Aerobic Cultivation of Mucor Species Enables the Deacidification of Yogurt Acid Whey and the Production of Fungal Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Microorganisms and Preparation of the Culture Substrates
2.3. Starter Preparation
2.4. Inoculation and Biomass Cultivation
2.5. Sampling and Analysis Methods
2.6. Determinations of Fatty Acid Profile in Fungal Lipids and Biochemical Oxygen Demand in Spent Cultivation Media
2.7. Statistical Analyses
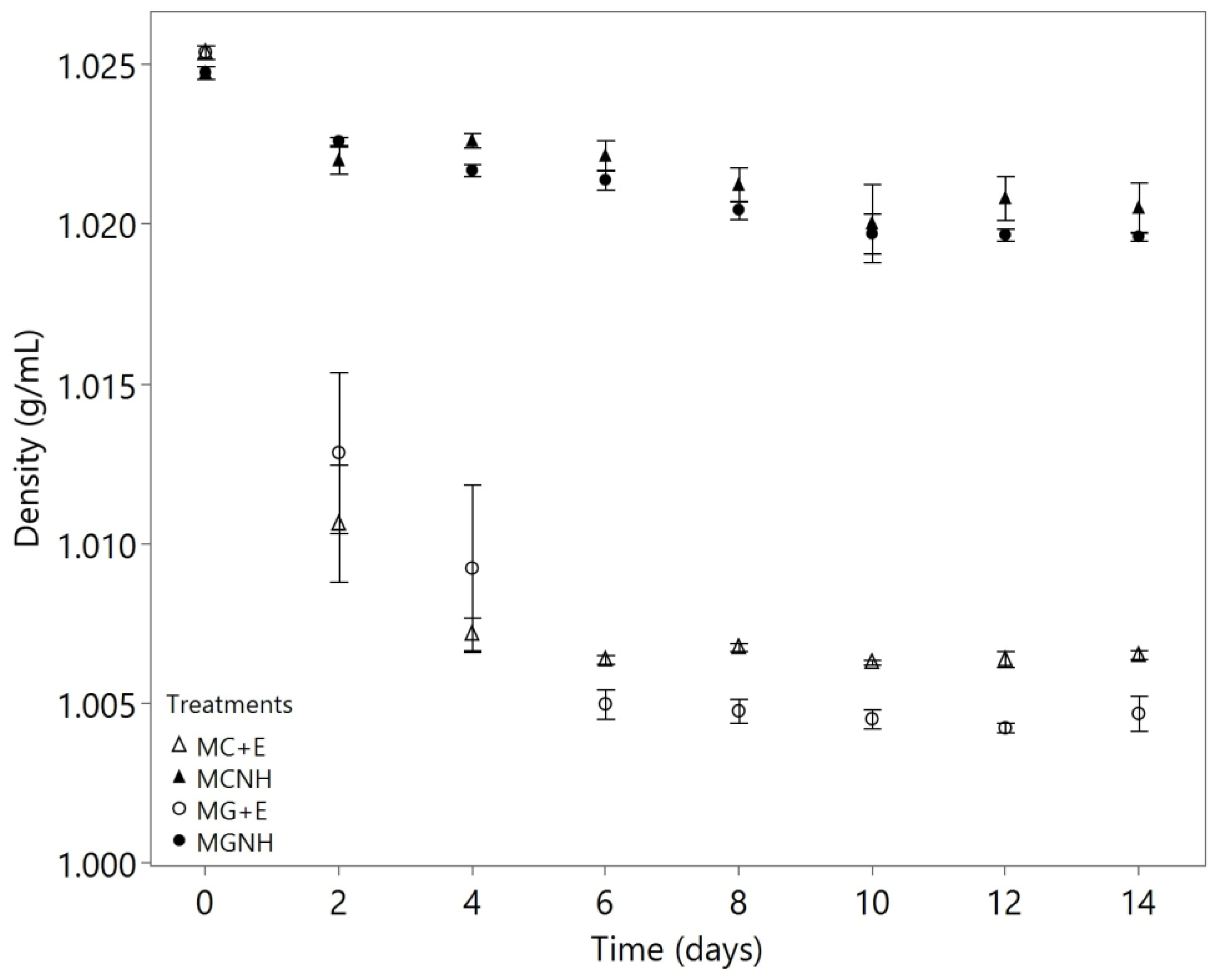
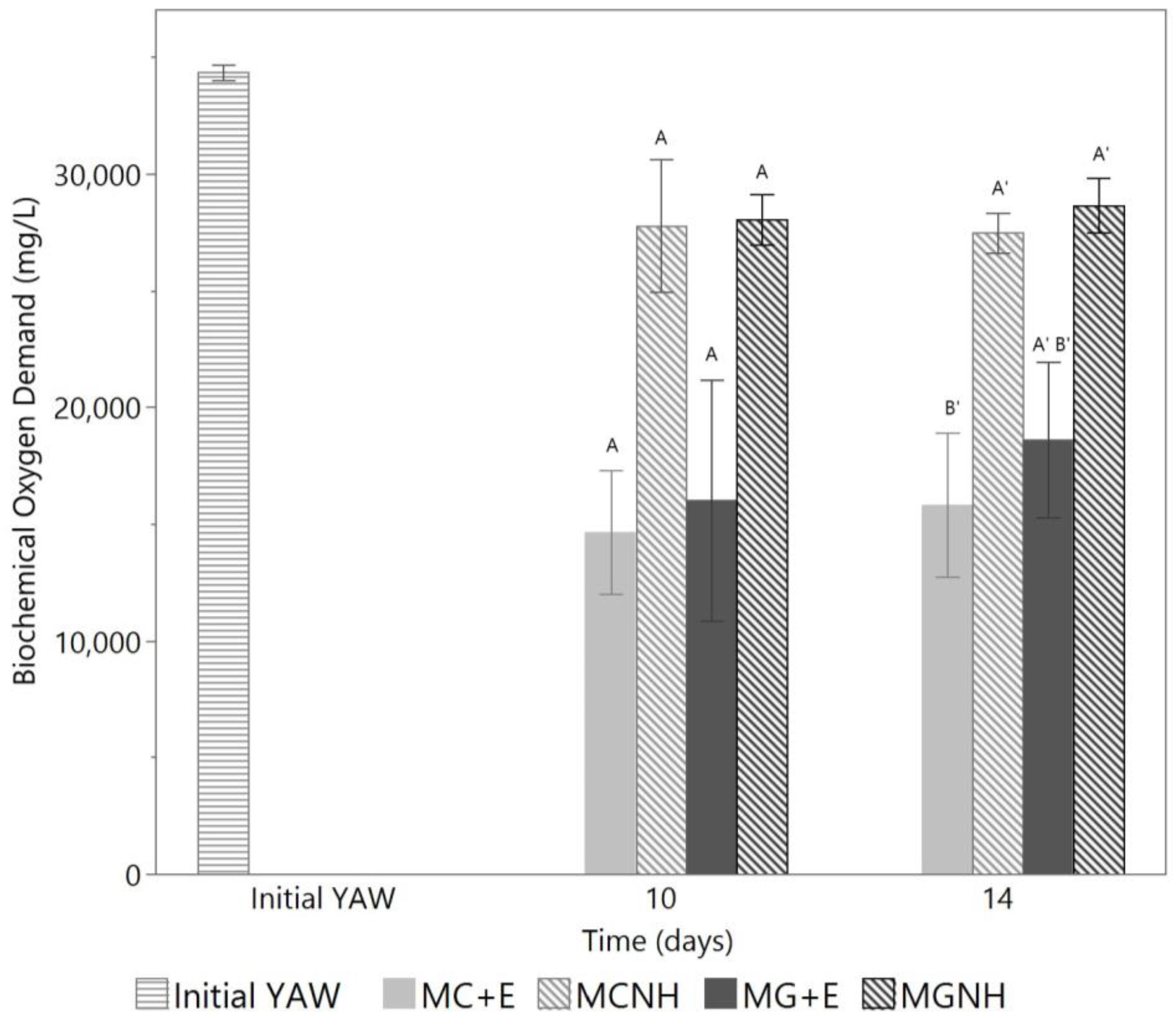
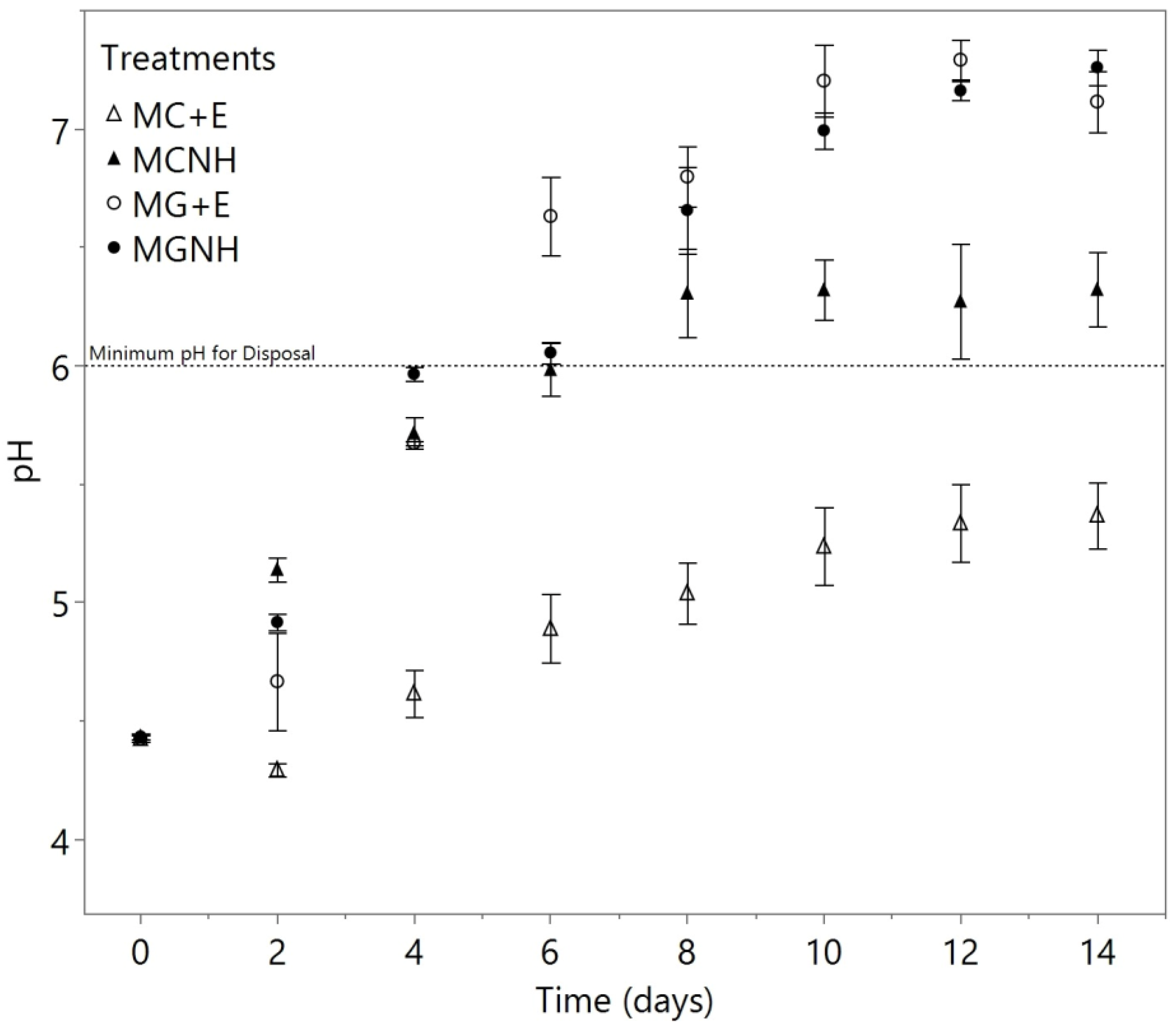
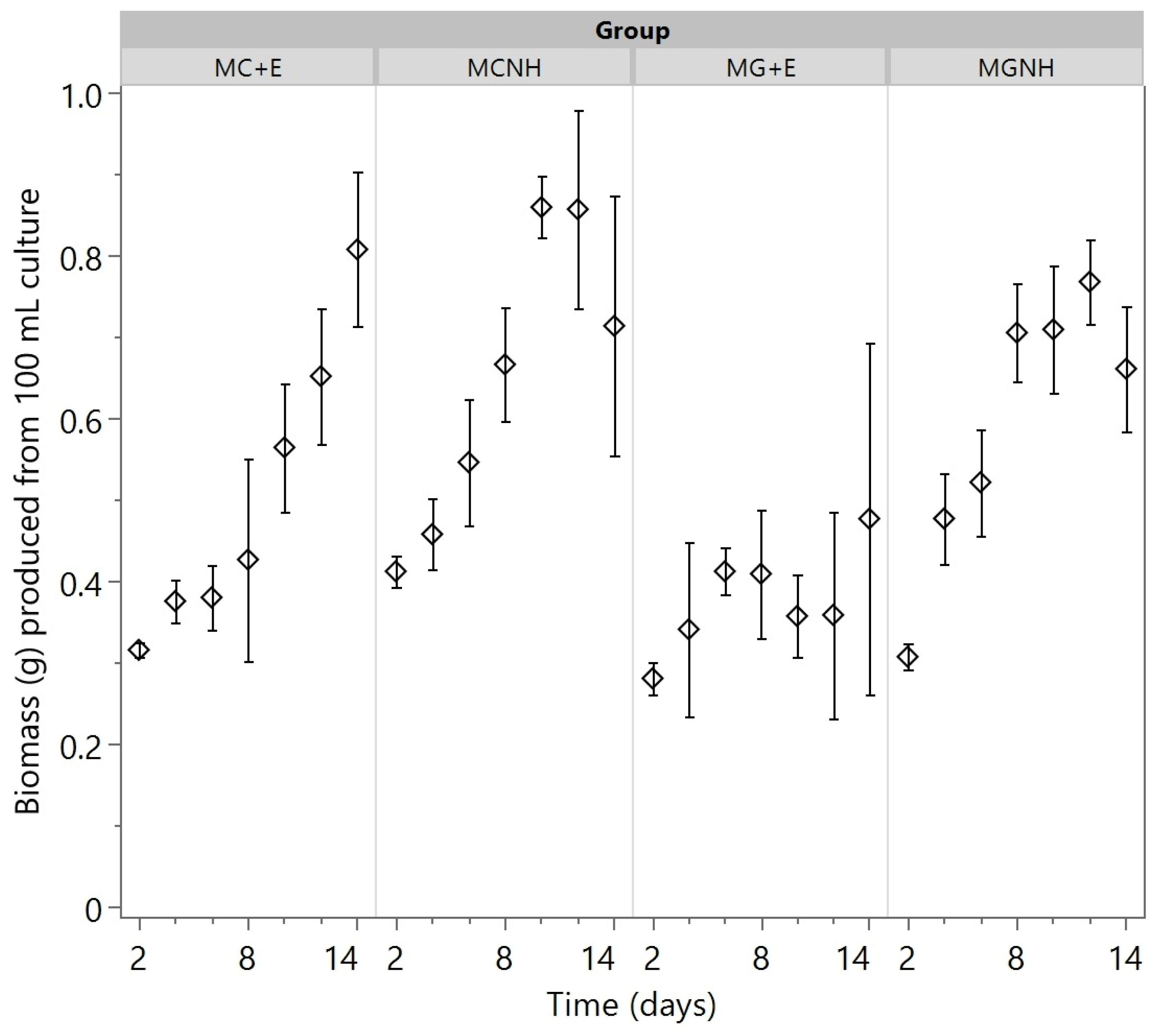
3. Results and Discussion
3.1. Utilizations of Lactose, Galactose, and Glucose by Mucor Species
3.2. Deacidification of Yogurt Acid Whey
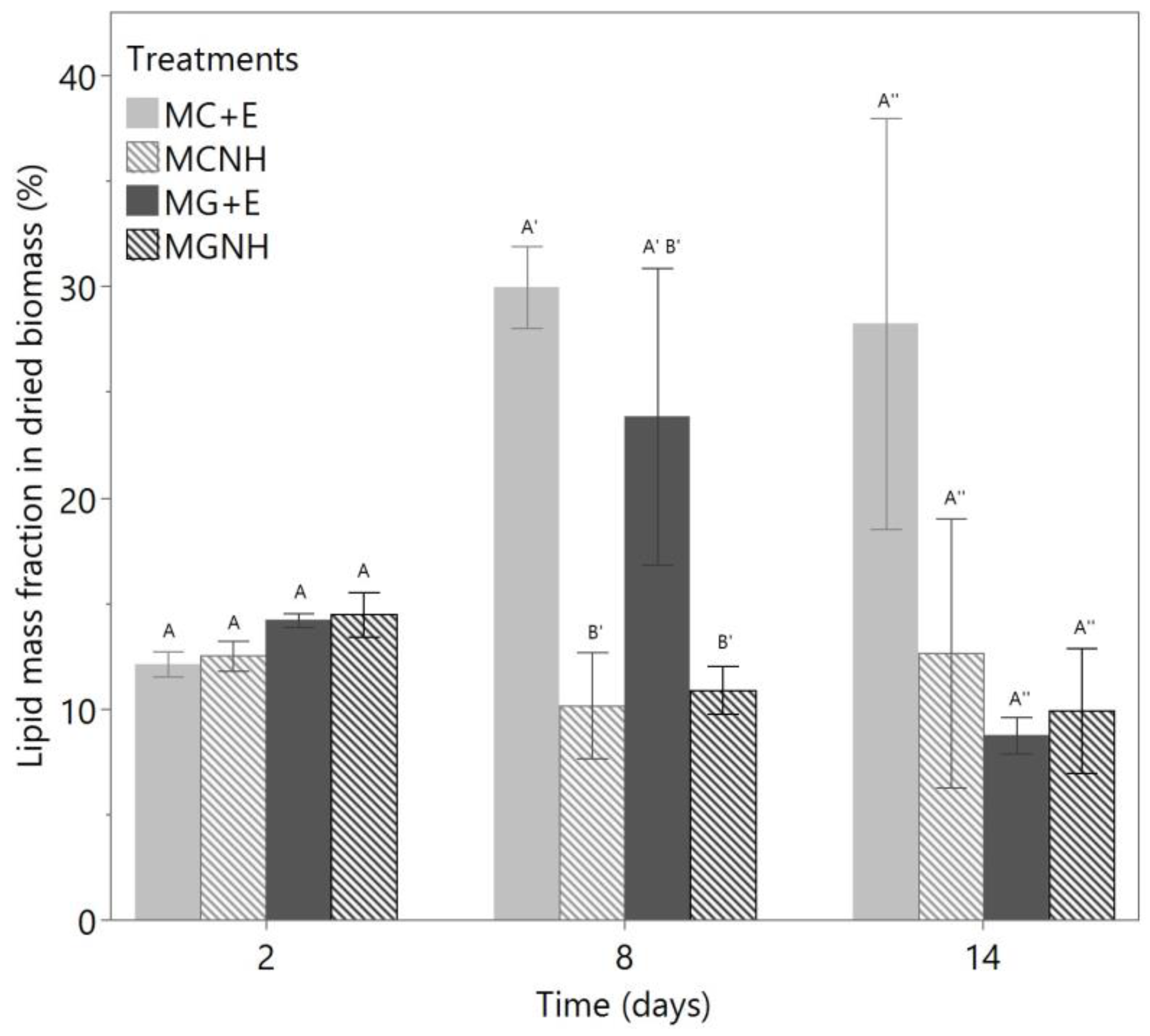
3.3. Biomass and Fungal Oil Production
3.4. Fungal Oil Fatty Acids Composition
