Influence of Fermentation Container Type on Chemical and Microbiological Parameters of Spontaneously Fermented Cow and Goat Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Milk Samples and Fermented Milk Preparation
2.2. Acidification and Lactic Acid Bacteria Growth Kinetics
2.3. Proximate Composition and Apparent Viscosity
2.4. Whiteness Index (WI)
2.5. Fatty Acid Methyl Esters (FAME) Analysis
2.6. Phenolic Compounds Analysis by LC-ESI-MS
2.7. Statistical Analysis
2.8. Microbial Community Composition
2.8.1. DNA Extraction
2.8.2. NGS, Bioinformatic and Statistical Analysis
3. Results
3.1. Effect of the Fermentation Container on Acidification and Lactic Acid Bacteria Growth
3.2. Effect of Fermentation Container on Apparent Viscosity and Whiteness Index
3.3. Effect of Type of Milk and Container on Nutritional Profile
3.4. Influence of Type of Milk and Container Used for Milk Fermentation on Fatty Acids and Phenolic Compounds
3.5. Microbial Profiles of Goat and Cow Milk Fermented in Glass and Clay Containers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, J.; Lee, S.I.; Rackerby, B.; Goddik, L.; Frojen, R.; Ha, S.-D.; Kim, J.H.; Park, S.H. Microbial communities of a variety of cheeses and comparison between core and rind region of cheeses. J. Dairy Sci. 2020, 103, 4026–4042. [Google Scholar] [CrossRef] [PubMed]
- Ladokun, O.; Oni, S. Fermented Milk Products from Different Milk Types. Food Nutr. Sci. 2014, 5, 1228–1233. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.J.L.; Duarte, A.M.; Batista, A.S.M.; De Figueirêdo, R.M.F.; De Sousa, E.P.; De Souza, E.L.; do Egypto, R.D.C.R. Physicochemical and sensory properties of fermented dairy beverages made with goat’s milk, cow’s milk and a mixture of the two milks. LWT 2013, 54, 18–24. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, structure, and digestive dynamics of milk from different species—A review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef]
- Turkmen, N. Chapter 35-The nutritional value and health benefits of goat milk components. In Nutrients in Dairy and Their Implications on Health and Disease; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 441–449. [Google Scholar] [CrossRef]
