A Green and Effective Polyethylene Glycols-Based Microwave-Assisted Extraction of Carnosic and Rosmarinic Acids from Rosmarinus officinalis Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Determination of CA and RA
2.3. Extraction of CA and RA from ROLs
2.3.1. The Developed MAE Method
2.3.2. Reported Extraction Methods
The MAE Methods
The UAE Method
The HRE Method
Maceration Methods
2.4. Measurements of the DPPH Radical Scavenging Activity of the PEG-400 Extract
2.5. Experimental Design Using Response Surface Methodology (RSM)
2.6. Statistical Analyses
3. Results and Discussion
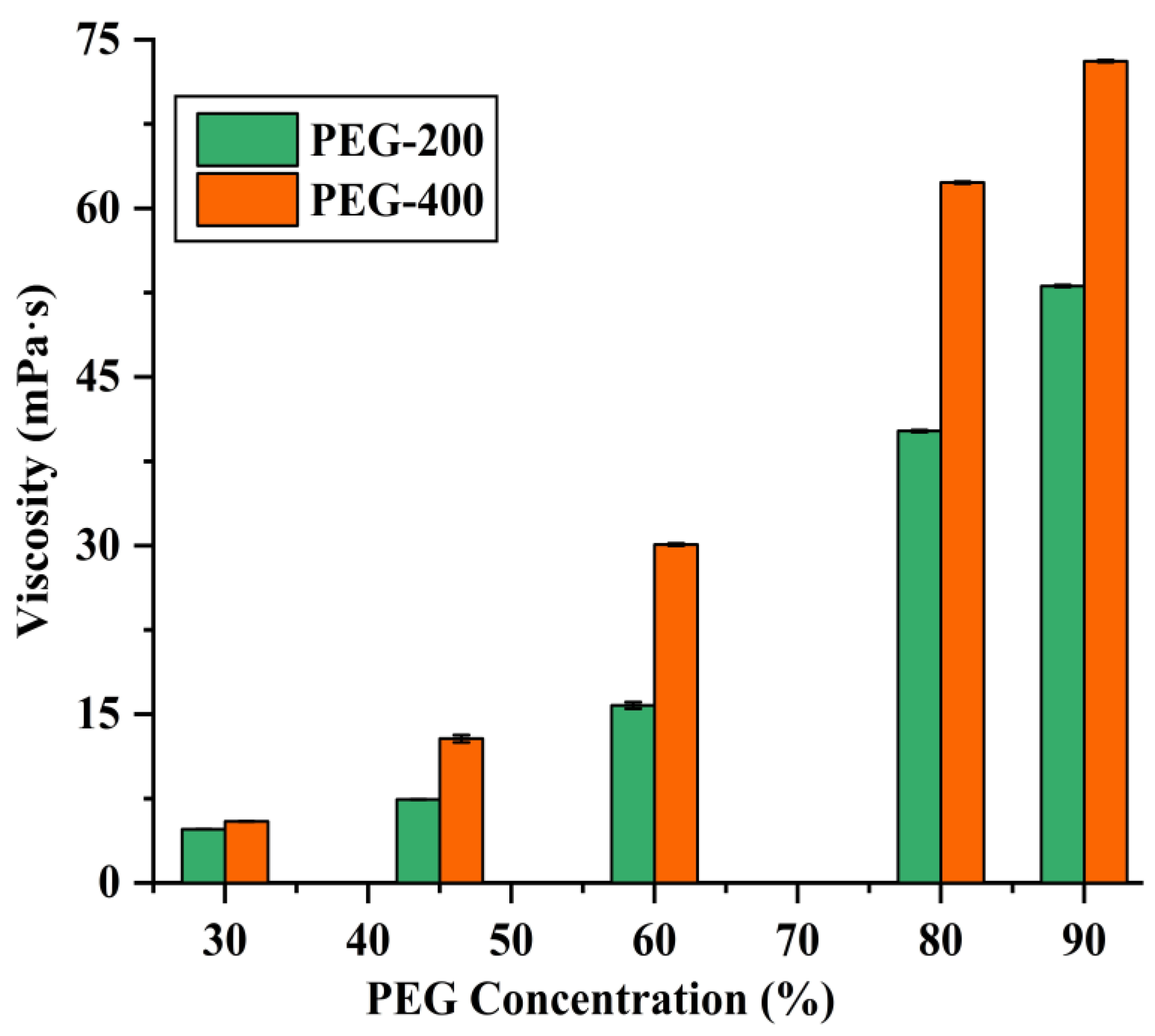
3.1. Selection of the Extraction Solvents
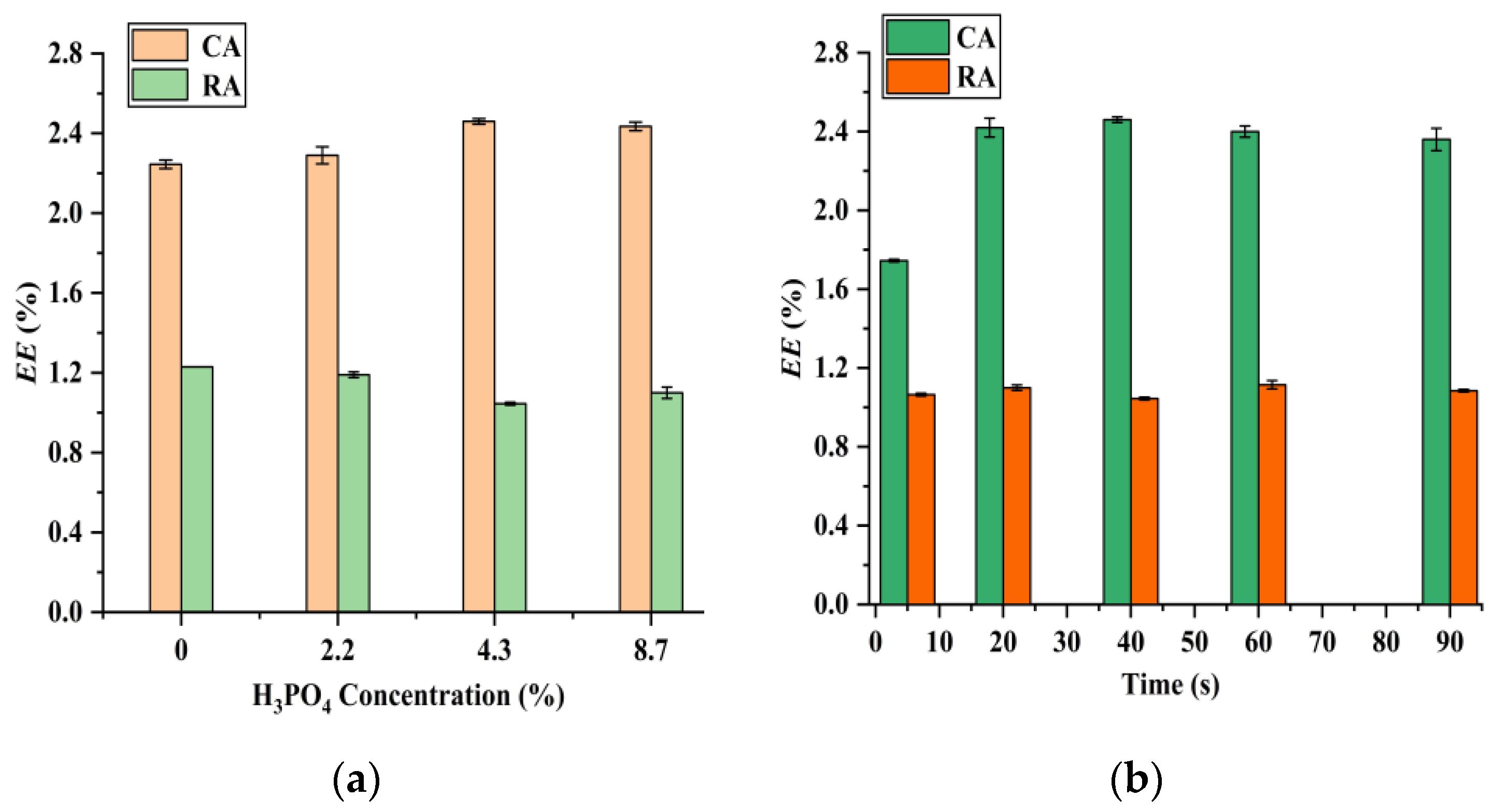
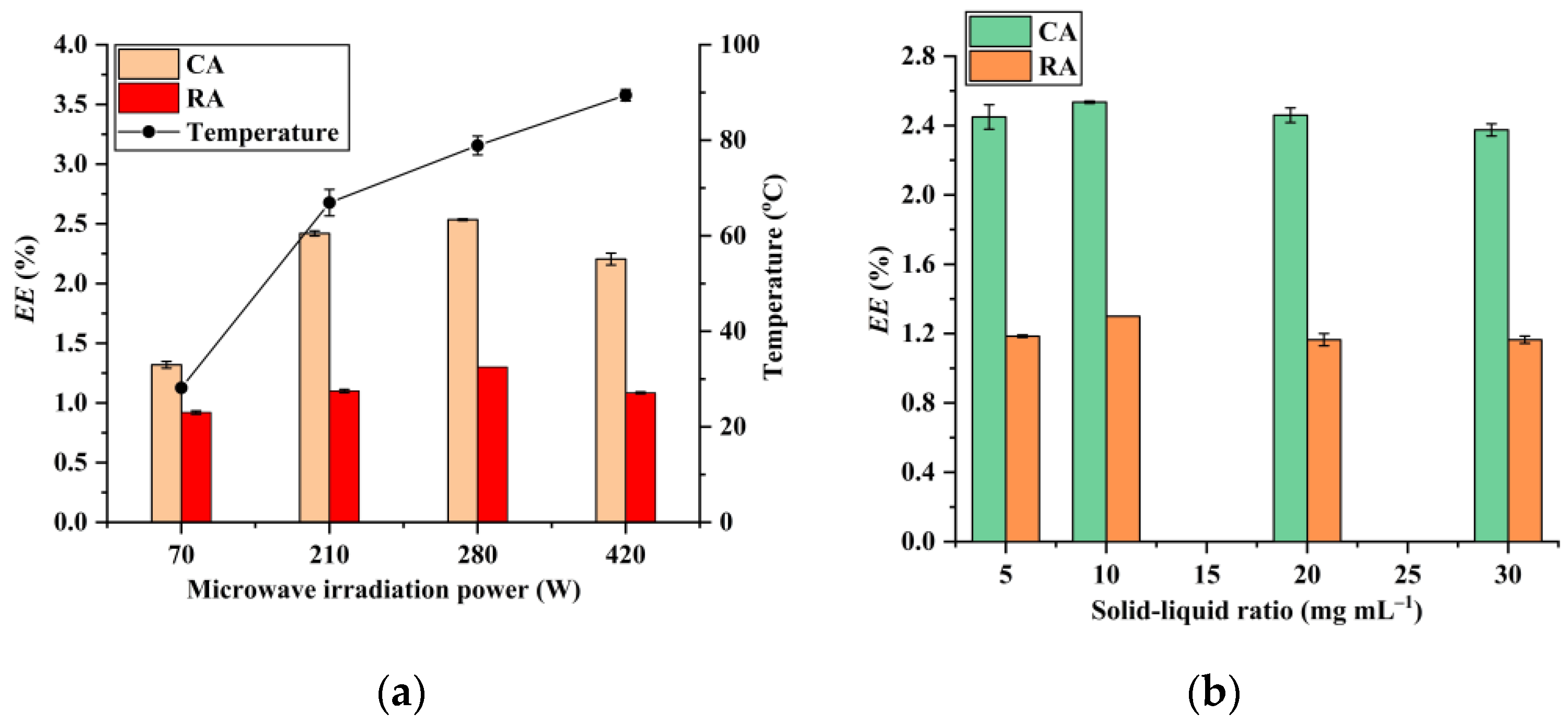
3.2. Effects of H3PO4Concentration, Microwave Irradiation Time, Microwave Irradiation Power, and Solid–Liquid Ratio on the Extraction Efficiencies of CA and RA
3.3. Analysis of RSM
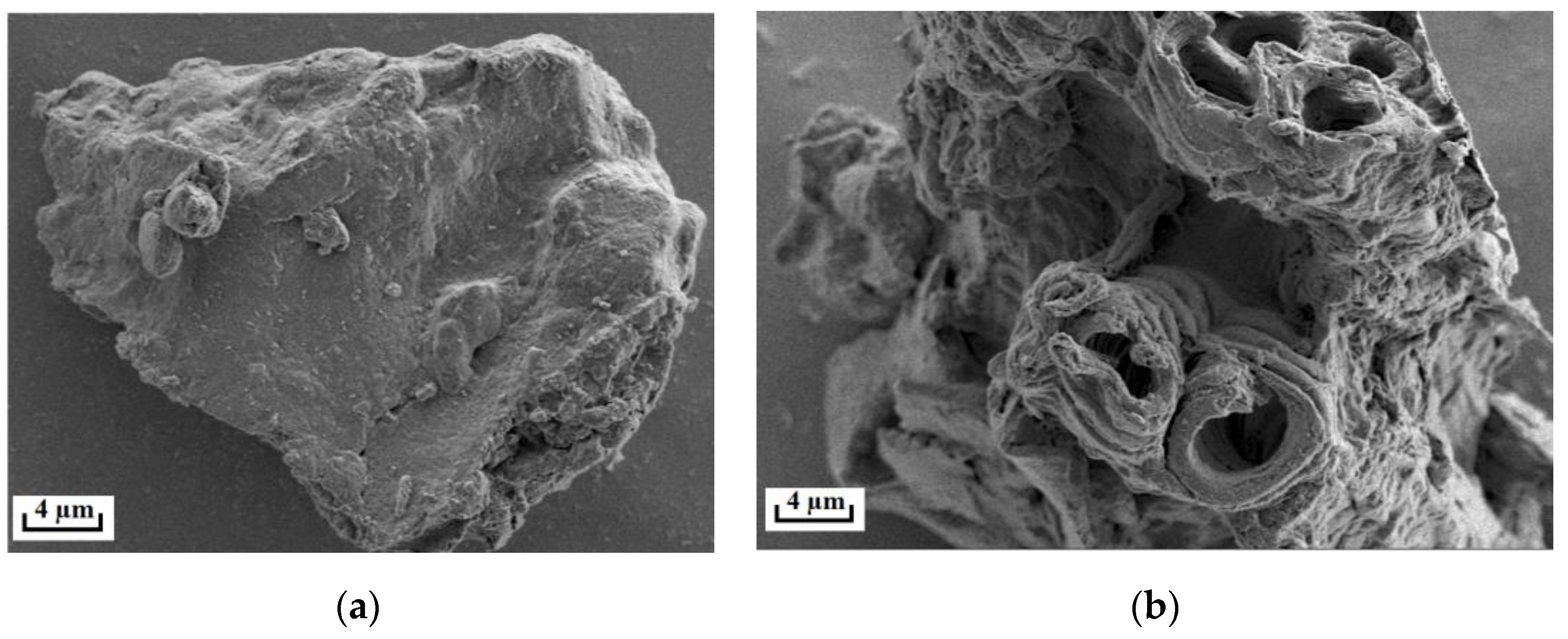
3.4. Microstructural Characteristics of ROLs
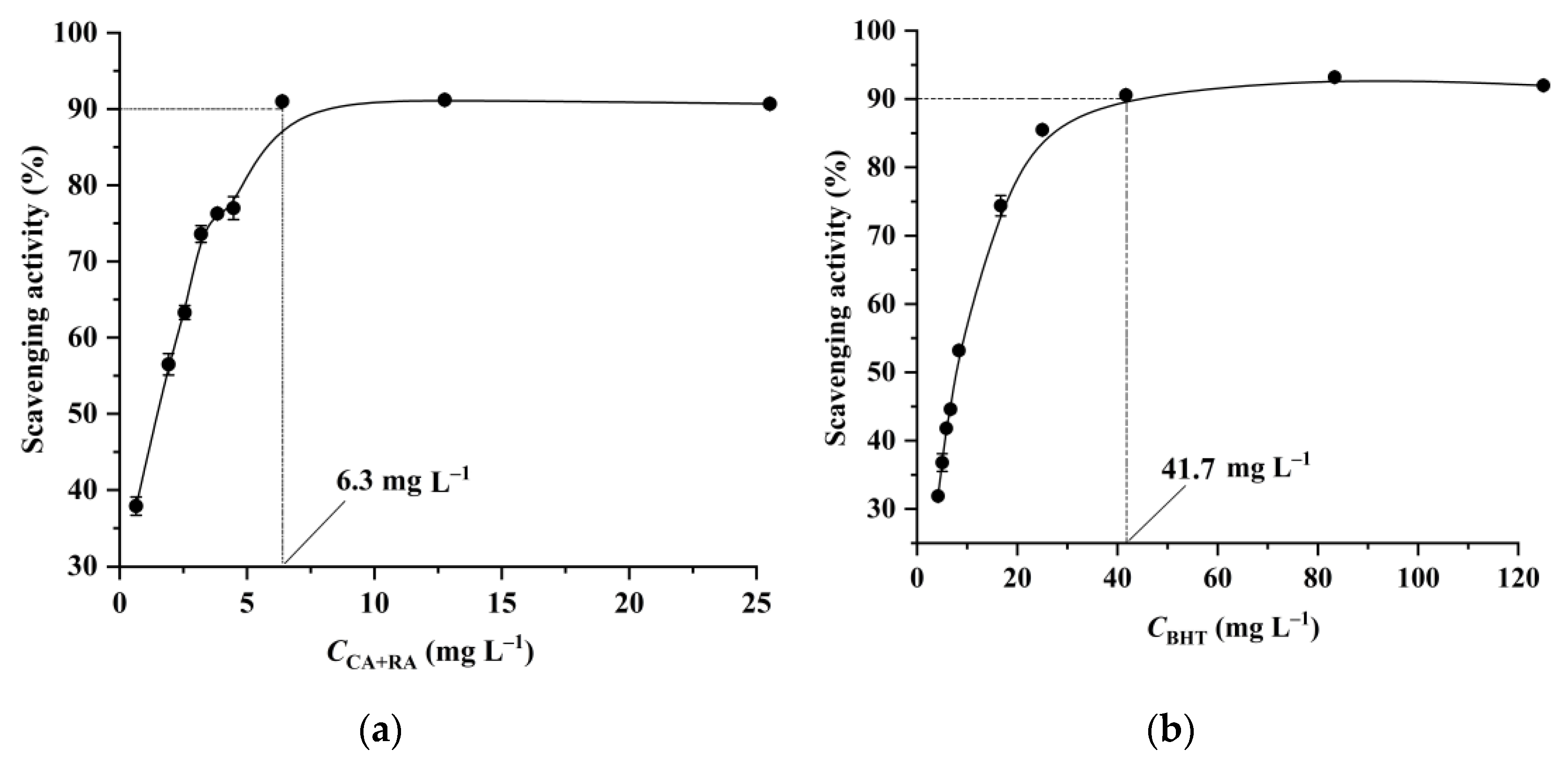
3.5. Antioxidant Activity of the Extract
3.6. Comparison with the Reported Extraction Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus Officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef] [PubMed]