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahbandeh, M.U.S. Greek Yogurt Market—Statistics & Facts. Available online: https://www.statista.com/topics/2351/greek-yogurt/ (accessed on 5 February 2023).
- Menchik, P.; Zuber, T.; Zuber, A.; Moraru, C.I. Short Communication: Composition of Coproduct Streams from Dairy Processing: Acid Whey and Milk Permeate. J. Dairy Sci. 2019, 102, 3978–3984. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.E. Acid Whey: Is the Waste Product an Untapped Goldmine? Available online: https://cen.acs.org/articles/95/i6/Acid-whey-waste-product-untapped.html (accessed on 5 February 2023).
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited Review: Acid Whey Trends and Health Benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef]
- 40 CFR 405 40 CFR Part 405—Dairy Products Processing Point Source Category. Available online: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-N/part-405 (accessed on 20 February 2023).
- Lindsay, M.J.; Molitor, M.S.; Goculdas, T.B.; Zhao, J.; Featherman, J.R.; Li, M.; Miller, J.B.; Avraamidou, S.; Rankin, S.A.; Dumesic, J.A.; et al. Production of Glucose-Galactose Syrup and Milk Minerals from Greek Yogurt Acid Whey. Green Chem. 2022, 24, 8538–8551. [Google Scholar] [CrossRef]
- Ketterings, Q.; Czymmek, K.; Gami, S.; Godwin, G.; Ganoe, K. Guidelines for Land Application of Acid Whey; Cornell University, Department of Animal Science: Ithaca, NY, USA, 2017; Volume 247. [Google Scholar]
- Rivera Flores, V.K.; DeMarsh, T.A.; Gibney, P.A.; Alcaine, S.D. Fermentation of Dairy-Relevant Sugars by Saccharomyces, Kluyveromyces, and Brettanomyces: An Exploratory Study with Implications for the Utilization of Acid Whey, Part I. Fermentation 2021, 7, 266. [Google Scholar] [CrossRef]