- Peng, C.; Sun, Z.; Sun, Y.; Ma, T.; Li, W.; Zhang, H. Characterization and association of bacterial communities and nonvolatile components in spontaneously fermented cow milk at different geographical distances. J. Dairy Sci. 2021, 104, 2594–2605. [Google Scholar] [CrossRef]
- Montel, M.-C.; Buchin, S.; Mallet, A.; Delbes-Paus, C.; Vuitton, D.A.; Desmasures, N.; Berthier, F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014, 177, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Yu, J.; Jin, H.; Hou, Q.; Yao, C.; Ren, D.; An, X.; Tsogtgerel, T.; Zhang, H. Investigating the bacterial microbiota of traditional fermented dairy products using propidium monoazide with single-molecule real-time sequencing. J. Dairy Sci. 2019, 102, 3912–3923. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; In Lee, S.; Rackerby, B.; Frojen, R.; Goddik, L.; Ha, S.-D.; Park, S.H. Assessment of overall microbial community shift during Cheddar cheese production from raw milk to aging. Appl. Microbiol. Biotechnol. 2020, 104, 6249–6260. [Google Scholar] [CrossRef]
- Chileshe, J.; van den Heuvel, J.; Handema, R.; Zwaan, B.J.; Talsma, E.F.; Schoustra, S. Nutritional composition and microbial communities of two non-alcoholic traditional fermented beverages from Zambia: A study of mabisi and munkoyo. Nutrients 2020, 12, 1628. [Google Scholar] [CrossRef]
- Moonga, H.B.; Schoustra, S.E.; Linnemann, A.R.; van den Heuvel, J.; Shindano, J.; Smid, E.J. Influence of fermentation temperature on microbial community composition and physicochemical properties of mabisi, a traditionally fermented milk. LWT 2021, 136, 110350. [Google Scholar] [CrossRef]
- Moonga, H.B.; Shoustra, S.E.; Linnemann, A.R.; Kuntashula, E.; Shindano, J.; Smid, E.J. The art of mabisi production: A traditional fermented milk. PLoS ONE 2019, 14, e0213541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simatende, P.; Gadaga, T.H.; Nkambule, S.J.; Siwela, M. Methods of preparation of Swazi traditional fermented foods. J. Ethn. Foods 2015, 2, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Saleena, L.A.K.; Phing, P.L.; Gan, R.Y.; Al-Nabulsi, A.; Osaili, T.; Kamal-Eldin, A.; Ayyash, M. Fermented dairy products from Middle Eastern and Northern African (MENA) countries: Insight on production and physiochemical characteristics. Int. Dairy J. 2023, 141, 105614. [Google Scholar] [CrossRef]
