Alginate Based Core–Shell Capsules Production through Coextrusion Methods: Recent Applications
Abstract
1. Introduction
2. Oil and Essential Oil Encapsulation
2.1. Reduced Oxidation
2.2. Reduced Evaporation and Release
2.3. Reduced Taste
3. Probiotics Encapsulation
| Shell and/or Coating Material | Probiotics | Prebiotics | Parameters | Capsules Size (µm) | EE (%) | SGC (ph, Duration) | Survivability (%) | Refs | |
|---|---|---|---|---|---|---|---|---|---|
| Encapsulated Probiotics | Free Probiotics | ||||||||
| Alginate | Lactobacillus acidophilus | Apple skin Polyphenols | Frequency: 2723 Hz | 423–486 | 96.7 | SGF (pH 2, 2 h) | 73.9 | 54.1 | [80] |
| Alginate | Lactiplantibacillus plantarum | Inulin | Inner/outer nozzle: 150/300 µm, Frequency: 300 Hz, Air pressure: 600 mbar | 685 | 95 | SGF (pH 2, 2 h) SIF (pH 7.5, 5 h) | 92.0 97.5 | 88.4 94.5 | [65] |
| Alginate | Bifidobacterium lactis Bi-07 | Galacto-oligosaccharides | Inner/outer nozzle: 200/300 µm, Frequency: 300 Hz, Air pressure: 600 mbar | 736 | 94 | SGF (pH 2, 2 h) SIF (pH 7.5, 5 h) | 91.3 82 | 80.5 75.0 | [83] |
| Alginate–low-methoxylated | Bifidobacterium infantis | / | Inner/outer nozzle:150/300 µm | 520–568 | ND | ND | ND | ND | [74] |
| Alginate/pectin | Lactobacillus rhamnosus GG | Black bean extract | Inner/outer nozzle: 150/300 µm, Frequency: 500 mL, Air pressure: 300 Hz | 715 | 98.3 | SGF (pH 2, 2 h) SIF (pH 7.4, 4 h) | 94.1 79.9 | 90.1 62.5 | [72] |
| Alginate/chitosan | Lactobacillus plantarum 299v | Oligofructose | Inner/outer nozzle: 150/300 µm, Air pressure: 600 mbar, Frequency: 300 Hz | 648–790 | 93.0 | SGF (pH 2, 2 h) SIF (pH 7.5, 5 h) | 97.0 85.0 | 80.0 75.0 | [73] |
| Alginate–chitosan | Bifidobacterium animalis subsp. lactis BB-12 | Mannitol | Inner/outer nozzle: 200/300 µm Air pressure: 600 mbar, Frequency: 300 Hz | 800 | 89.2 | SGF (pH 2, 2 h) SIF (pH 7.5, 3 h) | 97.3 86.7 | 74 97.5 | [84] |
| Alginate–chitosan | Lactobacillus plantarum 299v | / | Inner/outer nozzle: 150/300 µm Air pressure: 600 mbar, Frequency: 300 Hz | 620 | 97.7 | SGF (pH 2, 2 h) SIF (pH 7.2, 4 h) | 97.0 95.5 | 93.5 0.0 | [85] |
| Alginate– chitosan | Lactobacillus rhamnosus GG | Flaxseed mucilage | Inner/outer nozzle: 200/300 µm, Inner/outer rate: 1.0/7.8 mL/min | 780 | 98.8 | SGF (pH 2, 2 h) SIF (pH 7.0, 4 h) | 84.9 93.2 | 35.4 47.9 | [82] |
| Alginate– chitosan | Lactobacillus acidophilus 5 | Isomalto-oligosaccharide | Inner/outer nozzle: 200/300 µm, Air pressure: 600 mbar, Frequency: 300 Hz | 616 | 92.2 | SGF (pH 2, 2 h) SIF (pH 7.2, 2 h) | 60.9 0.0 | 0.0 0.0 | [86] |
| Alginate– alginate/shellac | Lactobacillus paracasei BGP-1 in sunflower oil/coconut fat | / | Inner/outer nozzle: 450/700 µm, Inner/outer rate: 2/16 mL/min, Frequency: 100 Hz | 710–860 | ND | SGF (pH 1.8, 2 h) SIF (pH 6.5, 3 h) | 95.0 100 | 50.0 70.0 | [61] |
| Alginate–Alginate/Shellac | Lactobacillus acidophilus LA3 in sunflower oil | / | Inner/outer nozzle: 450/700 µm, Inner/outer rate: 2/16 mL/min Frequency: 100 Hz | 690–760 | ND | SGF (pH 1.8, 2 h) SIF (pH 6.5, 3 h) | 92.0 93.0 | ND ND | [71] |
| Alginate–Alginate/Poly-L-lysine | Lactobacillus rhamnosus GG | Isomalto-oligosaccharide | Inner/outer nozzle: 200/300 µm, Air pressure: 600 mbar, Frequency: 300 Hz | 491–541 | 90.4 | SGF (pH 2, 2 h) SIF (pH 7.5, 2 h) | 75.0 0.0 | 39.2 0.0 | [78] |
| Alginate/locust bean gum | Lactobacillus acidophilus NCFM | Mannitol | Inner/outer nozzle: 200/300 µm, Air pressure: 600 mbar, Frequency: 300 Hz | 550–700 | 96.8 | SGF (pH 2, 2 h) SIF (pH 7.5, 3 h) | 78.4 70.2 | 64.9 0.0 | [59] |
4. Encapsulation of Other Ingredients
