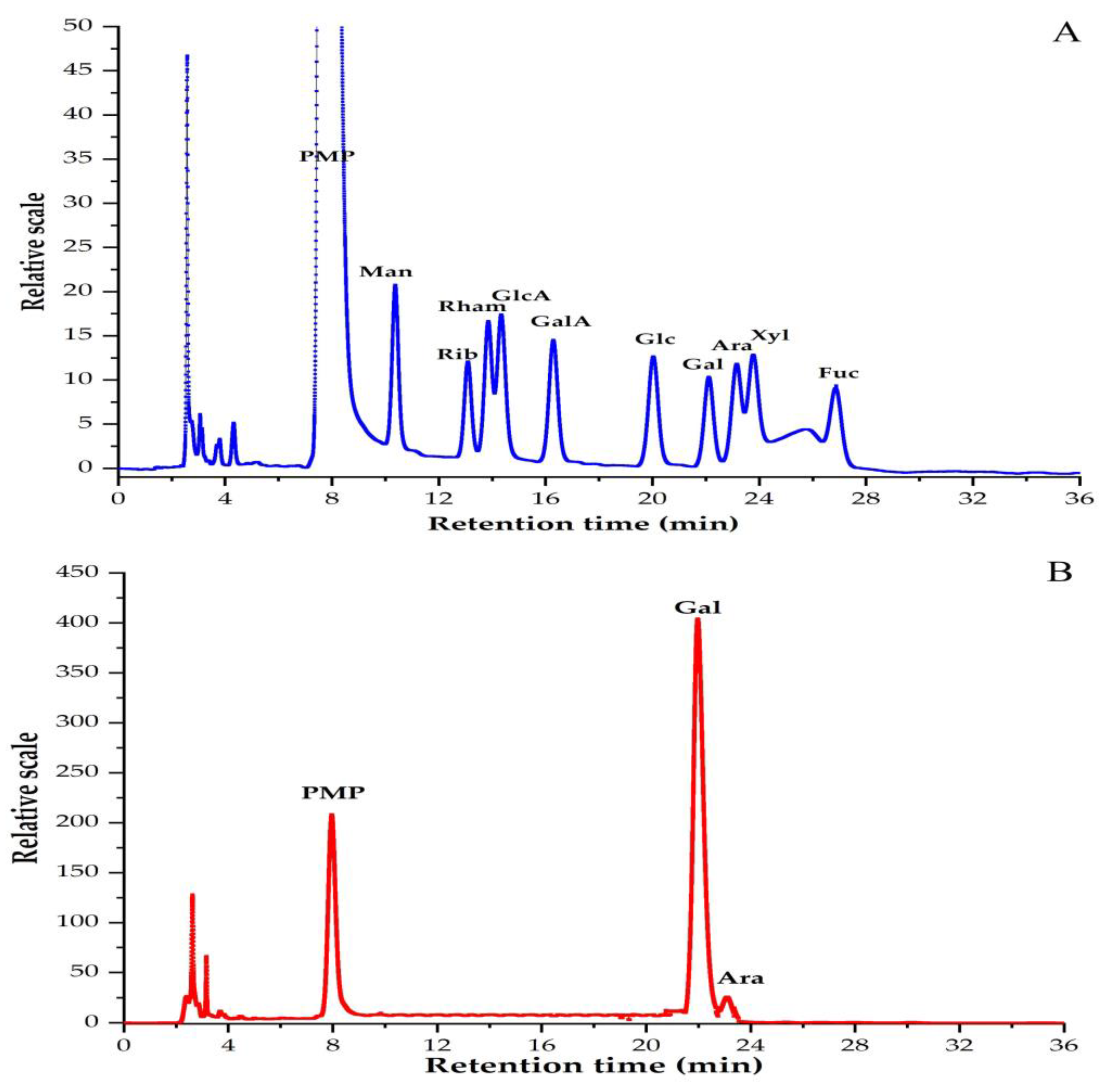
3.2. Monosaccharide Compositions of PBSR1
3.3. Homogeneity and Molecular Weight of PBSR1
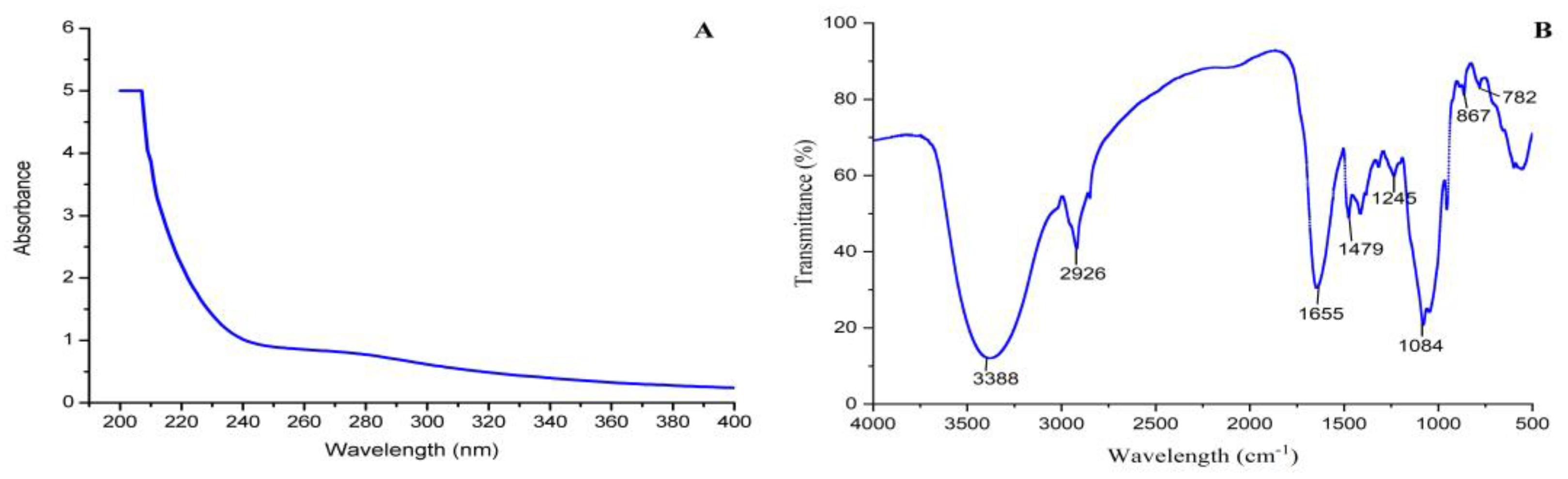
3.4. UV and FT-IR Analysis
3.5. Methylation Analysis
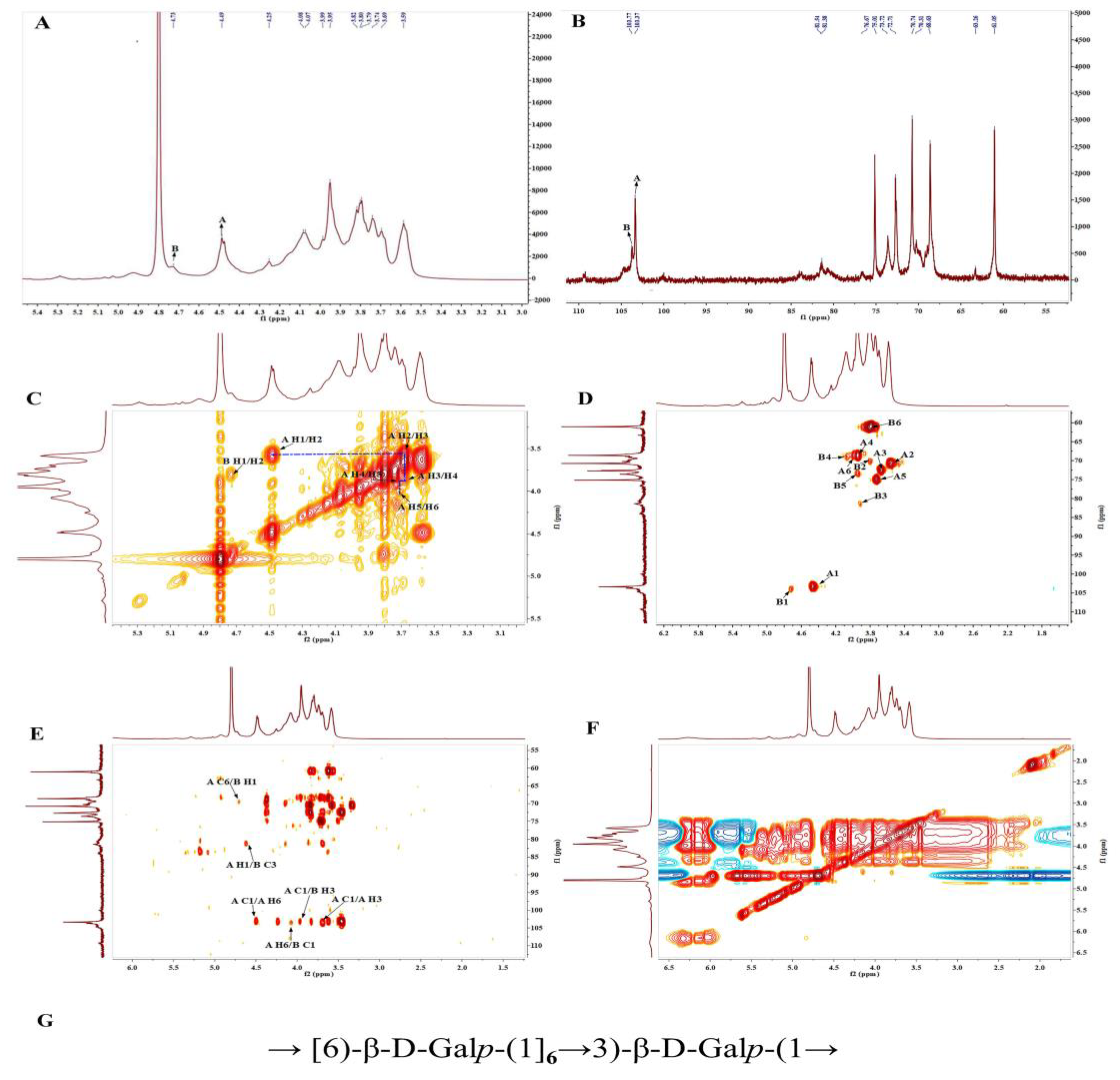
3.6. NMR Analysis of PBSR1
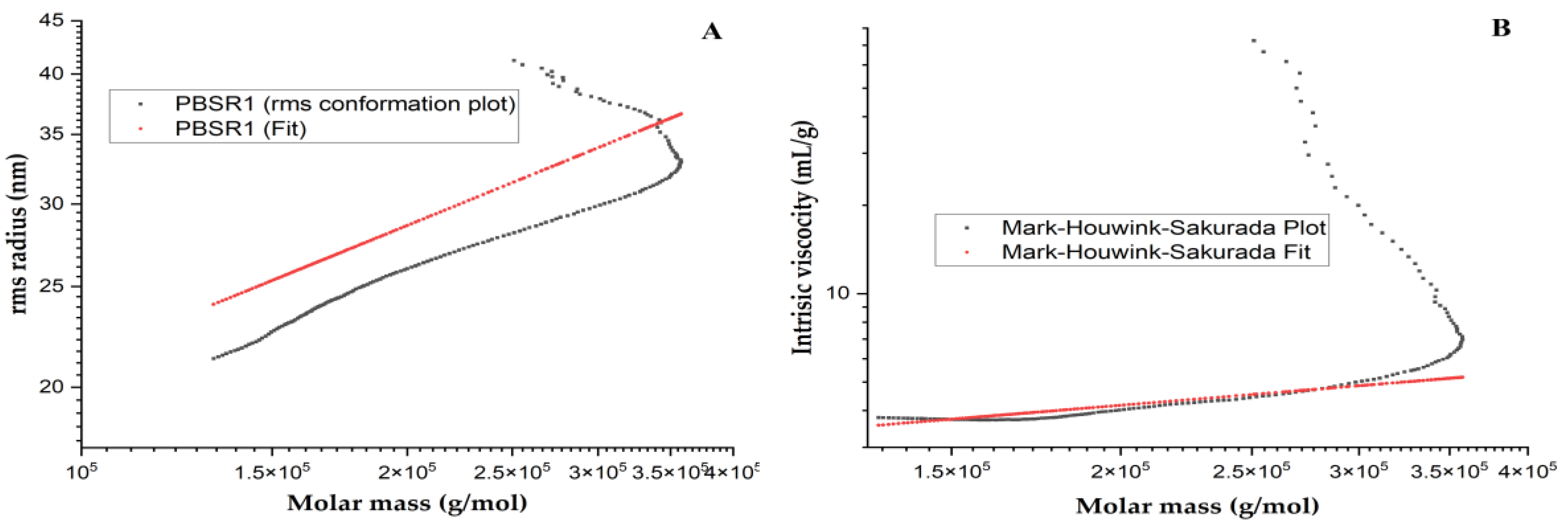
3.7. Advanced Conformation and Physical Property of PBSR1
3.7.1. Chain Conformation
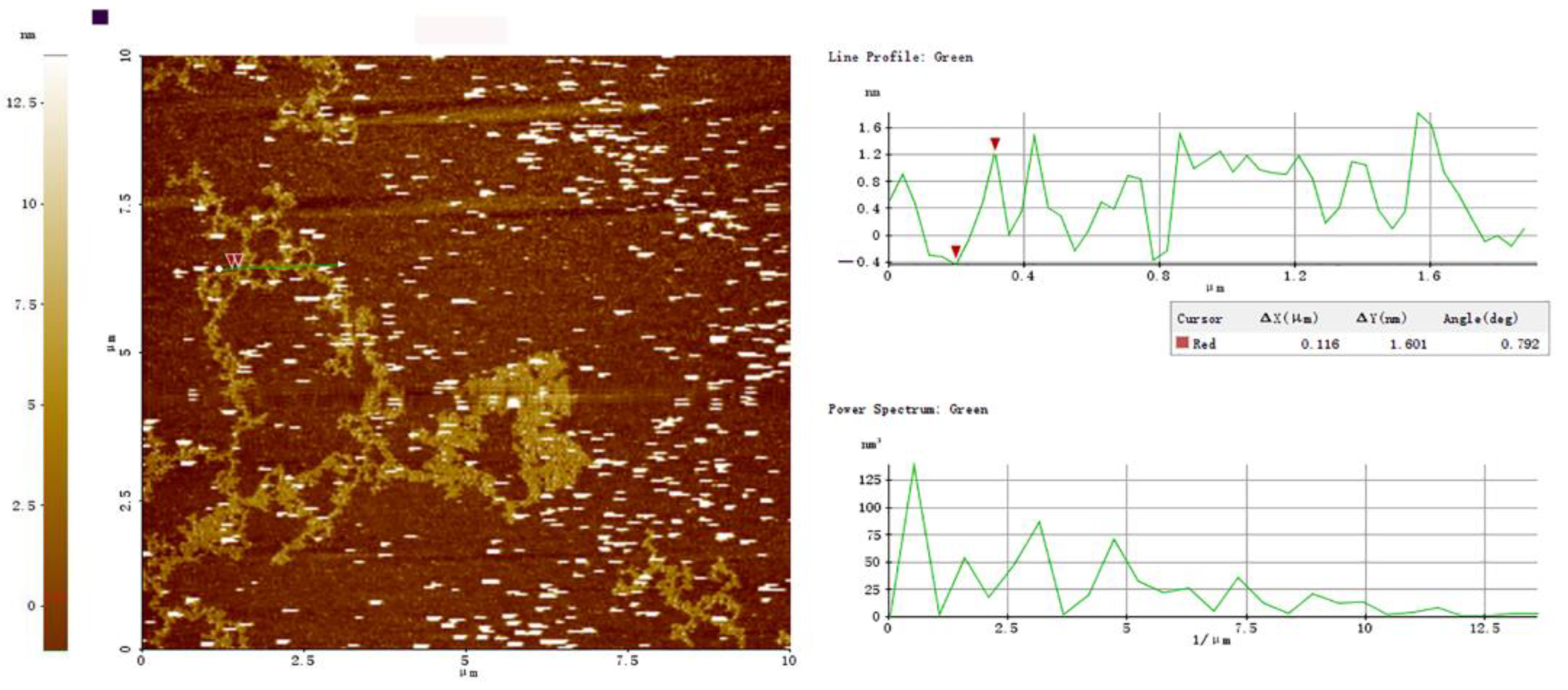
3.7.2. AFM of PBSR1
3.7.3. Congo Red Analysis
3.7.4. TG Analysis
3.8. Antioxidant Activities In Vitro
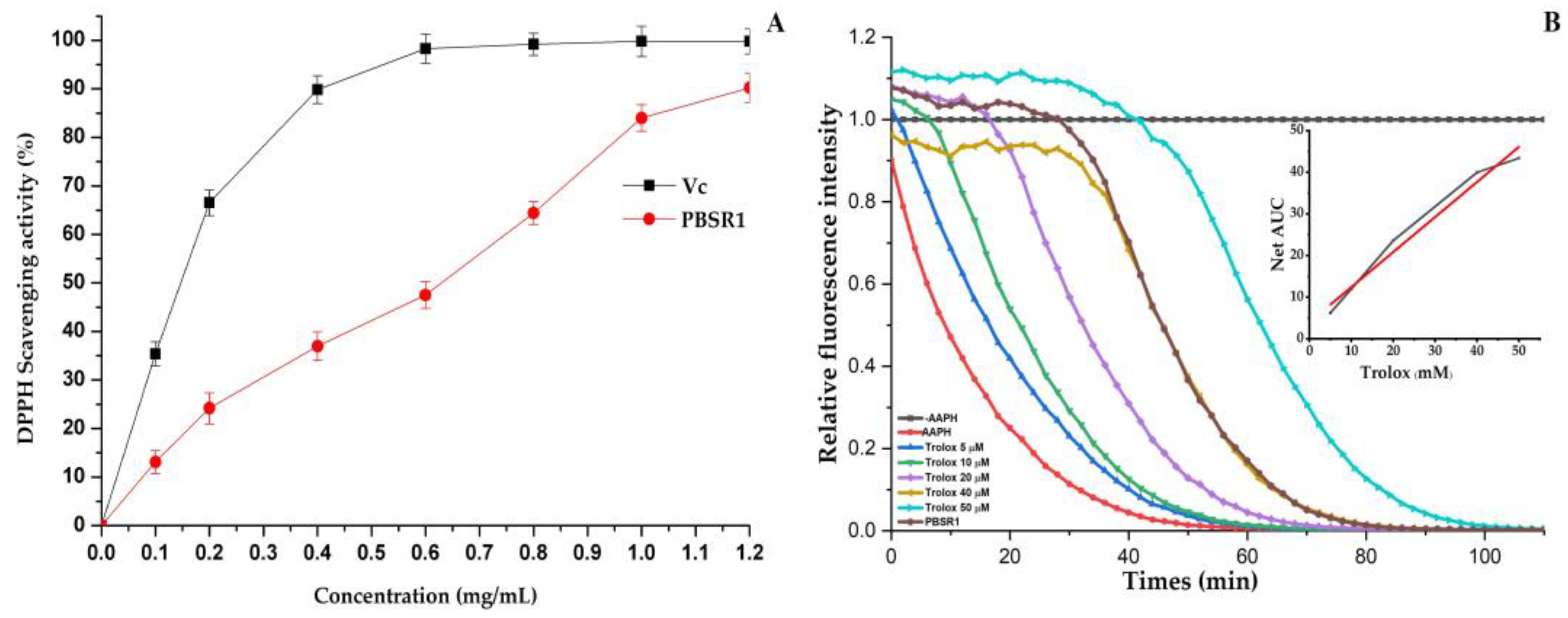
3.8.1. DPPH Scavenging Ability
3.8.2. ORAC Capacity
3.9. Immunomodulatory Activity of PBSR1 on RAW 264.7 Cells
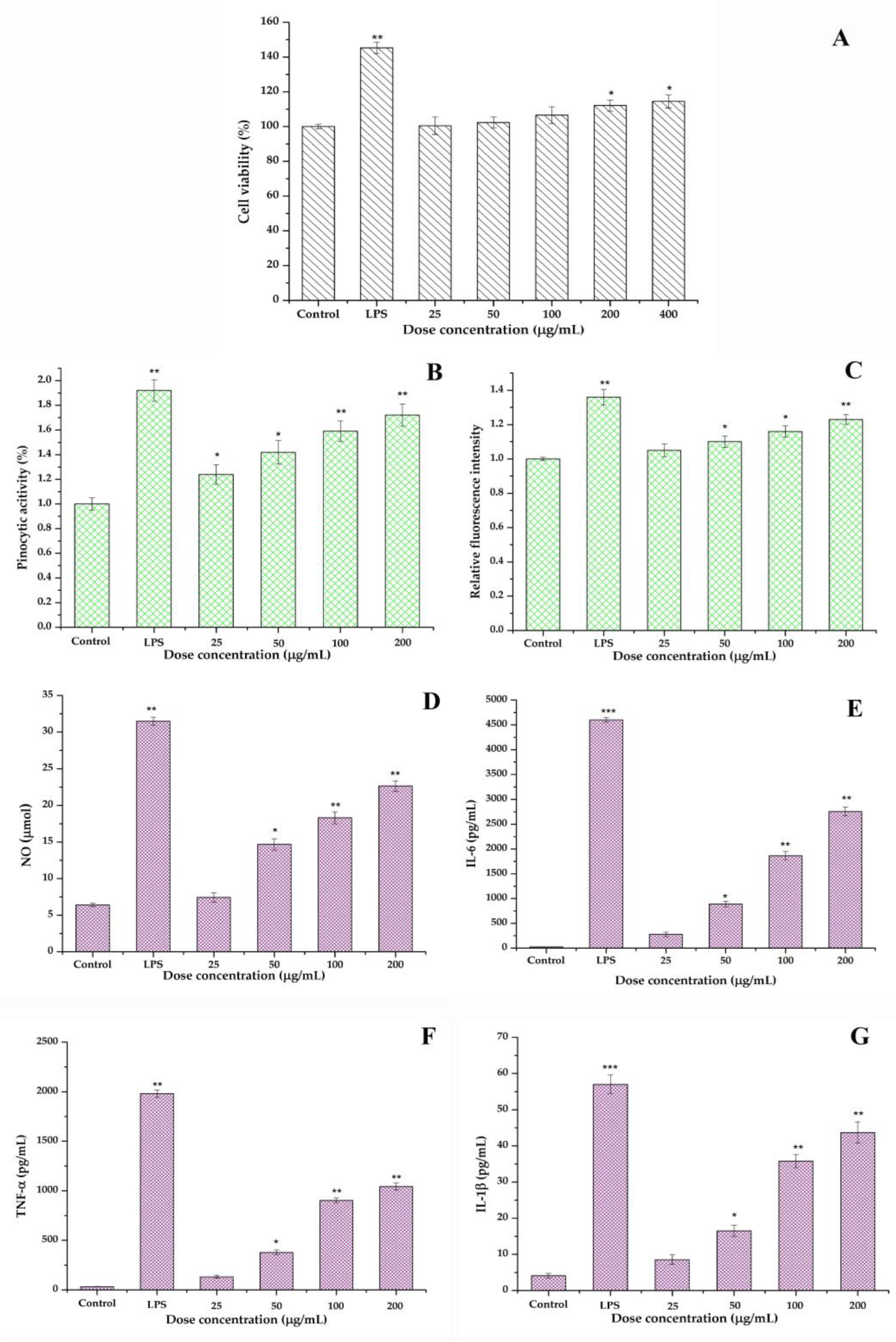
3.9.1. Cell Viability
3.9.2. Pinocytotic Activity
3.9.3. ROS Analysis
3.9.4. Cytokines Production and the Relationship of Structure–Function
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singhal, P.; Bal, L.M.; Satya, S.; Sudhakar, P.; Naik, S.N. Bamboo shoots: A novel source of nutrition and medicine. Crit. Rev. Food Sci. 2013, 53, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kobayashi, M. Plantation future of bamboo in China. J. For. Res. 2004, 15, 327–334. [Google Scholar] [CrossRef]
- Chongtham, N.; Bisht, M.S.; Haorongbam, S. Nutritional properties of bamboo shoots: Potential and prospects for utilization as a health food. Compr. Rev. Food Sci. 2011, 10, 153–168. [Google Scholar] [CrossRef]
- Satya, S.; Bal, L.M.; Singhal, P.; Naik, S.N. Bamboo shoot processing: Food quality and safety aspect (a review). Trends Food Sci. Technol. 2010, 21, 181–189. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, J.; Xia, X.; Kan, J. Purification and Structural Identification of polysaccharides from bamboo shoots (Dendrocalamus latiflorus). Int. J. Mol. Sci. 2015, 16, 15560–15577. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, H.Y.; Li, W.J.; Ye, J.Z. Chemical constituents and structural characterization of polysaccharides from four typical bamboo species leaves. Molecules 2015, 20, 4162–4179. [Google Scholar] [CrossRef]