- Hotessa, N.; Robe, J. Ethiopian Indigenous Traditional Fermented Beverage: The Role of the Microorganisms toward Nutritional and Safety Value of Fermented Beverage. Int. J. Microbiol. 2020, 2020, 8891259. [Google Scholar] [CrossRef] [PubMed]
- Ruvalcaba-Gómez, J.M.; Delgado-Macuil, R.J.; Zelaya-Molina, L.X.; Maya-Lucas, O.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; la Mora, Z.V.-D.; la Mora, D.A.L.-D.; Arteaga-Garibay, R.I. Bacterial Succession through the Artisanal Process and Seasonal Effects Defining Bacterial Communities of Raw-Milk Adobera Cheese Revealed by High Throughput DNA Sequencing. Microorganisms 2020, 9, 24. [Google Scholar] [CrossRef]
- Graffa, G. Studying dynamics of microbial populations during food fermentation. FEMS Microbiol. Rev. 2004, 28, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Kochetkova, T.V.; Grabarnik, I.P.; Klyukina, A.A.; Zayulina, K.; Elizarov, I.M.; Shestakova, O.O.; Gavirova, L.A.; Malysheva, A.D.; Shcherbakova, P.A. Microbial Communities of Artisanal Fermented Milk Products from Russia. Microorganisms 2022, 10, 2140. [Google Scholar] [CrossRef]
- Kebede, A.; Viljoen, B.; Gadaga, T.; Narvhus, J.; Lourens-Hattingh, A. The effect of container type on the growth of yeast and lactic acid bacteria during production of Sethemi, South African spontaneously fermented milk. Food Res. Int. 2007, 40, 33–38. [Google Scholar] [CrossRef]
- Association Française de Normalisation (AFNOR). Controle de la Qualite des Produits Alimentaires; Lait et Produits Laitiers: Paris, France, 1993. [Google Scholar]
- ISO 7889:2003 (IDF 117:2003); Yogurt e Enumeration of Characteristic Microorganisms e Colony Count Technique at 37 °C. International Organization for Standardization: Geneva, Switzerland, 2003.
- Flores-Mendoza, A.P.; Hernández-García, H.; Cocotle-Ronzón, Y.; Hernandez-Martinez, E. Methanogenesis of raw cheese whey: pH and substrate–inoculum ratio evaluation at mesophyll temperature range. J. Chem. Technol. Biotechnol. 2020, 95, 1946–1952. [Google Scholar] [CrossRef]