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rashedy, S.H.; El Hafez, M.S.M.A.; Dar, M.A.; Cotas, J.; Pereira, L. Evaluation and Characterization of Alginate Extracted from Brown Seaweed Collected in the Red Sea. Appl. Sci. 2021, 11, 6290. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cano, B.; Mendoza-Meneses, C.J.; García-Trejo, J.F.; Macías-Bobadilla, G.; Aguirre-Becerra, H.; Soto-Zarazúa, G.M.; Feregrino-Pérez, A.A. Review and Perspectives of the Use of Alginate as a Polymer Matrix for Microorganisms Applied in Agro-Industry. Molecules 2022, 27, 4248. [Google Scholar] [CrossRef] [PubMed]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Leirvåg, I.T. Strategies for Stabilising Calcium Alginate Gel Beads: Studies of Chitosan Oligomers, Alginate Molecular Weight and Concentration. Master’s Thesis, National Taiwan Normal University, Taipei, Taiwan, 2017. [Google Scholar]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Feng, L.; Cao, Y.; Xu, D.; Wang, S.; Zhang, J. Molecular weight distribution, rheological property and structural changes of sodium alginate induced by ultrasound. Ultrason. Sonochemistry 2017, 34, 609–615. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Zabot, G.L.; Rodrigues, F.S.; Ody, L.P.; Tres, M.V.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of Bioactive Compounds for Food and Agricultural Applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and Controlled Release of Resveratrol Within Functionalized Mesoporous Silica Nanoparticles for Prostate Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Sobel, R.; Versic, R.; Gaonkar, A.G. Introduction to Microencapsulation and Controlled Delivery in Foods. In Microencapsulation in the Food Industry: A Practical Implementation Guide; Elsevier: Amsterdam, The Netherlands, 2023; pp. 26–34. [Google Scholar] [CrossRef]
- Ben Messaoud, G.; Sánchez-González, L.; Probst, L.; Desobry, S. Influence of internal composition on physicochemical properties of alginate aqueous-core capsules. J. Colloid Interface Sci. 2016, 469, 120–128. [Google Scholar] [CrossRef]
- Brandau, T. Annular Nozzle in Laminar Flow Encapsulation Processes. In Microencapsulation in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 137–154. ISBN 9780128216835. [Google Scholar]
- Ben Messaoud, G.; Sánchez-González, L.; Probst, L.; Jeandel, C.; Arab-Tehrany, E.; Desobry, S. Physico-chemical properties of alginate/shellac aqueous-core capsules: Influence of membrane architecture on riboflavin release. Carbohydr. Polym. 2016, 144, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Oxley, J.D. Coextrusion for Food Ingredients and Nutraceutical Encapsulation: Principles and Technology; Elsevier Masson SAS: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Optimization of core-shell capsules properties (Olive oil/alginate) obtained by dripping coextrusion process. LWT 2022, 167, 113879. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Rodrigues, R.C.; Renard, D. Oil encapsulation techniques using alginate as encapsulating agent: Applications and drawbacks. J. Microencapsul. 2017, 34, 754–771. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng. Rev. 2014, 7, 452–490. [Google Scholar] [CrossRef]
- Wang, P.; Ding, M.; Zhang, T.; Wu, T.; Qiao, R.; Zhang, F.; Wang, X.; Zhong, J. Electrospraying Technique and Its Recent Application Advances for Biological Macromolecule Encapsulation of Food Bioactive Substances. Food Rev. Int. 2020, 38, 566–588. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Maudhuit, A.; Gaiani, C.; Desobry, S. Encapsulation of bioactive compounds using competitive emerging techniques: Electrospraying, nano spray drying, and electrostatic spray drying. J. Food Eng. 2023, 339, 111260. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, Y.; Qu, Q.; Xiong, R.; Tang, H.; Huang, C. Encapsulated Microstructures of Beneficial Functional Lipids and Their Applications in Foods and Biomedicines. J. Agric. Food Chem. 2022, 70, 8165–8187. [Google Scholar] [CrossRef]