- Mao, J.W.; Yin, J.; Ge, Q.; Jiang, Z.L.; Gong, J.Y. In vitro antioxidant activities of polysaccharides extracted from moso bamboo-leaf. Int. J. Biol. Macromol. 2013, 55, 1–5. [Google Scholar] [CrossRef]
- Azmi, A.F.M.N.; Mustafa, S.; Hashim, D.M.; Manap, Y.A. Prebiotic activity of polysaccharides extracted from Gigantochloa levis (Buluh beting) shoots. Molecules 2012, 17, 1635–1651. [Google Scholar] [CrossRef]
- He, S.D.; Wang, X.; Zhang, Y.; Wang, J.; Sun, H.J.; Wang, J.H.; Cao, X.D.; Ye, Y.K. Isolation and prebiotic activity of water-soluble polysaccharides fractions from the bamboo shoots (Phyllostachys praecox). Carbohyd. Polym. 2016, 151, 295–304. [Google Scholar] [CrossRef]
- Kweon, M.H.; Hwang, H.J.; Sung, H.C. Isolation and characterzaition of anticomplementary β-glucans from the shoots of bamboo Phyllostachys edulis. Plant Med. 2003, 69, 56–62. [Google Scholar] [CrossRef]
- Chen, G.J.; Chen, X.H.; Yang, B.; Yu, Q.Q.; Wei, X.Y.; Ding, Y.B.; Kan, J.Q. New insight into bamboo shoot (Chimonobambusa quadrangularis) polysaccharides: Impact of extraction processes on its prebiotic activity. Food Hydrocoll. 2019, 95, 367–377. [Google Scholar] [CrossRef]
- Chen, G.J.; Bu, F.; Chen, X.H.; Li, C.F.; Wang, S.S.; Kan, J.Q. Ultrasonic extraction, structural characterization, physicochemical properties and antioxidant activities of polysaccharides from bamboo shoots (Chimonobambusa quadrangularis) processing by-products. Int. J. Biol. Macromol. 2018, 112, 656–666. [Google Scholar] [CrossRef]