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100–1112. [Google Scholar]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Sequential extraction of carnosic acid, rosmarinic acid and pigments (carotenoids and chlorophylls) from rosemary by online supercritical fluid extraction-supercritical fluid chromatography. J. Chromatogr. A 2021, 1639, 461709. [Google Scholar] [CrossRef] [PubMed]
- Bellumori, M.; Innocenti, M.; Binello, A.; Boffa, L.; Mulinacci, N.; Cravotto, G. Selective recovery of rosmarinic and carnosic acids from rosemary leaves under ultrasound- and microwave-assisted extraction procedures. C. R. Chim. 2016, 19, 699–706. [Google Scholar] [CrossRef]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six Lamiaceae plants as determined by HPLC-DAD measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef]
- Mazaud, A.; Lebeuf, R.; Laguerre, M.; Nardello-Rataj, V. Hydrotropic extraction of carnosic acid from rosemary with shortchain alkyl polyethylene glycol ethers. ACS Sustain. Chem. Eng. 2020, 8, 15268–15277. [Google Scholar] [CrossRef]
- Oliveira, G.d.A.R.; de Oliveira, A.E.; da Conceição, E.C.; Leles, M.I.G. Multiresponse optimization of an extraction procedure of carnosol and rosmarinic and carnosic acids from rosemary. Food Chem. 2016, 211, 465–473. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from Rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Q.; Li, P.; Yang, H. Deep-eutectic solvents/ionic liquids/water mixture as a novel type of green thermo-switchable solvent system for selective extraction and separation of natural products from Rosmarinus officinalis leaves. Food Chem. 2022, 390, 133225. [Google Scholar] [CrossRef]
- Masilela, M.; Ndlovu, S. Extraction of Ag and Au from chloride electronic waste leach solutions using ionic liquids. J. Environ. Chem. Eng. 2019, 7, 102810. [Google Scholar] [CrossRef]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef] [PubMed]
- Log, T.; Moi, A.L. Ethanol and methanol burn risks in the home environment. Int. J. Environ. Res. Public Health 2018, 15, 2379. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.K.; Jones, N. The biodegradation of polyethylene glycols by sewage bacteria. Water Res. 1977, 11, 95–100. [Google Scholar] [CrossRef]
- Marchal, R.; Nicolau, E.; Ballaguet, J.P.; Bertoncini, F. Biodegradability of polyethylene glycol 400 by complex microfloras. Int. Biodeter. Biodegr. 2008, 62, 384–390. [Google Scholar] [CrossRef]
- Hermansky, S.J.; Neptun, D.A.; Loughran, K.A.; Leung, H.W. Effects of polyethylene glycol 400 (PEG 400) following 13 weeks of gavage treatment in fischer-344 rats. Food Chem. Toxicol. 1995, 33, 139–149. [Google Scholar] [CrossRef]