- Ma, S.; Yuan, G.; Zhang, Y.; Yang, N.; Li, Y.; Chen, Q. Development of encapsulation strategies towards the commercialization of perovskite solar cells. Energy Environ. Sci. 2021, 15, 13–55. [Google Scholar] [CrossRef]
- Miletić, A.; Pavlić, B.; Ristić, I.; Zeković, Z.; Pilić, B. Encapsulation of Fatty Oils into Electrospun Nanofibers for Cosmetic Products with Antioxidant Activity. Appl. Sci. 2019, 9, 2955. [Google Scholar] [CrossRef]
- Belostozky, A.; Bretler, S.; Kolitz-Domb, M.; Grinberg, I.; Margel, S. Solidification of oil liquids by encapsulation within porous hollow silica microspheres of narrow size distribution for pharmaceutical and cosmetic applications. Mater. Sci. Eng. C 2019, 97, 760–767. [Google Scholar] [CrossRef]
- Lopez, M.D.; Maudhuit, A.; Pascual-Villalobos, M.J.; Poncelet, D. Development of Formulations to Improve the Controlled-Release of Linalool to Be Applied As an Insecticide. J. Agric. Food Chem. 2012, 60, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Jerobin, J.; Sureshkumar, R.; Anjali, C.; Mukherjee, A.; Chandrasekaran, N. Biodegradable polymer based encapsulation of neem oil nanoemulsion for controlled release of Aza-A. Carbohydr. Polym. 2012, 90, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhong, F.; Wen, J.; McGillivray, D.; Quek, S.Y. Properties and Stability of Spray-Dried and Freeze-Dried Microcapsules Co-Encapsulated with Fish Oil, Phytosterol Esters, and Limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- Wang, P.; Li, M.; Wei, D.; Ding, M.; Tao, L.; Liu, X.; Zhang, F.; Tao, N.; Wang, X.; Gao, M.; et al. Electrosprayed Soft Capsules of Millimeter Size for Specifically Delivering Fish Oil/Nutrients to the Stomach and Intestines. ACS Appl. Mater. Interfaces 2020, 12, 6536–6545. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Miskelly, G.; Wibisono, R.; Wadhwa, S. Stability of encapsulated olive oil in the presence of caffeic acid. Food Chem. 2011, 126, 1049–1056. [Google Scholar] [CrossRef]
- Waterhouse, G.; Wang, W.; Sun-Waterhouse, D. Stability of canola oil encapsulated by co-extrusion technology: Effect of quercetin addition to alginate shell or oil core. Food Chem. 2014, 142, 27–38. [Google Scholar] [CrossRef]
- Dolçà, C.; Ferrándiz, M.; Capablanca, L.; Franco, E.; Mira, E.; López, F.; García, D. Microencapsulation of Rosemary Essential Oil by Co-Extrusion/Gelling Using Alginate as a Wall Material. J. Encapsulation Adsorpt. Sci. 2015, 05, 121–130. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, T.; Xue, Y.; Xue, C.; Wang, Y. Preparation of alginate core–shell beads with different M/G ratios to improve the stability of fish oil. LWT—Food Sci. Technol. 2017, 80, 304–310. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Linder, M.; Khanji, A.N.; Probst, L.; Desobry, S. Study and optimization of core-shell capsules produced by annular jet breaking coextrusion. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 629, 127475. [Google Scholar] [CrossRef]
- Rodríguez-Dorado, R.; Landin, M.; Altai, A.; Russo, P.; Aquino, R.P.; Del Gaudio, P. A novel method for the production of core-shell microparticles by inverse gelation optimized with artificial intelligent tools. Int. J. Pharm. 2018, 538, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mania, S.; Tylingo, R.; Michałowska, A. The Drop-in-Drop Encapsulation in Chitosan and Sodium Alginate as a Method of Prolonging the Quality of Linseed Oil. Polymers 2018, 10, 1355. [Google Scholar] [CrossRef]
- Jin, H.; Wang, L.; Yang, S.; Wen, J.; Zhang, Y.; Jiang, L.; Sui, X. Producing mixed-soy protein adsorption layers on alginate microgels to controlled-release β-carotene. Food Res. Int. 2023, 164, 112319. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D.; Penin-Peyta, L.; Wadhwa, S.S.; Waterhouse, G.I.N. Storage Stability of Phenolic-Fortified Avocado Oil Encapsulated Using Different Polymer Formulations and Co-extrusion Technology. Food Bioprocess Technol. 2011, 5, 3090–3102. [Google Scholar] [CrossRef]