- Li, Q.; Wu, W.J.; Fang, X.J.; Chen, H.J.; Han, Y.C.; Liu, R.L.; Niu, B.; Gao, H.Y. Structural characterization of a polysaccharide from bamboo (Phyllostachys edulis) shoot and its prevention effect on colitis mouse. Food Chem. 2022, 387, 132807. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.V.; Zelaya, V.M.; Cobello, L.; Vega, A.S.; Ciancia, M. Glucuronoarabinoxylans and other cell wall polysaccharides from shoots of Guadua chacoensis obtained by extraction in different conditions. Carbohyd. Polym. 2019, 226, 115313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Yang, L.Q.; Zhang, L.M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohyd. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Wang, X.M.; Yu, S.C.; Zhao, M.X. Isolation and antioxidant activities of polysaccharides extracted from the shoots of Phyllostachys edulis (Carr.). Int. J. Biol. Macromol. 2011, 49, 454–457. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. A quick and specific assay for hydroxyproline. Anal. Biochem. 1973, 55, 288–291. [Google Scholar] [CrossRef]
- Liu, H.Y.; He, P.F.; He, L.; Li, Q.; Cheng, J.W.; Wang, Y.B.; Yang, G.; Yang, B. Structure characterization and hypoglycemic activity of an arabinogalactan from Phyllostachys heterocycla bamboo shoot shell. Carbohyd. Polym. 2018, 201, 189–200. [Google Scholar] [CrossRef]
- Wang, Y.B.; He, P.F.; He, L.; Huang, Q.R.; Cheng, J.W.; Li, W.Q.; Liu, Y.; Wei, C.Y. Structural elucidation, antioxidant and immunomodulatory activities of a novel heteropolysaccharide from cultured Paecilomyces cicadae (Miquel.) Samson. Carbohyd. Polym. 2019, 216, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Li, W.Q.; Shao, S.S.; He, L.; Cheng, J.W.; Han, S.F.; Liu, Y. Structure and chain conformation of a neutral intracellular heteropolysaccharide from mycelium of Paecilomyces cicadae. Carbohyd. Polym. 2016, 136, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Zhou, X.D.; Tian, X.; Li, R.R.; Sui, W.J.; Liu, R.; Wu, T.; Zhang, M. Isolation and purification, structural characterization and antioxidant activities of a novel hetero-polysaccharide from stream explored wheat germ. Food 