- Prentice, D.E.; Majeed, S.K. Oral toxicity of polyethylene glycol (PEG 200) in monkeys and rats. Toxicol. Lett. 1978, 2, 119–122. [Google Scholar] [CrossRef]
- Kleber, C.; Andrade, Z.; Alves, L.M. Environmentally benign solvents in organic synthesis: Current topics. Curr. Org. Chem. 2005, 9, 195–218. [Google Scholar]
- Arslan, D.; Özcan, M.M. Evaluation of drying methods with respect to drying kinetics, mineral content and colour characteristics of rosemary leaves. Energy Convers. Manag. 2008, 49, 1258–1264. [Google Scholar] [CrossRef]
- Aleksovski, S.A.; Sovová, H. Supercritical CO2 extraction of Salvia officinalis L. J. Supercrit. Fluids 2007, 40, 239–245. [Google Scholar] [CrossRef]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014, 62, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, K.H.; Lee, M.H.; Kim, H.T.; Seo, W.D.; Kim, J.Y.; Baek, I.Y.; Jang, D.S.; Ha, T.J. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar] [CrossRef]
- Oelmeier, S.A.; Dismer, F.; Hubbuch, J. Molecular dynamics simulations on aqueous two-phase systems—Single PEG-molecules in solution. BMC Biophys. 2012, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Aydinli, M.; Tutas, M. Water sorption and water vapour permeability properties of polysaccharide (locust bean gum) based edible films. LWT Food Sci. Technol. 2000, 33, 63–67. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar]