- Goh, K.M.; Low, S.S.; Nyam, K.L. The changes of chemical composition of microencapsulated roselle ( Hibiscus sabdariffa L.) seed oil by co-extrusion during accelerated storage. Int. J. Food Sci. Technol. 2021, 56, 6649–6655. [Google Scholar] [CrossRef]
- Chew, S.C.; Nyam, K.-L. Oxidative Stability of Microencapsulated Kenaf Seed Oil Using Co-extrusion Technology. J. Am. Oil Chem. Soc. 2016, 93, 607–615. [Google Scholar] [CrossRef]
- Chew, S.-C.; Nyam, K.-L. Microencapsulation of kenaf seed oil by co-extrusion technology. J. Food Eng. 2016, 175, 43–50. [Google Scholar] [CrossRef]
- Hue, W.L.; Nyam, K.L. Physiochemical Properties of Kenaf Seed Oil Microcapsules before and after Freeze Drying and Its Storage Stability. Int. Food Res. J. 2018, 25, 1502–1509. [Google Scholar]
- Sun-Waterhouse, D.; Wang, W.; Waterhouse, G.I.N. Canola Oil Encapsulated by Alginate and Its Combinations with Starches of Low and High Amylose Content: Effect of Quercetin on Oil Stability. Food Bioprocess Technol. 2013, 7, 2159–2177. [Google Scholar] [CrossRef]
- Chew, S.-C.; Tan, C.-P.; Long, K.; Nyam, K.-L. In-vitro evaluation of kenaf seed oil in chitosan coated-high methoxyl pectin-alginate microcapsules. Ind. Crop. Prod. 2015, 76, 230–236. [Google Scholar] [CrossRef]
- Leong, M.-H.; Tan, C.-P.; Nyam, K.-L. Effects of Accelerated Storage on the Quality of Kenaf Seed Oil in Chitosan-Coated High Methoxyl Pectin-Alginate Microcapsules. J. Food Sci. 2016, 81, C2367–C2372. [Google Scholar] [CrossRef] [PubMed]
- Atencio, S.; Maestro, A.; Santamaría, E.; Gutiérrez, J.M.; González, C. Encapsulation of ginger oil in alginate-based shell materials. Food Biosci. 2020, 37, 100714. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderon, F.; Bustamante, M. Food-grade Pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef]
- Solomando, J.C.; Antequera, T.; Perez-Palacios, T. Evaluating the use of fish oil microcapsules as omega-3 vehicle in cooked and dry-cured sausages as affected by their processing, storage and cooking. Meat Sci. 2019, 162, 108031. [Google Scholar] [CrossRef]
- de Moura, S.C.S.R.; Schettini, G.N.; Garcia, A.O.; Gallina, D.A.; Alvim, I.D.; Hubinger, M.D. Stability of Hibiscus Extract Encapsulated by Ionic Gelation Incorporated in Yogurt. Food Bioprocess Technol. 2019, 12, 1500–1515. [Google Scholar] [CrossRef]
- Ngamnikom, P.; Phawaphuthanon, N.; Kim, M.; Boonsupthip, W.; Shin, I.-S.; Chung, D. Fabrication of core-shell structured macrocapsules by electro-coextrusion with agar-hydrocolloid mixtures for precooked food applications: Textural and release characteristics. Int. J. Food Sci. Technol. 2017, 52, 2538–2546. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Aisyah, H.A.; Nordin, A.H.; Ngadi, N.; Zuhri, M.Y.M.; Asyraf, M.R.M.; Sapuan, S.M.; Zainudin, E.S.; Sharma, S.; Abral, H.; et al. Natural-Fiber-Reinforced Chitosan, Chitosan Blends and Their Nanocomposites for Various Advanced Applications. Polymers 2022, 14, 874. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef]
- Mukurumbira, A.; Shellie, R.; Keast, R.; Palombo, E.; Jadhav, S. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control. 2022, 136, 108883. [Google Scholar] [CrossRef]
- Yu, J.; Xie, J.; Xie, H.; Hu, Q.; Wu, Z.; Cai, X.; Guo, Z.; Lin, J.; Han, L.; Zhang, D. Strategies for Taste Masking of Orodispersible Dosage Forms: Time, Concentration, and Perception. Mol. Pharm. 2022, 19, 3007–3025. [Google Scholar] [CrossRef] [PubMed]
- Almurisi, S.H.; Doolaanea, A.A.; Akkawi, M.E.; Chatterjee, B.; Sarker, Z.I. Taste masking of paracetamol encapsulated in chitosan-coated alginate beads. J. Drug Deliv. Sci. Technol. 2020, 56, 101520. [Google Scholar] [CrossRef]
- Bamidele, O.P.; Emmambux, M.N. Encapsulation of bioactive compounds by “extrusion” technologies: A review. Crit. Rev. Food Sci. Nutr. 2020, 61, 3100–3118. [Google Scholar] [CrossRef] [PubMed]