2022, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Rem, H.; Li, Z.Y.; Gao, R.; Zhao, T.X.; Luo, D.; Yu, Z.H.; Zhang, S.; Qi, C.; Wang, Y.Q.; Qiao, H.Z.; et al. Structural characterization of Rehmannia glutinosa polysaccharides treated using different decolorization processes and their antioxidant effects in intestinal epithelial cells. Food 2022, 11, 3449. [Google Scholar]
- Wang, Z.; Wang, C.; Quan, Y. Extraction of polysaccharides from Phellinus nigricans mycelia and their antioxidant activities in vitro. Carbohyd. Polym. 2014, 99, 110–115. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Gao, X.; Qi, J.Y.; Ho, C.; Li, B.; Mu, J.J.; Zhang, Y.T.; Hu, H.P.; Mo, W.P.; Chen, Z.Z.; Xie, Y.Z. Structural characterization and immunomodulatory activity of a water-soluble polysaccharide from Ganoderma leucocontextum fruiting bodies. Carbohyd. Polym. 2020, 249, 116874. [Google Scholar] [CrossRef]
- Wang, X.M.; Tian, J.J.; Zhang, X.L.; Tang, N.Y.; Rui, X.; Zhang, Q.Q.; Dong, M.S.; Li, W. Characterization and immunological activity of exopolysaccharide from Lacticaseibacillus paracasei GL1 isolated from Tibetan Kefir grains. Food 2022, 11, 3330. [Google Scholar] [CrossRef]
- Shaim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Zhang, S.; Wang, Q.; Lu, X.; Lin, L.M.; Tian, Y.T.; Xiao, J.B.; Zheng, B.D. Characterization and hypoglycemic activity of a β-pyran polysaccharides from bamboo shoot (Leleba oldhami Nakal) shells. Carbohyd. Polym. 2016, 14, 438–446. [Google Scholar] [CrossRef]
- Saeidy, S.; Petera, B.; Pierre, G.; Fenoradosoa, T.A.; Djomdi, D.; Michaud, P.; Delattre, C. Plants arabinogalactans: From structures to physico-chemical and biological properties. Biotechnol. Adv. 2021, 53, 107771. [Google Scholar] [CrossRef]