- Mkadem, W.; Belguith, K.; Semmar, N.; Zid, M.B.; ElHatmi, H.; Boudhrioua, N. Effect of process parameters on quality attributes of Lben: Correlation between physicochemical and sensory properties. LWT 2021, 155, 112987. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Alhammadi, A.; Gharsallaoui, A.; Hamed, F.; Ghnimi, S. Physicochemical, rheological, and micro-structural properties of yogurts produced from mixtures of camel and bovine milks. NFS J. 2020, 19, 26–33. [Google Scholar] [CrossRef]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin–Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Jrad, Z.; Oussaief, O.; Zaidi, S.; Khorchani, T.; El-Hatmi, H. Co-fermentation process strongly affect the nutritional, texture, syneresis, fatty acids and aromatic compounds of dromedary UF-yogurt. J. Food Sci. Technol. 2020, 58, 1727. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, A.; Oliveri, C.; Lucchi, A.; Savini, F.; Manfreda, G.; Sala, C. Pilot Study on Poultry Meat from Antibiotic Free and Conventional Farms: Can Metagenomics Detect Any Difference? Foods 2022, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Busetta, G.; Gannuscio, R.; Settanni, L.; Licitra, G.; Todaro, M. A Multivariate Approach to Study the Bacterial Diversity Associated to the Wooden Shelves Used for Aging Traditional Sicilian Cheeses. Foods 2022, 11, 774. [Google Scholar] [CrossRef]
- Mallappa, R.H.; Balasubramaniam, C.; Nataraj, B.H.; Ramesh, C.; Kadyan, S.; Pradhan, D.; Muniyappa, S.K.; Grover, S. Microbial diversity and functionality of traditional fermented milk products of India: Current scenario and future perspectives. Int. Dairy J. 2021, 114, 104941. [Google Scholar] [CrossRef]
- Maleke, M.S.; Adefisoye, M.A.; Doorsamy, W.; Adebo, O.A. Processing, nutritional composition and microbiology of amasi: A Southern African fermented milk product. Sci. Afr. 2021, 12, e00795. [Google Scholar] [CrossRef]
- Priyashantha, H.; Ranadheera, C.S.; Rasika, D.M.D.; Vidanarachchi, J.K. Traditional Sri Lankan fermented buffalo (Bubalus bubalis) milk gel (Meekiri): Technology, microbiology and quality characteristics. J. Ethn. Food 2021, 8, 27. [Google Scholar] [CrossRef]
- DATE, A.W. Heat and mass transfer analysis of a clay-pot refrigerator. Int. J. Heat Mass Transf. 2012, 55, 3977–3983. [Google Scholar] [CrossRef]
- Groenenboom, A.E.; Shindano, J.; Cheepa, N.; Smid, E.J.; Schoustra, S.E. Microbial population dynamics during traditional production of Mabisi, a spontaneous fermented milk product from Zambia: A field trial. World J. Microbiol. Biotechnol. 2020, 36, 184. [Google Scholar] [CrossRef]