- Yee, W.L.; Yee, C.L.; Lin, N.K.; Phing, P.L. Microencapsulation of Lactobacillus acidophilus NCFM incorporated with mannitol and its storage stability in mulberry tea. Ciência e Agrotecnologia 2019, 43, 64–75. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Ribas, M.M.; Penning, M.; Fávaro-Trindade, C.S.; Poncelet, D. Microcapsules loaded with the probiotic Lactobacillus paracasei BGP-1 produced by co-extrusion technology using alginate/shellac as wall material: Characterization and evaluation of drying processes. Food Res. Int. 2016, 89, 582–590. [Google Scholar] [CrossRef]
- Champagne, C.P.; Ross, R.P.; Saarela, M.; Hansen, K.F.; Charalampopoulos, D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int. J. Food Microbiol. 2011, 149, 185–193. [Google Scholar] [CrossRef]
- Sanders, M.E. How Do We Know When Something Called “ Probiotic ” Is Really a Probiotic ? A Guideline for Con-sumers and Health Care Professionals. Funct. Food Rev. 2009, 1, 3–12. [Google Scholar]
- How, Y.; Lai, K.; Pui, L.; In, L.L. Co-extrusion and extrusion microencapsulation: Effect on microencapsulation efficiency, survivability through gastrointestinal digestion and storage. J. Food Process. Eng. 2022, 45, e13985. [Google Scholar] [CrossRef]
- Chean, S.X.; Hoh, P.Y.; How, Y.H.; Nyam, K.L.; Pui, L.P. Microencapsulation of Lactiplantibacillus plantarum with inulin and evaluation of survival in simulated gastrointestinal conditions and roselle juice. Braz. J. Food Technol. 2021, 24, 105–119. [Google Scholar] [CrossRef]
- Sarkar, S. Approaches for enhancing the viability of probiotics: A review. Br. Food J. 2010, 112, 329–349. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Lipan, L.; Rusu, B.; Sendra, E.; Hernández, F.; Vázquez-Araújo, L.; Vodnar, D.C.; Carbonell-Barrachina, Á.A. Spray drying and storage of probiotic-enriched almond milk: Probiotic survival and physicochemical properties. J. Sci. Food Agric. 2020, 100, 3697–3708. [Google Scholar] [CrossRef]
- Pour, H.M.; Marhamatizadeh, M.H.; Fattahi, H. Encapsulation of Different Types of Probiotic Bacteria within Con-ventional/Multilayer Emulsion and Its Effect on the Properties of Probiotic Yogurt. J. Food Qual. 2022, 2022, 7923899. [Google Scholar]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Liu, W.; Tromp, R.H. Microencapsulation of probiotics in multi-polysaccharide microcapsules by electro-hydrodynamic atomization and incorporation into ice-cream formulation. Food Struct. 2020, 25, 100147. [Google Scholar] [CrossRef]
- Silva, M.P.; Tulini, F.L.; Martins, E.; Penning, M.; Fávaro-Trindade, C.S.; Poncelet, D. Comparison of extrusion and co-extrusion encapsulation techniques to protect Lactobacillus acidophilus LA3 in simulated gastrointestinal fluids. LWT—Food Sci. Technol. 2018, 89, 392–399. [Google Scholar] [CrossRef]
- How, Y.H.; Hubert, C.; Pui, L.P. Encapsulation of probiotic strain Lactobacillus rhamnosus GG with black bean extract in alginate-pectin microcapsules. Malays. J. Microbiol. 2021, 17, 190–199. [Google Scholar] [CrossRef]
- Ng, S.L.; Lai, K.W.; Nyam, K.L.; Pui, L.P. Microencapsulation of Lactobacillus plantarum 299v incorporated with oligofructose in chitosan coated-alginate beads and its storage stability in ambarella juice. Malays. J. Microbiol. 2019, 15, 408–418. [Google Scholar] [CrossRef]
- Jasinska, U.T.; Skąpska, S.; Owczarek, L.; Dekowska, A.; Lewińska, D. Immobilization of Bifidobacterium infantis Cells in Selected Hydrogels as a Method of Increasing Their Survival in Fermented Milkless Beverages. J. Food Qual. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Shahabivand, S.; Mortazavi, S.S.; Mahdavinia, G.R.; Darvishi, F. Phenol biodegradation by immobilized Rhodococcus qingshengii isolated from coking effluent on Na-alginate and magnetic chitosan-alginate nanocomposite. J. Environ. Manag. 2022, 307, 114586. [Google Scholar] [CrossRef] [PubMed]