- Zhang, F.S.; Ran, C.X.; Zheng, J.; Ding, Y.B.; Chen, G.J. Polysaccharides obtained from bamboo shoots (Chimonobambusa quadrangularis) processing by-products: New insight into ethanol precipitation and characterization. Int. J. Biol. Macromol. 2018, 112, 951–960. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Vasilieva, N.Y.; Malyar, Y.N.; Karacharov, A.A.; Kondrasenko, A.A.; Levdanskiy, A.V.; Borovkova, V.S.; Miroshnikova, A.V.; Issaoui, N.; Kazachenko, A.S.; et al. Sulfation of arabinogalactan with ammonium sulfamate. Biomass Convers. Bior. 2022, 2, 1–13. [Google Scholar] [CrossRef]
- Wei, C.Y.; Zhang, Y.; He, L.; Cheng, J.W.; Li, J.H.; Tao, W.Y.; Mao, G.Z.; Zhang, H.; Linhardt, R.J.; Ye, X.Q.; et al. Structural characterization and anti-proliferative activities of partially degraded polysaccharides from peach gum. Carbohyd. Polym. 2019, 203, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Babkin, V.A.; Neverova, N.A.; Medvedeva, E.N.; Fedorova, T.E.; Levchuk, A.A. Investigation of physicochemical properties of arabinogalactan of different larch species. Russ. J. Bioorg. Chem. 2016, 42, 707–711. [Google Scholar] [CrossRef]
- Makarova, E.N.; Shakhmatov, E.G.; Udoratina, E.V.; Kutchin, A.V. Structural and chemical charactertistics of pectins, arabinogalactans, and arabinogalactan proteins from conifers. Russ. Chem. B. 2015, 64(6), 1302–1318. [Google Scholar] [CrossRef]
- Nagel, A.; Conrad, J.; Leitenberger, M.; Carle, R.; Neidhart, S. Structural studies of the arabinogalactans in Mangifera indica L. fruit exudate. Food Hydrocoll. 2016, 61, 555–566. [Google Scholar] [CrossRef]
- Taguchi, I.; Kiyohara, H.; Matsumoto, T.; Yamada, H. Structure of oligosaccharide side chains of an intestinal immune system modulating arabinogalactan isolated from rhizomes of Atractylodes lancea DC. Carbohydr. Res. 2004, 339, 763–770. [Google Scholar] [CrossRef]
- Goubet, F.; Morvan, C. Synthesis of cell wall galactans from flax (Linum usitatissimum L.) suspension-cultured cells. Plant Cell Physiol. 1994, 35, 719–727. [Google Scholar] [CrossRef]
- Tao, Y.Z.; Zhang, L.; Yan, F.; Wu, X.J. Chain conformation of water-insoluble hyperbranched polysaccharides from fungus. Biomacromolecules 2007, 8, 2321–2328. [Google Scholar] [CrossRef]