- Zainal-Abidin, M.H.; Hayyan, M.; Hayyan, A.; Jayakumar, N.S. New horizons in the extraction of bioactive compounds using deep eutectic solvents: A review. Anal. Chim. Acta 2017, 979, 1–23. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Efficient extraction of proanthocyanidin from Ginkgo biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2018, 158, 317–326. [Google Scholar] [CrossRef]
- Bonifácio-Lopes, T.; Teixeira, J.A.; Pintado, M. Currentextraction techniques towards bioactive compounds from brewer’s spent grain—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2730–2741. [Google Scholar] [CrossRef]
- Sarada, R.; Vidhyavathi, R.; Usha, D.; Ravishankar, G.A. An efficient method for extraction of astaxanthin from green alga Haematococcuspluvialis. J. Agric. Food Chem. 2006, 54, 7585–7588. [Google Scholar] [CrossRef]
- Sui, X.; Liu, T.; Ma, C.; Yang, L.; Zu, Y.; Zhang, L.; Wang, H. Microwave irradiation to pretreat rosemary (Rosmarinus officinalis L.) for maintaining antioxidant content during storage and to extract essential oil simultaneously. Food Chem. 2012, 131, 1399–1405. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus of ficinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef] [PubMed]


| Independent Variable | Coded Variable Level | ||
|---|---|---|---|