- Khangwal, I.; Shukla, P. Potential prebiotics and their transmission mechanisms: Recent approaches. J. Food Drug Anal. 2019, 27, 649–656. [Google Scholar] [CrossRef]
- Sredkova, P.; Batsalova, T.; Moten, D.; Dzhambazov, B. Prebiotics can change immunomodulatory properties of probiotics. Central Eur. J. Immunol. 2020, 45, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Siang, S.C.; Wai, L.K.; Lin, N.K.; Phing, P.L. Effect of added prebiotic (Isomalto-oligosaccharide) and Coating of Beads on the Survival of Microencapsulated Lactobacillus rhamnosus GG. Food Sci. Technol. 2019, 39, 601–609. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Shinde, T.; Sun-Waterhouse, D.; Brooks, J. Co-extrusion Encapsulation of Probiotic Lactobacillus acidophilus Alone or Together with Apple Skin Polyphenols: An Aqueous and Value-Added Delivery System Using Alginate. Food Bioprocess Technol. 2013, 7, 1581–1596. [Google Scholar] [CrossRef]
- Kaur, R.; Simnani, F.Z.; Singh, S. Enhancement of Probiotics for Functional Food. In Recent Advances in Food Biotechnology; Kumar, A., Patruni, K., Singh, V., Eds.; Springer: Singapore, 2022; pp. 97–137. ISBN 978-981-16-8125-7. [Google Scholar]
- Lai, K.; How, Y.; Pui, L. Storage stability of microencapsulated Lactobacillus rhamnosus GG in hawthorn berry tea with flaxseed mucilage. J. Food Process. Preserv. 2020, 44, e14965. [Google Scholar] [CrossRef]
- Lai, P.Y.; How, Y.H.; Pui, L.P. Microencapsulation of Bifidobacterium Lactis Bi-07 with Galactooligosaccharides Using Co-Extrusion Technique. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e2416. [Google Scholar] [CrossRef]
- Yong, A.K.L.; Lai, K.W.; Ghazali, H.M.; Chang, L.S.; Pui, L.P. Microencapsulation of Bifidobacterium animalis subsp. lactis BB-12 with mannitol. Asia Pac. J. Mol. Biol. Biotechnol. 2020, 28, 32–42. [Google Scholar] [CrossRef]
- Lai, J.T.; Lai, K.W.; Zhu, L.Y.; Nyam, K.L.; Pui, L.P. Microencapsulation of Lactobacillus plantarum 299v and its storage in kuini juice. Malays. J. Microbiol. 2020, 16, 235–244. [Google Scholar] [CrossRef]
- Chan, L.Y.; Pui, L.P. Microencapsulation of Lactobacillus Acidophilus 5 with Isomalto-Oligosaccharide. Carpathian J. Food Sci. Technol. 2020, 12, 26–36. [Google Scholar] [CrossRef]
- Bouhlel, W.; Kui, J.; Bibette, J.; Bremond, N. Encapsulation of Cells in a Collagen Matrix Surrounded by an Alginate Hydrogel Shell for 3D Cell Culture. ACS Biomater. Sci. Eng. 2022, 8, 2700–2708. [Google Scholar] [CrossRef] [PubMed]
- Gryshkov, O.; Mutsenko, V.; Tarusin, D.; Khayyat, D.; Naujok, O.; Riabchenko, E.; Nemirovska, Y.; Danilov, A.; Petrenko, A.Y.; Glasmacher, B. Coaxial Alginate Hydrogels: From Self-Assembled 3D Cellular Constructs to Long-Term Storage. Int. J. Mol. Sci. 2021, 22, 3096. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Tao, T.; Liu, H.; Wang, Y.; Cui, K.; Guo, Y.; Qin, J. Controllable Fabrication of Composite Core–Shell Capsules at a Macroscale as Organoid Biocarriers. ACS Appl. Bio Mater. 2021, 4, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.J.; Luquet, E.; Pletenka, J.; Leonard, A.; Warter, E.; Gurchenkov, B.; Carrere, J.; Rieu, C.; Hardouin, J.; Moncaubeig, F.; et al. Engineering 3D micro-compartments for highly efficient and scale-independent expansion of human pluripotent stem cells in bioreactors. Biomaterials 2023, 29, 122033. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, K.; Sarangi, B.R.; Gurchenkov, V.V.; Sinha, B.; Kießling, T.R.; Fetler, L.; Rico, F.; Scheuring, S.; Lamaze, C.; Simon, A.; et al. Cellular capsules as a tool for multicellular spheroid production and for investigating the mechanics of tumor progression in vitro. Proc. Natl. Acad. Sci. USA 2013, 110, 14843–14848. [Google Scholar] [CrossRef]