- Rivera Flores, V.K.; DeMarsh, T.A.; Gibney, P.A.; Alcaine, S.D. Fermentation of Dairy-Relevant Sugars by Saccharomyces, Kluyveromyces, and Brettanomyces: An Exploratory Study with Implications for the Utilization of Acid Whey, Part II. Fermentation 2022, 8, 257. [Google Scholar] [CrossRef]
- Xu, J.; Hao, J.; Guzman, J.J.L.; Spirito, C.M.; Harroff, L.A.; Angenent, L.T. Temperature-Phased Conversion of Acid Whey Waste Into Medium-Chain Carboxylic Acids via Lactic Acid: No External e-Donor. Joule 2018, 2, 280–295. [Google Scholar] [CrossRef][Green Version]
- Sarenkova, I.; Sáez-Orviz, S.; Ciprovica, I.; Rendueles, M.; Díaz, M. Lactobionic Acid Production by Pseudomonas Taetrolens in a Fed-Batch Bioreactor Using Acid Whey as Substrate. Int. J. Dairy Technol. 2022, 75, 361–371. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of Galactooligosaccharides by Cryptococcus Laurentii and Aspergillus Oryzae Using Different Kinds of Acid Whey. Int. Dairy J. 2021, 112, 104867. [Google Scholar] [CrossRef]
- Lindsay, M.J.; Walker, T.W.; Dumesic, J.A.; Rankin, S.A.; Huber, G.W. Production of Monosaccharides and Whey Protein from Acid Whey Waste Streams in the Dairy Industry. Green Chem. 2018, 20, 1824–1834. [Google Scholar] [CrossRef]
- Camacho Flinois, J.; Dando, R.; Padilla-Zakour, O.I. Effects of Replacing Buttermilk with Yogurt Acid Whey in Ranch Dressing. J. Dairy Sci. 2019, 102, 7874–7883. [Google Scholar] [CrossRef][Green Version]
- Camacho Flinois, J.; Dando, R.; Padilla-Zakour, O.I. Yogurt Acid Whey Utilization for Production of Baked Goods: Pancakes and Pizza Crust. Foods 2019, 8, 615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shahbandeh, M. Global Greek Yogurt Market Value Forecast 2027. Available online: https://www.statista.com/statistics/1022042/global-greek-yogurt-market-value/ (accessed on 18 February 2023).