- Yang, L.Q.; Fu, S.S.; Zhou, X.N.; Zhang, L.M.; Yang, Y.R.; Yang, X.M.; Liu, H. Hyperbranched acidic polysaccharide from green tea. Biomacromolecules 2010, 11, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.H.; Gu, X.M.; Wang, Y.; Zhou, J.Y.; Xu, X.B.; Yang, X.L. A β-D-glucan from the sclerotia of Pleurotus tuber-regium (Fr.) Sing. Carbohydr. Res. 2000, 328, 629–633. [Google Scholar]
- Lucyszyn, N.; Lubambo, A.F.; Ono, L.; Jo, T.A.; Souza, C.F.D.; Sierakowski, M.R. Chemical, physico-chemical and cytotoxicity characterisation of xyloglucan from Guibourtia hymenifolia (Moric.) J. Leonard seeds. Food Hydrocoll. 2011, 25, 1242–1250. [Google Scholar] [CrossRef]
- Chen, G.J.; Chen, K.W.; Zhang, R.F.; Chen, X.L.; Hu, P. Polysaccharides from bamboo shoots processing by-products: New insight into extraction and characterization. Food Chem. 2018, 245, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Li, C.F.; Wang, S.S.; Mei, X.F.; Zhang, H.X.; Kan, J.Q. Characterization of physicochemical properties and antioxidant activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis): Effect of drying procedures. Food Chem. 2019, 292, 281–293. [Google Scholar] [CrossRef]
- Yang, G.; Qu, Y.H.; Meng, Y.; Wang, Y.S.; Song, C.C.; Cheng, H.R.; Li, X.M.; Sun, L.; Zhou, Y.F. A novel linear 3-O-methylated galactan isolated from Cantharellus cibarius activates macrophages. Carbohyd. Polym. 2019, 214, 34–43. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Zhang, X.; Zhang, F.M.; Linhardt, R.J. Structural and immunological studies on the polysaccharide from spores of a medicinal entomogenous fungus Paecilomyces cicadae. Carbohyd. Polym. 2021, 254, 117462. [Google Scholar] [CrossRef]