- Doméjean, H.; Pierre, M.D.L.M.S.; Funfak, A.; Atrux-Tallau, N.; Alessandri, K.; Nassoy, P.; Bibette, J.; Bremond, N. Controlled production of sub-millimeter liquid core hydrogel capsules for parallelized 3D cell culture. Lab Chip 2016, 17, 110–119. [Google Scholar] [CrossRef]
- Fransen, M.F.; Addario, G.; Bouten, C.V.; Halary, F.; Moroni, L.; Mota, C. Bioprinting of kidney in vitro models: Cells, biomaterials, and manufacturing techniques. Essays Biochem. 2021, 65, 587–602. [Google Scholar] [CrossRef]
- Nebel, S.; Lux, M.; Kuth, S.; Bider, F.; Dietrich, W.; Egger, D.; Boccaccini, A.R.; Kasper, C. Alginate Core–Shell Capsules for 3D Cultivation of Adipose-Derived Mesenchymal Stem Cells. Bioengineering 2022, 9, 66. [Google Scholar] [CrossRef]
- Windbergs, M.; Zhao, Y.; Heyman, J.; Weitz, D.A. Biodegradable Core–Shell Carriers for Simultaneous Encapsulation of Synergistic Actives. J. Am. Chem. Soc. 2013, 135, 7933–7937. [Google Scholar] [CrossRef]
- Whelehan, M.; von Stockar, U.; Marison, I.W. Removal of pharmaceuticals from water: Using liquid-core microcapsules as a novel approach. Water Res. 2010, 44, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Ebisawa, T.; Toyoshima, A.; Mori, Y.; Taniguchi, Y. Oleic acid esterification with methanol to methyl oleate under light irradiation using modified alginate capsules loaded with a solid acid catalyst. Chem. Eng. J. 2021, 429, 132524. [Google Scholar] [CrossRef]
- Moghaddam, M.K.; Mortazavi, S.M. Preparation, characterisation and thermal properties of calcium alginate/n-nonadecane microcapsules fabricated by electro-coextrusion for thermo-regulating textiles. J. Microencapsul. 2015, 32, 737–744. [Google Scholar] [CrossRef] [PubMed]

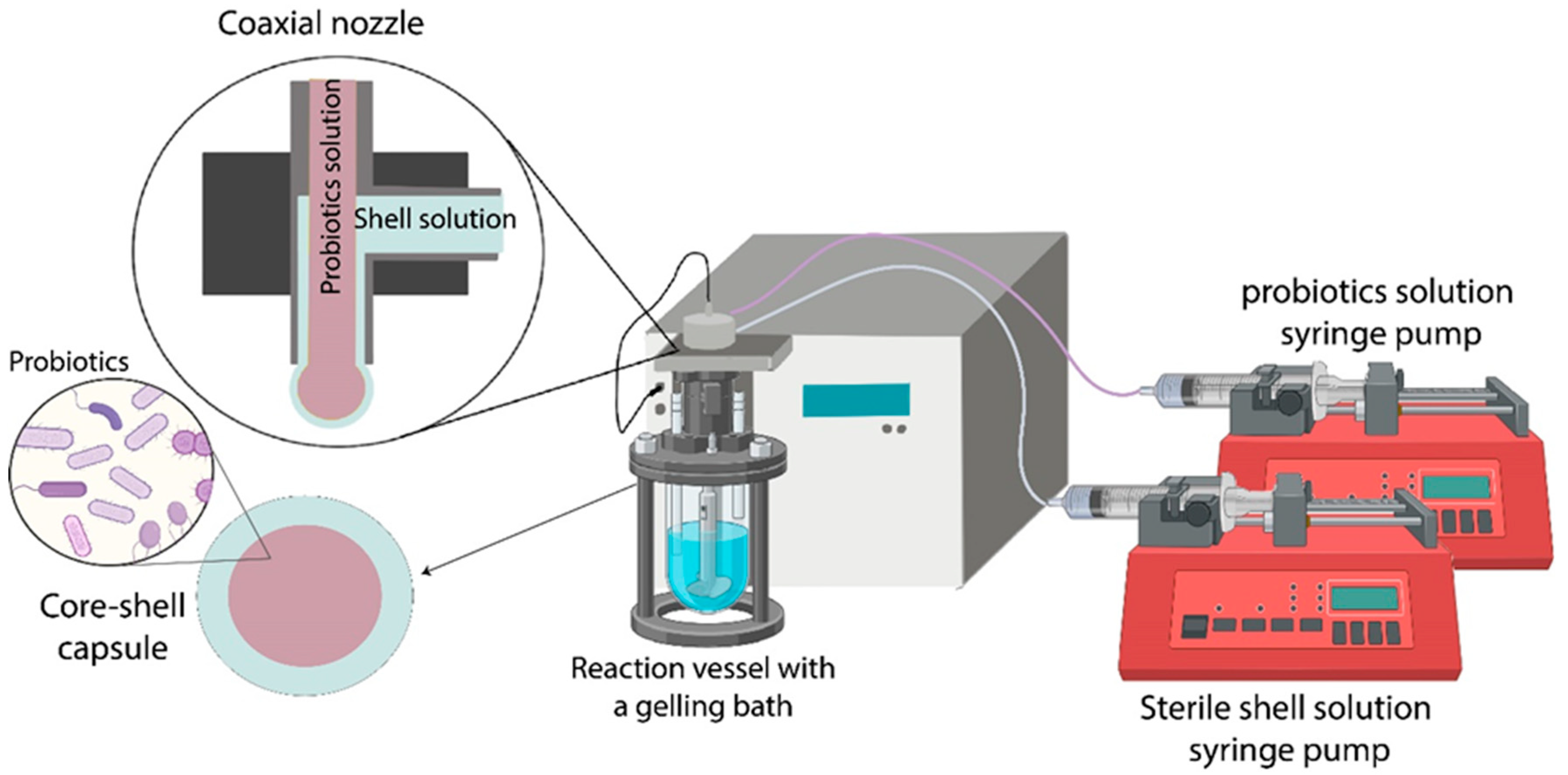
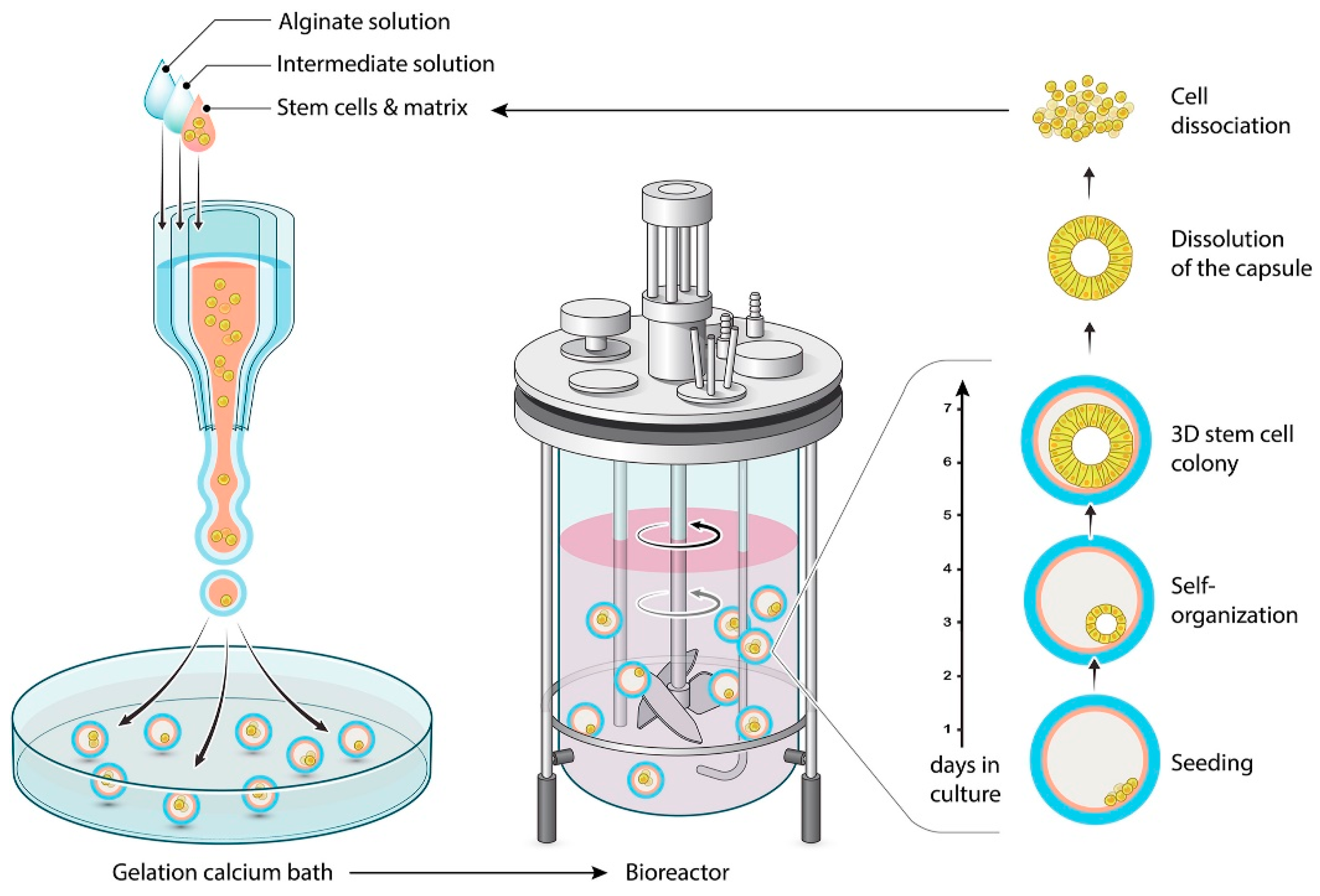
| Shell and/or Coating Material | Oil/Essential Oil | Key Parameters of the Coextrusion Method | Average Capsule Size (µm) | Treatment Post Encapsulation | EE (%) | Load (%) | References |
|---|---|---|---|---|---|---|---|
| Alginate | Olive | Frequency: 1706 Hz | ND | None | ND | ND | [31] |
| Alginate | Canola | Inner/Outer rate: 30/200 mL/h Outer nozzle: 200 µm Frequency: 1750 Hz | 400 | pH treatment, freeze drying | ND | 55.2 | [32] |
| Alginate | Rosemary EO | Inner/Outer nozzle: 200/400 µm Inner/Outer rate: 900/300 mL/h Frequency: 350 Hz | 950 (756 dried) | Oven-drying (60 °C for 2 h) | ND | ND | [33] |
| Alginate | Fish oil | Frequency: 1300 Hz | 600 | None | ND | ND | [34] |
| Alginate | Sunflower | Inner/Outer nozzle: 750/900 µm Inner/Outer rate: 14,55/34 mL/min. | 2060 | None | ND | 37 | [35] |
| Alginate | Sunflower (emulsion with CaCl2) | Inner/Outer nozzle: 400/600 µm | 800 | None | ND | ND | [36,37] |
| Alginate | Linseed | Dripping height: 5 cm | ND | None | 91 | 38.4 | [37] |
| Alginate | Rapeseed | Dripping height: 5 cm | ND | None | 91.6 | 39.9 | [37] |
| Alginate–Soy protein isolate | Sunflower + β-carotene | Inner/Outer nozzle: 150/300 µm Inner/Outer rate: 120/400 mL/h Frequency: 1000 Hz | 567 | None | 99.2 | 82.8 | [38] |
| Alginate–HPMC | Avocado | Frequency: 1706 Hz | 323–416 | Freeze-drying | 68 | ND | [39] |
| Alginate–HMP | Roselle seed | Inner/Outer nozzle: 300/400 µm Frequency: 300 Hz Air pressure: 600 mbar | ND | Oven-drying | ND | 95 | [40] |
| Alginate–HMP | Kenaf seed | Inner/Outer nozzle: 150/300 µm Inner/Outer rate: 0.2/7 mL/min Air pressure: 600 mbar | 900 (500 dried) | Air-drying–Freeze-drying | 33–67 | ND | [41,42] |
| Alginate–HMP | Kenaf seed | Inner/Outer nozzle: 200/300 µm Frequency: 500 Hz | 700–920 (330–500 dried) | Freeze-drying | 63 | ND | [43] |
| Alginate- low or high amylose content starch | Canola | Inner/Outer rate: 30/200 mL/h Outer nozzle: 200 µm Frequency: 1750 Hz | 310–380 | pH treatment Freeze-drying | ND | 58 | [44] |