- Marcus, J.F.; DeMarsh, T.A.; Alcaine, S.D. Upcycling of Whey Permeate through Yeast- and Mold-Driven Fermentations under Anoxic and Oxic Conditions. Fermentation 2021, 7, 16. [Google Scholar] [CrossRef]
- Chan, L.G.; Cohen, J.L.; Ozturk, G.; Hennebelle, M.; Taha, A.Y.; LN de Moura Bell, J.M. Bioconversion of Cheese Whey Permeate into Fungal Oil by Mucor Circinelloides. J. Biol. Eng. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed]
- Bento, H.B.S.; Carvalho, A.K.F.; Reis, C.E.R.; De Castro, H.F. Single Cell Oil Production and Modification for Fuel and Food Applications: Assessing the Potential of Sugarcane Molasses as Culture Medium for Filamentous Fungus. Ind. Crops Prod. 2020, 145, 112141. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Chapkin, R.S. Importance of Dietary γ-Linolenic Acid in Human Health and Nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef][Green Version]
- Kris-Etherton, P.M. Monounsaturated Fatty Acids and Risk of Cardiovascular Disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef][Green Version]
- Botha, A.; Kock, J.L.F.; Coetzee, D.J.; Botes, P.J. Physiological Properties and Fatty Acid Composition in Mucor Circinelloides f. Circinelloides. Antonie Van Leeuwenhoek 1997, 71, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, M. Mucor Dimorphism. Microbiol. Rev. 1991, 55, 234–258. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. AOAC Official Method 922.06; Fat in Flour. Acid Hydrolysis Method. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Mehrotra, B.R.; Kakkar, R.K. Utilization of Monosaccharides by Two Species of Mucor. Flora Oder Allg. Bot. Zeitung. Abt. A Physiol. Und Biochem. 1969, 160, 369–377. [Google Scholar] [CrossRef]
- Wierzbicki, L.E.; Kosikowski, F.V. Lactase Potential of Various Microorganisms Grown in Whey. J. Dairy Sci. 1973, 56, 26–32. [Google Scholar] [CrossRef]
- Deploey, J.J. Carbohydrate Nutrition of Mucor Miehei and M. Pusillus. Mycologia 1976, 68, 190–194. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, J.; Zhang, W.; Hu, B. A New Cultivation Method for Microbial Oil Production: Cell Pelletization and Lipid Accumulation by Mucor Circinelloides. Biotechnol. Biofuels 2011, 4, 15. [Google Scholar] [CrossRef][Green Version]
- Iqbal, Z.; Sajjad, R. Some Pathogenic Fungi Parasitizing Two Exotic Tropical Ornamental Fishes. Int. J. Agric. Biol. (Pak.) 2013, 15, 595–598. [Google Scholar]
- Saffari, M.; Langrish, T. Effect of Lactic Acid In-Process Crystallization of Lactose/Protein Powders during Spray Drying. J. Food Eng. 2014, 137, 88–94. [Google Scholar] [CrossRef]
- Silverman, A.M. Metabolic Engineering Strategies for Increasing Lipid Production in Oleaginous Yeast. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2015. [Google Scholar]
- Alves, M.H.; Campos-Takaki, G.M.; Porto, A.L.F.; Milanez, A.I. Screening of Mucor Spp. for the Production of Amylase, Lipase, Polygalacturonase and Protease. Braz. J. Microbiol. 2002, 33, 325–330. [Google Scholar] [CrossRef][Green Version]