- Bahramzadeh, S.; Tabarsa, M.; You, S.; Yelithao, K.; Klochkov, V.; Ilfat, R. An arabinogalactan isolated from Boswellia carterii: Purification, structural elucidation and macrophage stimulation via NF-κB and MAPK pathways. J. Funct. Foods 2019, 52, 450–458. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, J.; Du, Z.; Wang, P.; Ding, K. Structural elucidation and immune enhancing activity of an arabinogalactan from flowers of Carthamus tinctorius L. Carbohydr. Polym. 2018, 202, 134–142. [Google Scholar] [CrossRef]
- Dong, Q.; Yao, J.; Fang, J.N. Structural characterization of the water-extractable polysaccharides from Sophora subprostrata roots. Carbohydr. Polym. 2003, 54, 13–19. [Google Scholar] [CrossRef]
- Li, C.; Dong, Z.; Zhang, B.; Huang, Q.; Liu, G.; Fu, X. Structural characterization and immune enhancement activity of a novel polysaccharide from Moringa oleifera leaves. Carbohydr. Polym. 2020, 234, 115897. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.Y.; Watanable, S.T.; Chihara, C.; Rokutanda, M. Denaturation and renaturation of a β-1,6;1,3-glucan, lentinan, associated with expression of T-cell-mediated responses. Cancer Res. 1988, 48, 671–675. [Google Scholar] [PubMed]
- Yanaki, T.; Ito, W.; Tabata, K. Correlation between antitumor activity of schizophyllan and its triple helix. Agric. Biol. Chem. 1986, 509, 2415–2416. [Google Scholar]
- Yermak, I.M.; Barabanova, A.O.; Aminin, D.L.; Davydova, V.N.; Sokolova, E.V.; Solov’eva, T.F. Effects of structural peculiarities of carrageenans on their immunomodulatory and anticoagulant activities. Carbohydr. Polym. 2012, 87, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Rathee, P.; Sharma, K.; Chaugule, B.B.; Kar, N.; Bera, T. Immunomodulation effect of sulphated polysaccharide (porphyran) from Porphyra vietnamensis. Int. J. Biol. Macromol. 2013, 57, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.; Raptis, J.; Rice, P.J.; Kalbfleisch, J.H.; Stout, R.D.; Ensley, H.E.; Browder, W.; Williams, D.L. The influence of glucan polymer structure and solution conformation on binding to (1→3)-β-D-glucan receptors in a human monocyte-like cell line. Glycobiology 2000, 10, 339–346. [Google Scholar] [CrossRef]