| HMP alginate/chitosan | Kenaf seed | Inner/Outer nozzle: 200/300 µm Inner/Outer rate: 0.2/7 mL/min Frequency: 500 Hz | 475–775 | Oven-drying (50 °C for 2 h) | 33–65 | ND | [45] |
| Alginate-HMP-chitosan | Kenaf seed | Inner/Outer nozzle: 200/300 µm Frequency: 500 Hz | ND | Freeze-drying | ND | ND | [46] |
| Alginate/κ-carrageenan/ chitosan | Ginger oil | Inner/Outer nozzle: 450/900 µm Frequency: 40 Hz Air pressure: 400 mbar | 1600 µm | None | 85 | 76 | [47] |
| Shell and/or Coating Material | Oil/Essential Oil | Antioxidant | Oxidation Indicators | Storage Conditions | References | |
|---|---|---|---|---|---|---|
| Free Oil/Essential Oil | Encapsulated | |||||
| Alginate | Olive | Caffeic acid (300 ppm) | PV> 16 meq/kg p-AV > 3.2 FFA > 0.12% | PV < 14 meq/kg p-AV < 2.5 FFA <0.14% | 30 days at 37 °C | [31] |
| Alginate | Canola | Quercetin (200 ppm) | PV> 16 meq/kg p-AV > 3.90 FFA > 0.21% | PV < 10.2 meq/kg p-AV < 2.99 FFA < 0.23% | 60 days at 38°C | [32] |
| Alginate | Fish oil | / | PV> 11.3 meq/kg, p-AV > 11.7 DHA loss 2.72% EPA loss 0.84% | PV < 4.8 meq/kg, p-AV < 6.7 DHA reduction 0.68% EPA reduction 0.74% | 17 days at 37 °C | [34] |
| Alginate | Linseed | / | PV > 65 meq/kg, p-AV > 9.33 FFA > 1.22 | PV < 48.26 meq/kg, p-AV < 5.06 FFA < 1.26 | 4 weeks at 40 °C | [37] |
| Alginate | Rapeseed | / | PV > 65 meq/kg p-AV > 11.30 FFA > 0.30 | PV < 20.77 meq/kg p-AV < 6.99 FFA 0.32 | 4 weeks at 40 °C | [37] |
| Alginate | Ginger | / | PV > 23 meq/kg p-AV > 34 TBARS: 5.8 mg MDA/kg | PV > 21 meq/kg p-AV > 40 TBARS: 5.8 mg MDA/kg | 15 days at 4 °C | [47] |
| Alginate–HPMC | Avocado | Phloridzin or BHT (300 ppm) | Totox: 25.4 | Totox: 18.0/19.7 | 90 days at 37 °C | [39] |
| Alginate–HMP | Roselle seed | / | PHY loss: 35% TOCO loss: 34.6% | PHY loss: 13.78% TOCO loss: 87.6 | 24 days at 65 °C | [40] |
| Alginate–HMP | Kenaf seed | / | PV> 11.3 meq/kg, p-AV > 11.7 FFA > 1.6 | PV < 4.8 meq/kg, p-AV < 6.7 FFA < 1.41 | 24 days at 65 °C | [41,42] |
| Alginate–HMP | Kenaf seed | / | PHY loss: 59.7% TOCO loss: 51.2% | PHY loss: 32.8% TOCO loss: 12.9% | 24 days at 65 °C | [43] |
| Alginate–low- or high-amylose-content starch | Canola | Quercetin, Vitamin E or BHT (200 ppm) | PV> 16.4 meq/kg p-AV > 3.90 FFA > 0.22% | PV < 10.8 meq/kg, p-AV < 4.19 FFA < 0.19% | 60 days at 38 °C | [44] |
| Alginate–κ-carrageenan | Ginger | / | PV > 23 meq/kg p-AV > 34 TBARS: 5.8 mg MDA/kg | PV > 16 meq/kg p-AV > 25 TBARS: 4.86 mg MDA/kg | 15 days at 4 °C | [47] |
| Alginate–κ-carrageenan–chitosan | Ginger | / | PV > 23 meq/kg p-AV > 34 TBARS: 5.8 mg MDA/kg | PV > 15 meq/kg p-AV > 16 TBARS: 4.44 mg MDA/kg | 15 days at 4 °C | [47] |
| Alginate–HMP–chitosan | Kenaf seed | / | PV> 10.1 meq/kg, p-AV > 20.2 FFA > 2.26% | PV < 3.9 meq/kg, p-AV < 15.94 FFA < 1.72% | 24 days at 65 °C | [46] |
| Alginate–chitosan | Ginger | PV > 23 meq/kg p-AV > 34 | PV > 19 meq/kg p-AV > 26 TBARS: 5.2 mg MDA/kg | 15 days at 4 °C | [47] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennacef, C.; Desobry, S.; Probst, L.; Desobry-Banon, S. Alginate Based Core–Shell Capsules Production through Coextrusion Methods: Recent Applications. Foods 2023, 12, 1788. https://doi.org/10.3390/foods12091788
Bennacef C, Desobry S, Probst L, Desobry-Banon S. Alginate Based Core–Shell Capsules Production through Coextrusion Methods: Recent Applications. Foods. 2023; 12(9):1788. https://doi.org/10.3390/foods12091788
Chicago/Turabian StyleBennacef, Chanez, Stéphane Desobry, Laurent Probst, and Sylvie Desobry-Banon. 2023. "Alginate Based Core–Shell Capsules Production through Coextrusion Methods: Recent Applications" Foods 12, no. 9: 1788. https://doi.org/10.3390/foods12091788
APA StyleBennacef, C., Desobry, S., Probst, L., & Desobry-Banon, S. (2023). Alginate Based Core–Shell Capsules Production through Coextrusion Methods: Recent Applications. Foods, 12(9), 1788. https://doi.org/10.3390/foods12091788