- Awulachew, M.T. Common Ethiopian Fermented Products: Beverages-Alcoholic/Semi-Alkali, Dairy Products. Glob. Acad. J. Med. Sci. 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Vargas, M.; Cháfer, M.; Albors, A.; Chiralt, A.; González-Martínez, C. Physicochemical and sensory characteristics of yoghurt produced from mixtures of cows’ and goats’ milk. Int. Dairy J. 2008, 18, 1146–1152. [Google Scholar] [CrossRef]
- Sant’Ana, A.M.S.; Bezerril, F.F.; Madruga, M.S.; Batista, A.S.M.; Magnani, M.; Souza, E.L.; Queiroga, R.C.R.E. Nutritional and sensory characteristics of Minas fresh cheese made with goat milk, cow milk, or a mixture of both. J. Dairy Sci. 2013, 96, 7442–7453. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Saleh, H.A.; El Ahmady, S.; Elmassry, M.M. Dissecting yogurt: The impact of milk types, probiotics, and selected additives on yogurt quality. Food Rev. Int. 2021, 38 (Suppl. 1), 634–650. [Google Scholar] [CrossRef]
- Mani-Lopez, E.; Palou, E.; Lopez-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D.R. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef] [Green Version]
- Samet-Bali, O.; Ennouri, M.; Dhouib, A.; Attia, H. Characterisation of typical Tunisian fermented milk: Leben. Afr. J. Microbiol. Res. 2012, 6, 2169–2175. [Google Scholar] [CrossRef]
- Agyei, D.; Owusu-Kwarteng, J.; Akabanda, F.; Akomea-Frempong, S. Indigenous African fermented dairy products: Processing technology, microbiology and health benefits. Crit. Rev. Food Sci. Nutr. 2019, 60, 991–1006. [Google Scholar] [CrossRef]
- Pimentel, L.R.; da Silva, F.F.; Silva, R.R.; de Oliveira, E.S.R.; de Almeida, M.M.; Júnior, A.F.P.; Dicastro, D.S.; Gonçalo, M.S.; Pacheco, C.C.; de Oliveira, P.A. Fatty acid profile of milk from cows fed palm kernel cake. Semin. Cienc. Agrar. 2016, 37, 2773–2783. [Google Scholar] [CrossRef] [Green Version]
- Lucatto, L.N.; da Silva-Buzanello, R.A.; de Mendonça, S.N.T.G.; Lazarotto, T.C.; Sanchez, J.L.; Bona, E.; Drunkler, D.A. Performance of different microbial cultures in potentially probiotic and prebiotic yoghurts from cow and goat milks. Int. J. Dairy Technol. 2020, 73, 144–156. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Jordán, M.J.; Moñino, M.I.; Martínez, C.; Lafuente, A.; Sotomayor, J.A. Introduction of Distillate Rosemary Leaves into the Diet of the Murciano-Granadina Goat: Transfer of Polyphenolic Compounds to Goats’ Milk and the Plasma of Suckling Goat Kids. J. Agric. Food Chem. 2010, 58, 8265–8270. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Rock, E. In vitro and in vivo Antioxidant Potential of Milks, Yoghurts, Fermented Milks and Cheeses: A Narrative Review of Evidence. Nutr. Res. Rev. 2018, 31, 52–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Peng, S.; Hoffmann, W.; Bockelmann, W.; Hummerjohann, J.; Stephan, R.; Hammer, P. Fate of Shiga toxin-producing and generic Escherichia coli during production and ripening of semihard raw milk cheese. J. Dairy Sci. 2013, 96, 815–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shangpliang, H.N.J.; Rai, R.; Keisam, S.; Jeyaram, K.; Tamang, J.P. Bacterial community in naturally fermented milk products of Arunachal Pradesh and Sikkim of India analysed by high-throughput amplicon sequencing. Sci. Rep. 2018, 8, 1532. [Google Scholar] [CrossRef] [Green Version]
- Secchi, G.; Amalfitano, N.; Carafa, I.; Franciosi, E.; Gallo, L.; Schiavon, S.; Sturaro, E.; Tagliapietra, F.; Bittante, G. Milk metagenomics and cheese-making properties as affected by indoor farming and summer highland grazing. J. Dairy Sci. 2023, 106, 96–116. [Google Scholar] [CrossRef]
- Ryu, S.; Park, W.S.; Yun, B.; Shin, M.; Go, G.-W.; Kim, J.N.; Oh, S.; Kim, Y. Diversity and characteristics of raw milk microbiota from Korean dairy farms using metagenomic and culturomic analysis. Food Control 2021, 127, 108160. [Google Scholar] [CrossRef]
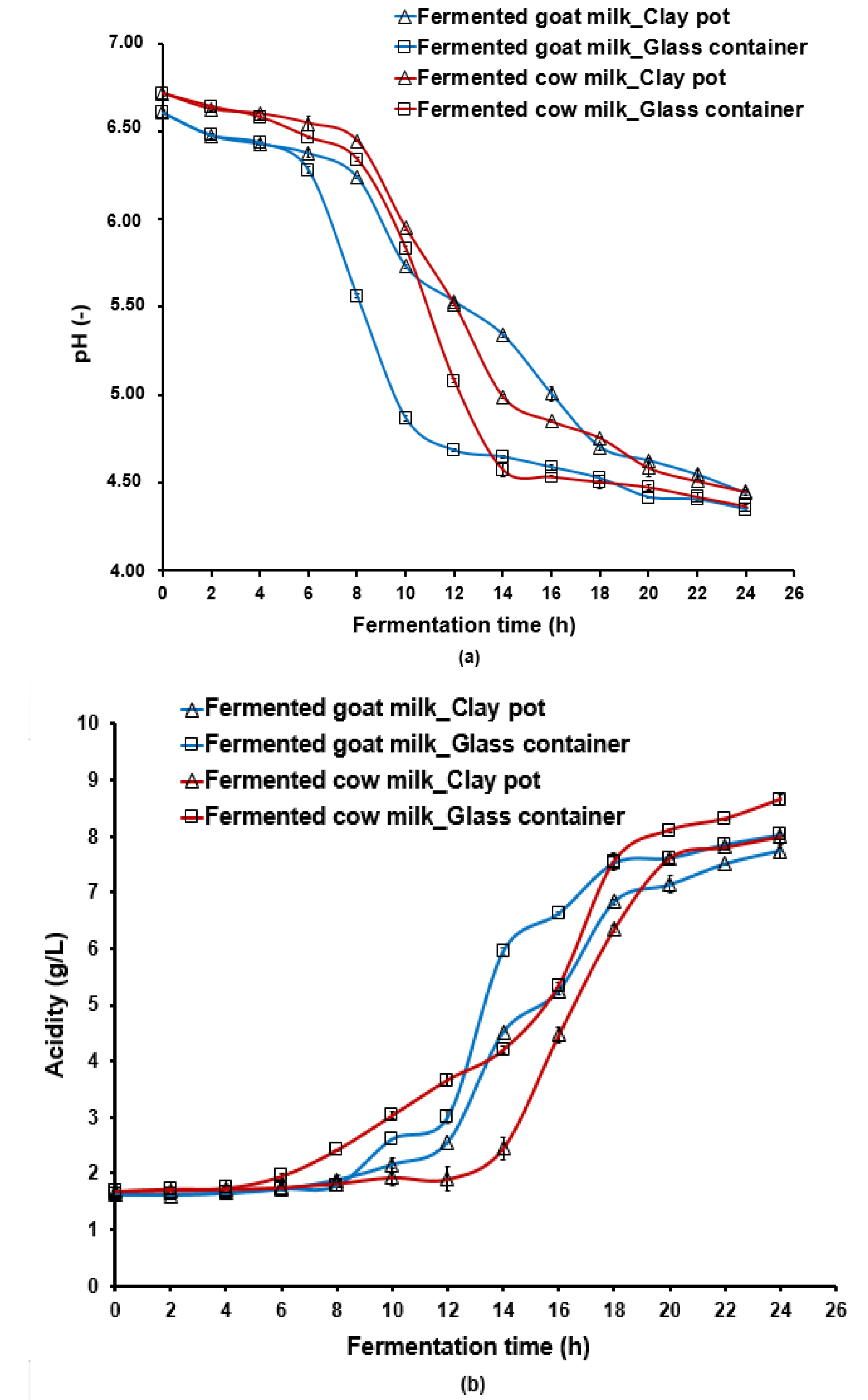
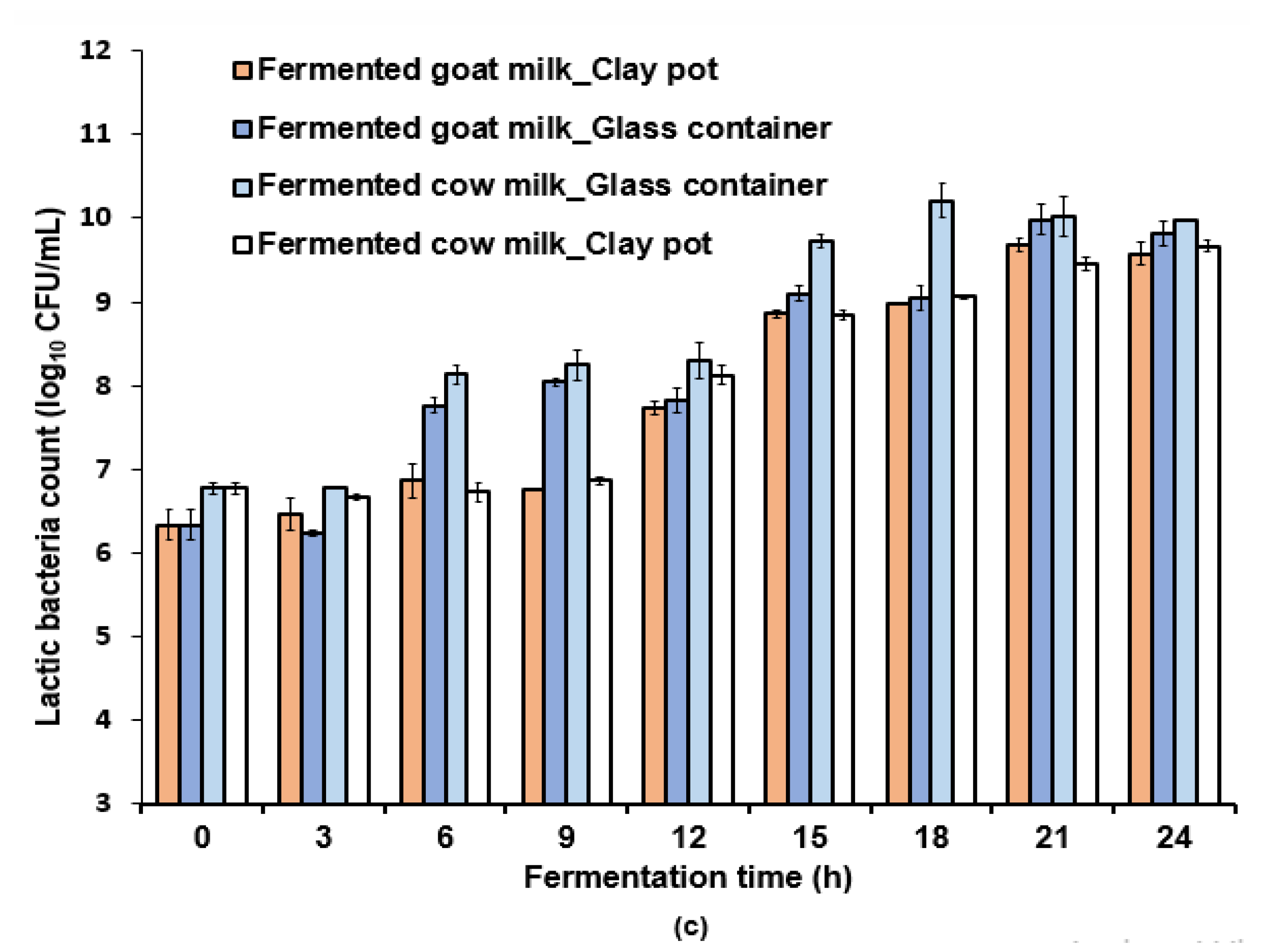

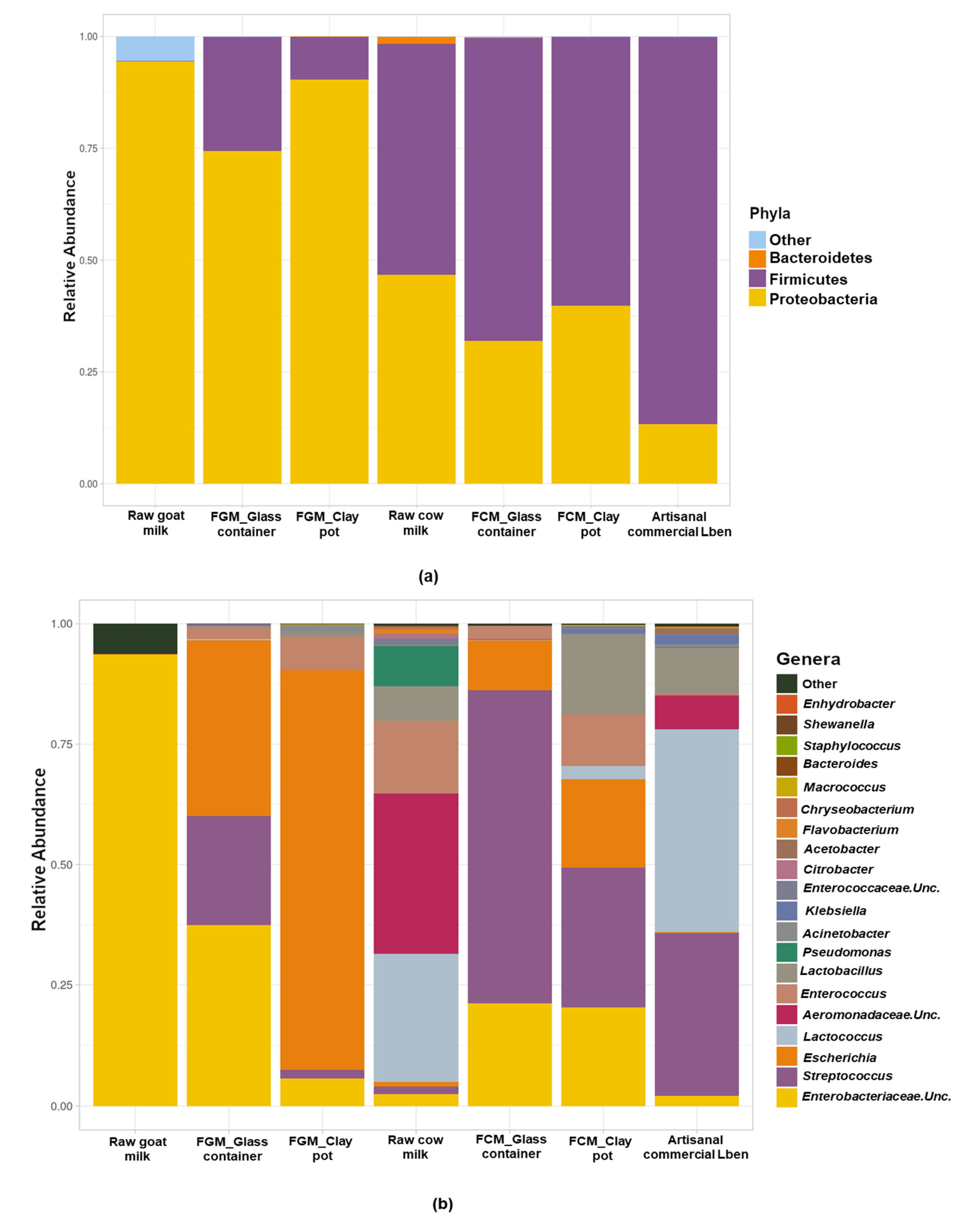
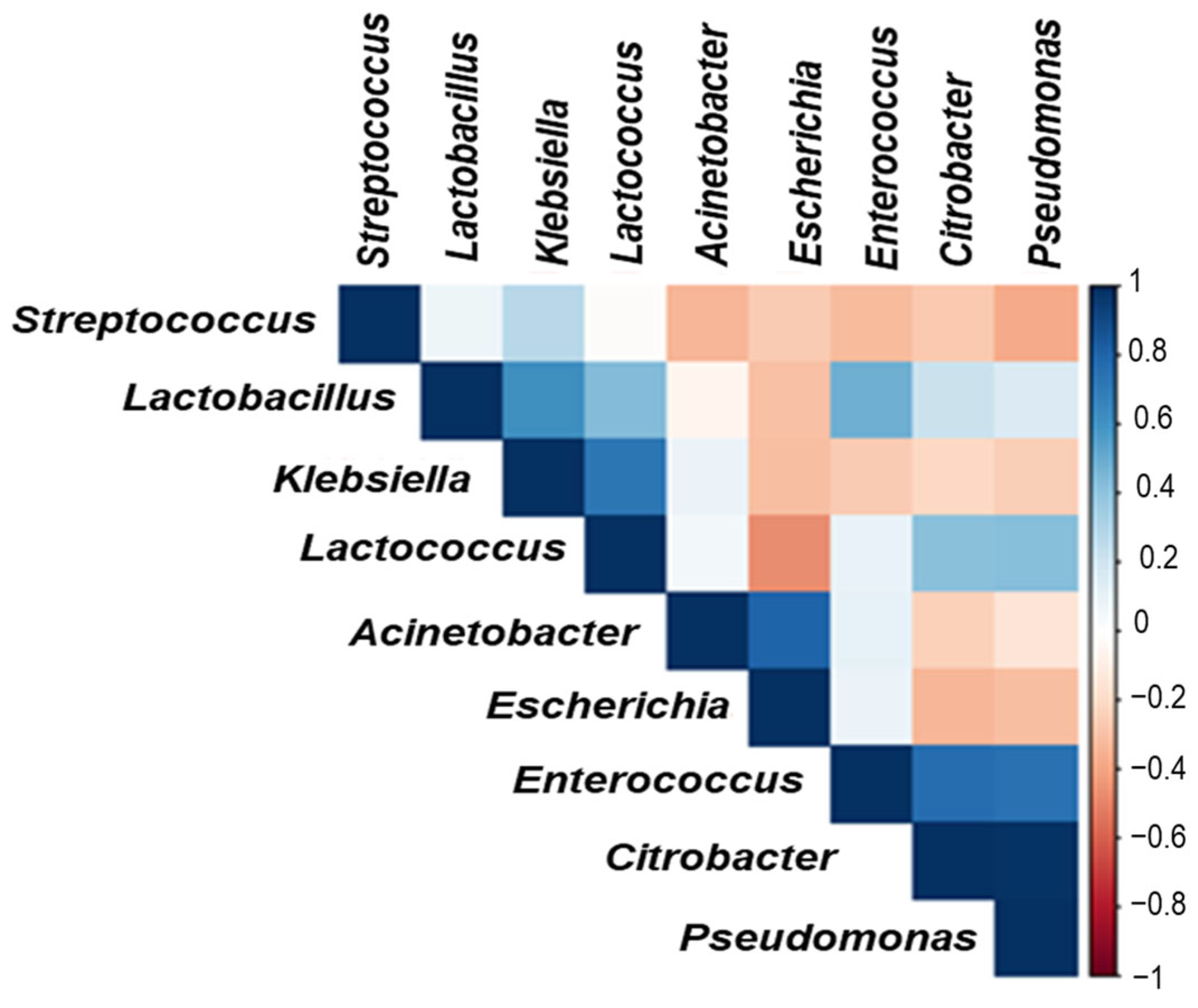
| Milk Samples | Aw (-) | Lactose (g/100 g) | Ash (g/100 g) | Total Solids (g/100 g) | Fat (g/100 g) | Protein (g/100 g) |
|---|---|---|---|---|---|---|
| Raw goat milk | 0.986 ± 0.001 a | 4.5 ± 0.1 a | 0.878 ± 0.002 a | 13.1 ± 0.9 a | 3.5 ± 0.2 a | 3.3 ± 0.1 a |
| FGM_Clay pot | 0.980 ± 0.004 b | 3.7 ± 0.1 b | 0.798 ± 0.003 b | 12.0 ± 0.8 ab | 3.3 ± 0.3 ab | 3.1 ± 0.2 ab |
| FGM_Glass container | 0.985 ± 0.001 a | 3.3 ± 0.1 cd | 0.700 ± 0.001 c | 11.5 ± 0.3 ab | 2.8 ± 0.3 b | 3.1 ± 0.1 ab |
| Raw cow milk | 0.980 ± 0.004 b | 4.2 ± 0.1 a | 0.82 ± 0.01 b | 12.8 ± 0.6 ab | 3.3 ± 0.2 ab | 3.2 ± 0.2 a |