| Low Level (−1) | Center Level (0) | High Level (+1) | |
| PEG-400 concentration (%), A | 30 | 45 | 60 |
| H3PO4concentration (%), B | 2.2 | 4.3 | 8.7 |
| Microwave irradiation time(s), C | 5 | 20 | 40 |
| Microwave irradiation power(W), D | 210 | 280 | 420 |
| Run | A | B | C | D | Response (EE, %) | |
|---|---|---|---|---|---|---|
| CA | RA | |||||
| 1 | 45 | 4.3 | 5 | 210 | 1.75 | 1.07 |
| 2 | 30 | 8.7 | 20 | 280 | 1.97 | 1.29 |
| 3 | 45 | 8.7 | 40 | 280 | 2.16 | 1.24 |
| 4 | 45 | 2.2 | 40 | 280 | 2.34 | 1.33 |
| 5 | 45 | 4.3 | 5 | 420 | 1.48 | 1.14 |
| 6 | 60 | 2.2 | 20 | 280 | 2.04 | 1.22 |
| 7 | 30 | 4.3 | 20 | 210 | 1.43 | 1.11 |
| 8 | 60 | 4.3 | 20 | 210 | 2.41 | 1.09 |
| 9 | 60 | 8.7 | 20 | 280 | 2.45 | 1.24 |
| 10 | 45 | 4.3 | 40 | 210 | 2.46 | 1.05 |
| 11 | 45 | 4.3 | 20 | 280 | 2.54 | 1.30 |
| 12 | 45 | 2.2 | 20 | 420 | 2.16 | 1.23 |
| 13 | 60 | 4.3 | 5 | 280 | 1.66 | 1.14 |
| 14 | 30 | 4.3 | 20 | 420 | 1.80 | 1.24 |
| 15 | 45 | 2.2 | 20 | 210 | 2.17 | 1.09 |
| 16 | 45 | 8.7 | 20 | 420 | 2.18 | 1.16 |
| 17 | 30 | 2.2 | 20 | 280 | 1.59 | 1.30 |
| 18 | 45 | 2.2 | 5 | 280 | 1.69 | 1.29 |
| 19 | 30 | 4.3 | 5 | 280 | 1.13 | 1.20 |
| 20 | 60 | 4.3 | 20 | 420 | 2.03 | 1.23 |
| 21 | 30 | 4.3 | 40 | 280 | 1.82 | 1.29 |
| 22 | 45 | 8.7 | 5 | 280 | 1.90 | 1.32 |
| 23 | 60 | 4.3 | 40 | 280 | 2.25 | 1.30 |
| 24 | 45 | 8.7 | 20 | 210 | 2.32 | 1.16 |