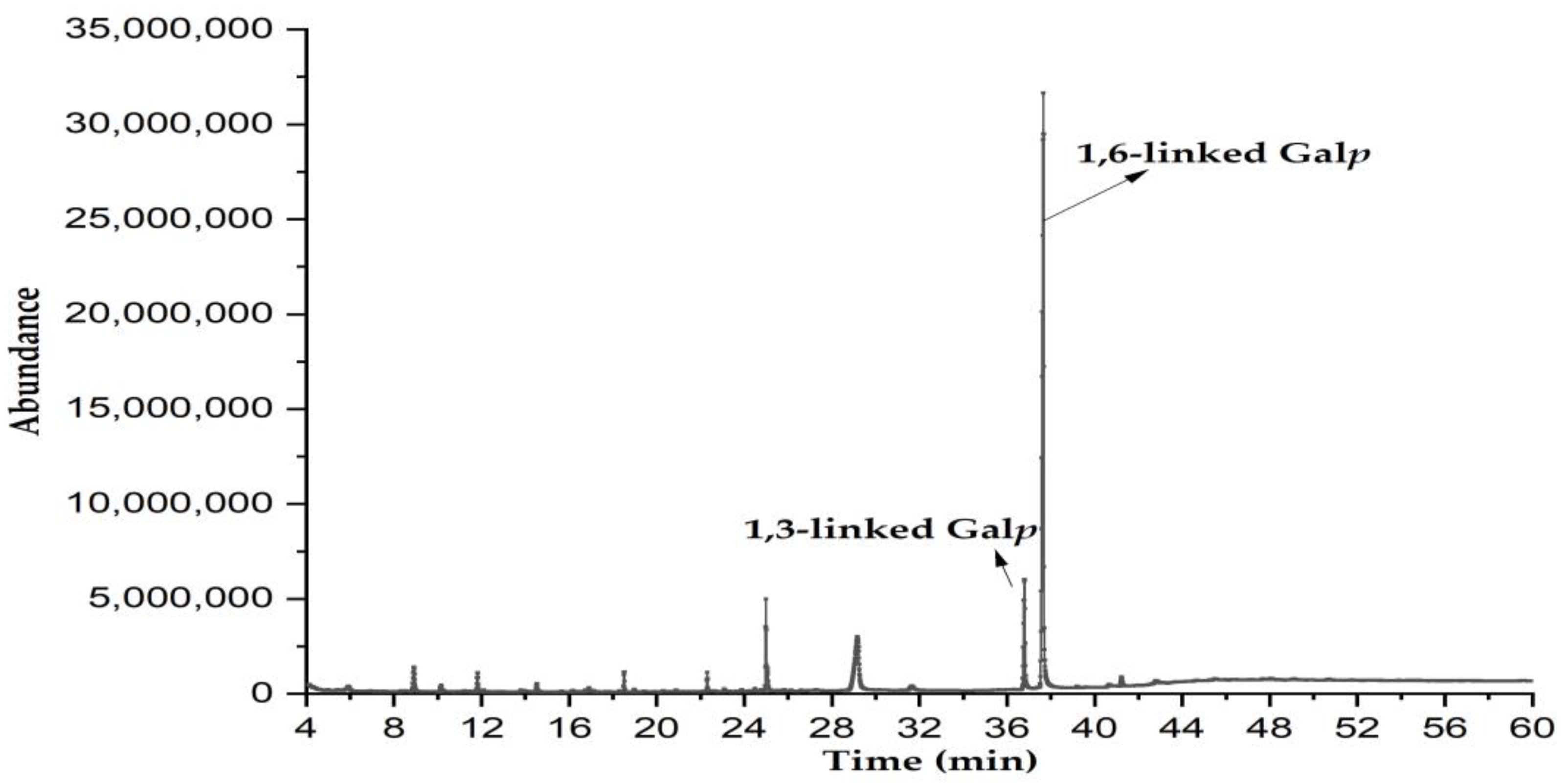

| Residues | Retention Time (min) | PMAAs | Type of Linkages | Molar Ratio (mol%) | Major Mass Fragments (m/z) |
|---|---|---|---|---|---|
| 1 | 36.52 | 2,4,6-Me3-Galp | 1,3-linked Galp | 14.3 | 87, 101, 118, 129, 143, 161, 174, 202, 217, 277 |
| 2 | 37.89 | 2,3,4-Me3-Galp | 1,6-linked Galp | 85.7 | 87, 99, 102, 118, 129, 145, 162, 189, 233 |
| Residue | Chemical Shifts (δ, ppm) | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| (A) β-1,6-D-Galp | 4.46/103.31 | 3.55/70.73 | 3.66/72.64 | 3.93/68.92 | 3.73/75.1 | 3.93, 4.06/69.01 |
| (B) β-1,3-D-Galp | 4.71/103.78 | 3.78/70.06 | 3.89/81.38 | 4.13/68.52 | 3.95/76.55 | 3.79/60.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhang, Y.; Xie, N.; Cheng, J.; Wang, Y.; Yuan, S.; Li, Q.; Shi, R.; He, L.; Chen, M. Molecular Characterization and Bioactivities of a Novel Polysaccharide from Phyllostachys pracecox Bamboo Shoot Residues. Foods 2023, 12, 1758. https://doi.org/10.3390/foods12091758
Huang X, Zhang Y, Xie N, Cheng J, Wang Y, Yuan S, Li Q, Shi R, He L, Chen M. Molecular Characterization and Bioactivities of a Novel Polysaccharide from Phyllostachys pracecox Bamboo Shoot Residues. Foods. 2023; 12(9):1758. https://doi.org/10.3390/foods12091758
Chicago/Turabian StyleHuang, Xubo, Yalan Zhang, Na Xie, Junwen Cheng, Yanbin Wang, Shaofei Yuan, Qin Li, Rui Shi, Liang He, and Min Chen. 2023. "Molecular Characterization and Bioactivities of a Novel Polysaccharide from Phyllostachys pracecox Bamboo Shoot Residues" Foods 12, no. 9: 1758. https://doi.org/10.3390/foods12091758
APA StyleHuang, X., Zhang, Y., Xie, N., Cheng, J., Wang, Y., Yuan, S., Li, Q., Shi, R., He, L., & Chen, M. (2023). Molecular Characterization and Bioactivities of a Novel Polysaccharide from Phyllostachys pracecox Bamboo Shoot Residues. Foods, 12(9), 1758. https://doi.org/10.3390/foods12091758