| FCM_Clay pot | 0.973 ± 0.001 c | 3.4 ± 0.1 bc | 0.79 ± 0.02 b | 11.5 ± 0.4 ab | 3.1 ± 0.2 ab | 3.1 ± 0.1 ab |
| FCM_Glass container | 0.980 ± 0.003 b | 3.1 ± 0.1 d | 0.71 ± 0.02 c | 11.4 ± 0.3 b | 3.1 ± 0.2 ab | 2.8 ± 0.1 b |
| Fatty Acids (%) | RGM | FGM_Clay Pot | FGM_Glass Container | RCM | FCM_Clay Pot | FCM_Glass Container |
|---|---|---|---|---|---|---|
| Saturated fatty acids | ||||||
| Butanoic acid (C4:0) | 2.40 ± 0.03 | 1.92 ± 0.2 ab | 2.4 ± 0.1 a | 2.3 ± 0.2 | 1.45 ± 0.02 b | 2.1 ± 0.1 a |
| Caproic acid (C6:0) | 2.72 ± 0.02 | 2.05 ± 0.1 ab | 2.9 ± 0.1 a | 1.4 ± 0.1 | 1.3 ± 0.1 b | 1.2 ± 0.1 b |
| Caprylic acid (C8:0) | 3.17 ± 0.01 | 2.4 ± 0.1 b | 3.5 ± 0.2 a | 0.864 ± 0.005 | 0.78 ± 0.01 c | 0.81 ± 0.02 c |
| Capric acid (C10:0) | 11.97 ± 0.03 | 7.5 ± 0.4 b | 11.8 ± 0.2 a | 2.14 ± 0.01 | 2.002 ± 0.004 c | 2.01 ± 0.01 c |
| Lauric acid (C12:0) | 4.3 ± 0.7 | 3.47 ± 0.03 b | 4.33 ± 0.03 a | 2.85 ± 0.03 | 2.71 ± 0.02 c | 2.68 ± 0.01 c |
| Myristic acid (C14:0) | 9.2 ± 0.1 | 10.2 ± 0.2 a | 10.20 ± 0.01 a | 10.40 ± 0.01 | 10.4 ± 0.2 a | 10.3 ± 0.2 a |
| Pentadecanoic acid (C15:0) | 1.01 ± 0.02 | 1.13 ± 0.01 a | 0.9 ± 0.1 a | 1.41 ± 0.04 | 1.3 ± 0.1 a | 1.34 ± 0.04 a |
| Palmitic acid (C16:0) | 26.9 ± 0.8 | 29.15 ± 0.05 a | 30.7 ± 0.2 a | 29.03 ± 0.3 | 30.31 ± 0.03 a | 30.3 ± 1.2 a |
| Heptadecanoic acid (C17:0) | 0.66 ± 0.01 | 0.92 ± 0.04 a | 0.5 ± 0.1 a | 0.73 ± 0.01 | 0.6 ± 0.1 a | 0.62 ± 0.04 |
| Stearic acid (C18:0) | 11.9 ± 0.1 | 14.1 ± 0.3 a | 11.4 ± 0.2 b | 9.7 ± 0.2 | 9.4 ± 0.2 b | 9.5 ± 0.1 b |
| Arachidic acid (C20:0) | 0.70 ± 0.01 | 0.3 ± 0.1 a | 0.27 ± 0.03 a | 2.23 ± 0.01 | 0.15 ± 0.01 a | 0.13 ± 0.01 |
| Mono-saturated fatty acids | ||||||
| Cis-9-hexadecenoic acid (C16:1) | 0.5 ± 0.01 | 0.6 ± 0.04 b | 0.30 ± 0.01 c | 2.33 ± 0.01 | 1.94 ± 0.04 a | 1.7 ± 0.2 a |
| Cis-10- heptadecenoic acid (C17:1) | 0.2 ± 0.04 | 0.3 ± 0.04 a | nd | 0.3 ± 0.1 | 0.29 ± 0.03 a | 0.34 ± 0.03 a |
| Oleic acid (C18:1) | 18.97 ± 0.1 | 25.2 ± 0.1 b | 20.7 ± 0.01 c | 30.0 ± 0.1 | 31.3 ± 0.3 a | 31.2 ± 0.9 a |
| Myristoleic acid (C14:1) | nd | nd | nd | 1.30 ± 0.02 | 1.20 ± 0.04 a | 1.22 ± 0.02 a |
| Polyunsaturated fatty acids | ||||||
| Linoleic acid (C18:2) | 2.14 ± 0.01 | 3.4 ± 0.2 a | 1.8 ± 0.1 b | 4.6 ± 0.1 | 4.3 ± 0.04 a | 4.2 ± 0.1 a |