| 25 | 45 | 4.3 | 40 | 420 | 2.09 | 1.21 |
| Source | SS | DF | MS | F-Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| RA | ||||||
| Model | 0.15 | 14 | 0.011 | 5.32 | 0.006 | Significant |
| A | 1.737 × 10−3 | 1 | 1.737 × 10−3 | 0.85 | 0.378 | |
| B | 2.567 × 10−3 | 1 | 2.567 × 10−3 | 1.26 | 0.288 | |
| C | 2.443 × 10−3 | 1 | 2.443 × 10−3 | 1.20 | 0.300 | |
| D | 0.022 | 1 | 0.022 | 10.81 | 0.008 | |
| AB | 2.737 × 10−5 | 1 | 2.737 × 10−5 | 0.013 | 0.910 | |
| AC | 1.328 × 10−3 | 1 | 1.328 × 10−3 | 0.65 | 0.439 | |
| AD | 1.438 × 10−4 | 1 | 1.438 × 10−4 | 0.070 | 0.796 | |
| BC | 6.248 × 10−3 | 1 | 6.248 × 10−3 | 3.06 | 0.111 | |
| BD | 5.949 × 10−3 | 1 | 5.949 × 10−3 | 2.92 | 0.118 | |
| CD | 1.079 × 10−3 | 1 | 1.079 × 10−3 | 0.53 | 0.484 | |
| A2 | 1.832 × 10−3 | 1 | 1.832 × 10−3 | 0.90 | 0.366 | |
| B2 | 5.594 × 10−5 | 1 | 5.594 × 10−5 | 0.027 | 0.872 | |
| C2 | 3.874 × 10−3 | 1 | 3.874 × 10−3 | 1.90 | 0.198 | |
| D2 | 0.063 | 1 | 0.063 | 30.92 | <0.0001 | |
| Residual | 0.020 | 10 | 2.040 × 10−3 | |||
| Cor Total | 0.17 | 24 | ||||
| CA | ||||||
| Model | 2.98 | 14 | 0.21 | 12.02 | <0.0001 | Significant |
| A | 0.43 | 1 | 0.43 | 24.11 | 0.001 | |
| B | 0.042 | 1 | 0.042 | 2.37 | 0.155 | |
| C | 0.61 | 1 | 0.61 | 34.67 | <0.0001 | |
| D | 0.061 | 1 | 0.061 | 3.46 | 0.092 | |