- Struszczyk, K.; Szczęsna-Antczak, M.; Walczak, M.; Pomianowska, E.; Antczak, T. Isolation and Purification of Mucor Circinelloides Intracellular Chitosanolytic Enzymes. Carbohydr. Polym. 2009, 78, 16–24. [Google Scholar] [CrossRef]
- Chan, L.G.; Dias, F.F.G.; Saarni, A.; Cohen, J.; Block, D.; Taha, A.Y.; de Moura Bell, J.M.L.N. Scaling up the Bioconversion of Cheese Whey Permeate into Fungal Oil by Mucor Circinelloides. J. Am. Oil Chem. Soc. 2020, 97, 703–716. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Kabir Khan, M.A.; Garre, V.; Vongsangnak, W.; Song, Y. Increased Lipid Accumulation in Mucor Circinelloides by Overexpression of Mitochondrial Citrate Transporter Genes. Ind. Eng. Chem. Res. 2019, 58, 2125–2134. [Google Scholar] [CrossRef]
- Vicente, G.; Bautista, L.F.; Gutiérrez, F.J.; Rodríguez, R.; Martínez, V.; Rodríguez-Frómeta, R.A.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Direct Transformation of Fungal Biomass from Submerged Cultures into Biodiesel. Energy Fuels 2010, 24, 3173–3178. [Google Scholar] [CrossRef]
- Vicente, G.; Bautista, L.F.; Rodríguez, R.; Gutiérrez, F.J.; Sádaba, I.; Ruiz-Vázquez, R.M.; Torres-Martínez, S.; Garre, V. Biodiesel Production from Biomass of an Oleaginous Fungus. Biochem. Eng. J. 2009, 48, 22–27. [Google Scholar] [CrossRef]
- Snyder, A.B.; Churey, J.J.; Worobo, R.W. Characterization and Control of Mucor Circinelloides Spoilage in Yogurt. Int. J. Food Microbiol. 2016, 228, 14–21. [Google Scholar] [CrossRef][Green Version]
- Gordon, P.A.; Stewart, P.R.; Clark-Walker, G.D. Fatty Acid and Sterol Composition of Mucor Genevensis in Relation to Dimorphism and Anaerobic Growth. J. Bacteriol. 1971, 107, 114–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gnoni, G.V.; Natali, F.; Geelen, M.J.H.; Siculella, L. Chapter 152—Oleic Acid as an Inhibitor of Fatty Acid and Cholesterol Synthesis. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 1365–1373. ISBN 978-0-12-374420-3. [Google Scholar]
- Mustonen, A.-M.; Nieminen, P. Dihomo-γ-Linolenic Acid (20:3n-6)—Metabolism, Derivatives, and Potential Significance in Chronic Inflammation. Int. J. Mol. Sci. 2023, 24, 2116. [Google Scholar] [CrossRef]
- Strong, P.J.; Self, R.; Allikian, K.; Szewczyk, E.; Speight, R.; O’Hara, I.; Harrison, M.D. Filamentous Fungi for Future Functional Food and Feed. Curr. Opin. Biotechnol. 2022, 76, 102729. [Google Scholar] [CrossRef]
- Karimi, K.; Zamani, A. Mucor Indicus: Biology and Industrial Application Perspectives: A Review. Biotechnol. Adv. 2013, 31, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, B.-B.; Wu, Z.-H. Studies on Mucor Racemosus Fermentation to Manufacture Gamma-Linolenic Acid Functional Food Douchi. Food Sci. Technol. Res. 2010, 16, 543–548. [Google Scholar] [CrossRef][Green Version]


| Cultures with Lactose Hydrolysis | Lactose (g/L) | Galactose (g/L) | Glucose (g/L) | |
|---|---|---|---|---|
| Uninoculated | day 0 | -- a | 25.48 ± 1.41 | 18.67 ± 0.84 |
| M. genevensis | day 6 | <0.04 | 0.04 ± 0.00 | 0.09 ± 0.01 |
| day 14 | <0.04 | 0.03 ± 0.00 | 0.07 ± 0.03 | |
| M. circinelloides | day 6 | <0.04 | <0.02 | 0.07 ± 0.03 |
| day 14 | <0.04 | <0.02 | <0.07 b | |