| Cis-9,12,15-octadecatrienoic (α-linolenic) (C18:3) | 0.21 ± 0.03 | 0.62 ± 0.02 a | 0.22 ± 0.01 b | 0.25 ± 0.02 | 0.25 ± 0.01 b | 0.21 ± 0.01 b |
| Peak | Retention Time (min) | Compound | Chemical Formula | [M-H] m/z | Concentration (mg/Lextract) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| RGM | FGM_Clay Pot | FGM_ Glass Container | RCM | FCM_ Clay Pot | FCM_ Glass Container | |||||
| 1 | 2.269 | quinic acid | C7H12O6 | 191 | 29.59 ± 0.10 | 42.67 ± 0.10 | 42.89 ± 0.10 | 25.2 ± 0.1 | 34.03 ± 0.1 | 26.2 ± 0.1 |
| 2 | 27.734 | Rutin | C27H30O16 | 609 | nd | 0.003 ± 0.003 | nd | nd | nd | nd |
| 3 | 33.292 | Salviolinic acid | C36H30O1 | 717 | 1.184 ± 0.001 | nd | nd | nd | nd | nd |
| 4 | 35.502 | quercetin | C15H10O7 | 301 | nd | 0.012 ± 0.002 | nd | nd | nd | nd |
| 5 | 36.915 | Naringenin | C15H12O5 | 271 | 0.007 ± 0.003 | nd | nd | nd | nd | nd |
| 6 | 38.107 | Luteolin | C21H20O11 | 285 | nd | nd | 0.038 ± 0.001 | nd | nd | nd |
| 7 | 38.816 | Apigenin | C15H10O5 | 269 | 0.018 ± 0.001 | 0.035 ± 0.001 | 0.003 ± 0.001 | 0.058 ± 0.001 | 0.043 ± 0.001 | 0.029 ± 0.001 |
| 8 | 39.015 | Cirsiliol | C17H14O7 | 329 | 0.010 ± 0.003 | 0.012 ± 0.003 | 0.018 ± 0.003 | 0.015 ± 0.003 | 0.017 ± 0.003 | 0.009 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkadem, W.; Indio, V.; Belguith, K.; Oussaief, O.; Savini, F.; Giacometti, F.; El Hatmi, H.; Serraino, A.; De Cesare, A.; Boudhrioua, N. Influence of Fermentation Container Type on Chemical and Microbiological Parameters of Spontaneously Fermented Cow and Goat Milk. Foods 2023, 12, 1836. https://doi.org/10.3390/foods12091836
Mkadem W, Indio V, Belguith K, Oussaief O, Savini F, Giacometti F, El Hatmi H, Serraino A, De Cesare A, Boudhrioua N. Influence of Fermentation Container Type on Chemical and Microbiological Parameters of Spontaneously Fermented Cow and Goat Milk. Foods. 2023; 12(9):1836. https://doi.org/10.3390/foods12091836
Chicago/Turabian StyleMkadem, Wafa, Valentina Indio, Khaoula Belguith, Olfa Oussaief, Federica Savini, Federica Giacometti, Halima El Hatmi, Andrea Serraino, Alessandra De Cesare, and Nourhene Boudhrioua. 2023. "Influence of Fermentation Container Type on Chemical and Microbiological Parameters of Spontaneously Fermented Cow and Goat Milk" Foods 12, no. 9: 1836. https://doi.org/10.3390/foods12091836