| AB | 2.662 × 10−4 | 1 | 2.662 × 10−4 | 0.015 | 0.905 | |
| AC | 3.625 × 10−3 | 1 | 3.625 × 10−3 | 0.20 | 0.660 | |
| AD | 0.11 | 1 | 0.11 | 6.33 | 0.031 | |
| BC | 0.064 | 1 | 0.064 | 3.63 | 0.086 | |
| BD | 5.257 × 10−3 | 1 | 5.257 × 10−3 | 0.30 | 0.598 | |
| CD | 3.486 × 10−3 | 1 | 3.486 × 10−3 | 0.20 | 0.666 | |
| A2 | 0.50 | 1 | 0.50 | 28.08 | <0.0001 | |
| B2 | 0.060 | 1 | 0.060 | 3.40 | 0.095 | |
| C2 | 0.55 | 1 | 0.55 | 30.92 | <0.0001 | |
| D2 | 0.093 | 1 | 0.093 | 5.26 | 0.045 | |
| Residual | 0.18 | 10 | 0.018 | |||
| Cor Total | 3.15 | 24 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Fan, Y.; Bai, X. A Green and Effective Polyethylene Glycols-Based Microwave-Assisted Extraction of Carnosic and Rosmarinic Acids from Rosmarinus officinalis Leaves. Foods 2023, 12, 1761. https://doi.org/10.3390/foods12091761
Zhu C, Fan Y, Bai X. A Green and Effective Polyethylene Glycols-Based Microwave-Assisted Extraction of Carnosic and Rosmarinic Acids from Rosmarinus officinalis Leaves. Foods. 2023; 12(9):1761. https://doi.org/10.3390/foods12091761
Chicago/Turabian StyleZhu, Chunyan, Yunchang Fan, and Xiujun Bai. 2023. "A Green and Effective Polyethylene Glycols-Based Microwave-Assisted Extraction of Carnosic and Rosmarinic Acids from Rosmarinus officinalis Leaves" Foods 12, no. 9: 1761. https://doi.org/10.3390/foods12091761