| Cultures without Lactose Hydrolysis | Lactose (g/L) | Galactose (g/L) | Glucose (g/L) | |
|---|---|---|---|---|
| Uninoculated | day 0 | 39.11 ± 1.19 | 6.72 ± 0.43 | 0.18 ± 0.01 |
| M. genevensis | day 6 | 40.66 ± 0.54 | 0.15 ± 0.01 | 0.14 ± 0.04 |
| day 14 | 40.96 ± 1.01 | 0.16 ± 0.03 | <0.07 a | |
| M. circinelloides | day 6 | 39.80 ± 2.71 | 0.13 ± 0.00 | 0.10 ± 0.01 |
| day 14 | 42.77 ± 2.81 | 0.24 ± 0.02 | <0.07 a | |
| % w/w in Total Fatty Acids | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fatty Acid a | MC+E b | MCNH b | MG+E b | MGNH b | ||||
| Day 10 | Day 14 | Day 10 | Day 14 | Day 10 | Day 14 | Day 10 | Day 14 | |
| Myristic acid (14:0) | 3.3 ± 0.2 | 3.2 ± 0.3 | 3.0 ± 0.4 | 3.4 ± 0.1 | 1.8 ± 0.4 | 1.7 ± 0.0 | 2.4 ± 0.1 | 1.0 ± 0.9 |
| Palmitic acid (16:0) | 17.8 ± 0.7 | 17.9 ± 0.1 | 17.5 ± 0.2 | 15.5 ± 0.2 | 13.2 ± 2.3 | 15.3 ± 2.3 | 18.4 ± 0.7 | 25.6 ± 0.9 |
| Palmitoleic acid (16:1) | 9.0 ± 0.5 | 7.1 ± 0.3 | 5.5 ± 0.2 | 5.4 ± 0.3 | 11.3 ± 1.5 | 9.2 ± 0.1 | 7.1 ± 1.7 | 4.9 ± 0.8 |
| Stearic acid (18:0) | 10.7 ± 0.0 | 10.6 ± 1.0 | 10.7 ± 0.2 | 12.3 ± 0.4 | 7.7 ± 3.5 | 7.9 ± 0.5 | 8.5 ± 1.4 | 12.1 ± 0.3 |
| Oleic acid (18:1) | 49.5 ± 1.5 | 49.9 ± 1.1 | 35.7 ± 1.4 | 40.1 ± 1.0 | 40.1 ± 1.8 | 30.3 ± 1.6 | 34.8 ± 2.1 | 29.2 ± 1.8 |
| Linoleic acid (18:2) | 2.6 ± 0.6 | 4.6 ± 0.9 | 10.1 ± 2.4 | 6.8 ± 0.2 | 6.6 ± 0.5 | 9.9 ± 3.0 | 8.5 ± 1.4 | 7.2 ± 0.9 |
| γ-linolenic acid (18:3, ω-6) | 1.2 ± 0.2 | 1.7 ± 0.4 | 10.5 ± 0.7 | 9.2 ± 1.5 | 7.3 ± 0.9 | 10.7 ± 2.9 | 11.4 ± 2.8 | 10.7 ± 0.3 |
| Lignoceric acid (24:0) | 0.2 ± 0.1 | 0.1 ± 0.0 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 2.0 ± 0.3 | 2.0 ± 0.1 | 2.1 ± 0.2 |
| Total SFAs c | 32.8 ± 1.0 | 32.4 ± 2.4 | 34.0 ± 0.8 | 34.3 ± 0.0 | 24.2 ± 6.9 | 27.6 ± 2.8 | 32.4 ± 0.8 | 42.4 ± 0.4 |
| Total MUFAs c | 59.2 ± 1.9 | 57.1 ± 0.4 | 41.7 ± 0.8 | 46.0 ± 1.7 | 57.1 ± 5.0 | 47.8 ± 2.3 | 43.7 ± 0.3 | 36.1 ± 0.8 |
| Total PUFAs c | 3.64 ± 0.7 | 6.1 ± 1.2 | 19.9 ± 1.8 | 15.3 ± 1.6 | 13.5 ± 1.4 | 20.3 ± 5.2 | 19.5 ± 0.9 | 17.2 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Rivera Flores, V.K.; DeMarsh, T.A.; deRiancho, D.L.; Alcaine, S.D. Aerobic Cultivation of Mucor Species Enables the Deacidification of Yogurt Acid Whey and the Production of Fungal Oil. Foods 2023, 12, 1784. https://doi.org/10.3390/foods12091784
Fan X, Rivera Flores VK, DeMarsh TA, deRiancho DL, Alcaine SD. Aerobic Cultivation of Mucor Species Enables the Deacidification of Yogurt Acid Whey and the Production of Fungal Oil. Foods. 2023; 12(9):1784. https://doi.org/10.3390/foods12091784
Chicago/Turabian StyleFan, Xingrui, Viviana K. Rivera Flores, Timothy A. DeMarsh, Dana L. deRiancho, and Samuel D. Alcaine. 2023. "Aerobic Cultivation of Mucor Species Enables the Deacidification of Yogurt Acid Whey and the Production of Fungal Oil" Foods 12, no. 9: 1784. https://doi.org/10.3390/foods12091784
APA StyleFan, X., Rivera Flores, V. K., DeMarsh, T. A., deRiancho, D. L., & Alcaine, S. D. (2023). Aerobic Cultivation of Mucor Species Enables the Deacidification of Yogurt Acid Whey and the Production of Fungal Oil. Foods, 12(9), 1784. https://doi.org/10.3390/foods12091784







