Protective Effect of Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Pomace Wine on Oxidative Stress of Hydrogen Peroxide by Regulating Keap1/Nrf2 Pathway in HepG2 Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Materials and Microbial Strains
2.3. Fermentation of Chinese Bayberry Pomace Wine
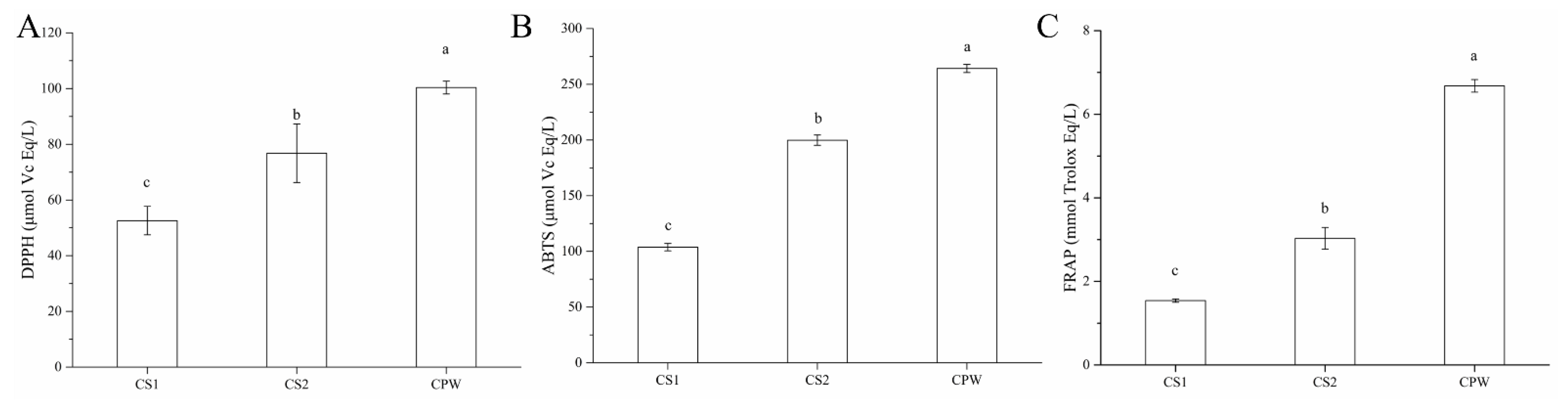
2.4. Antioxidant Capacity Analysis
2.5. Cell Culture
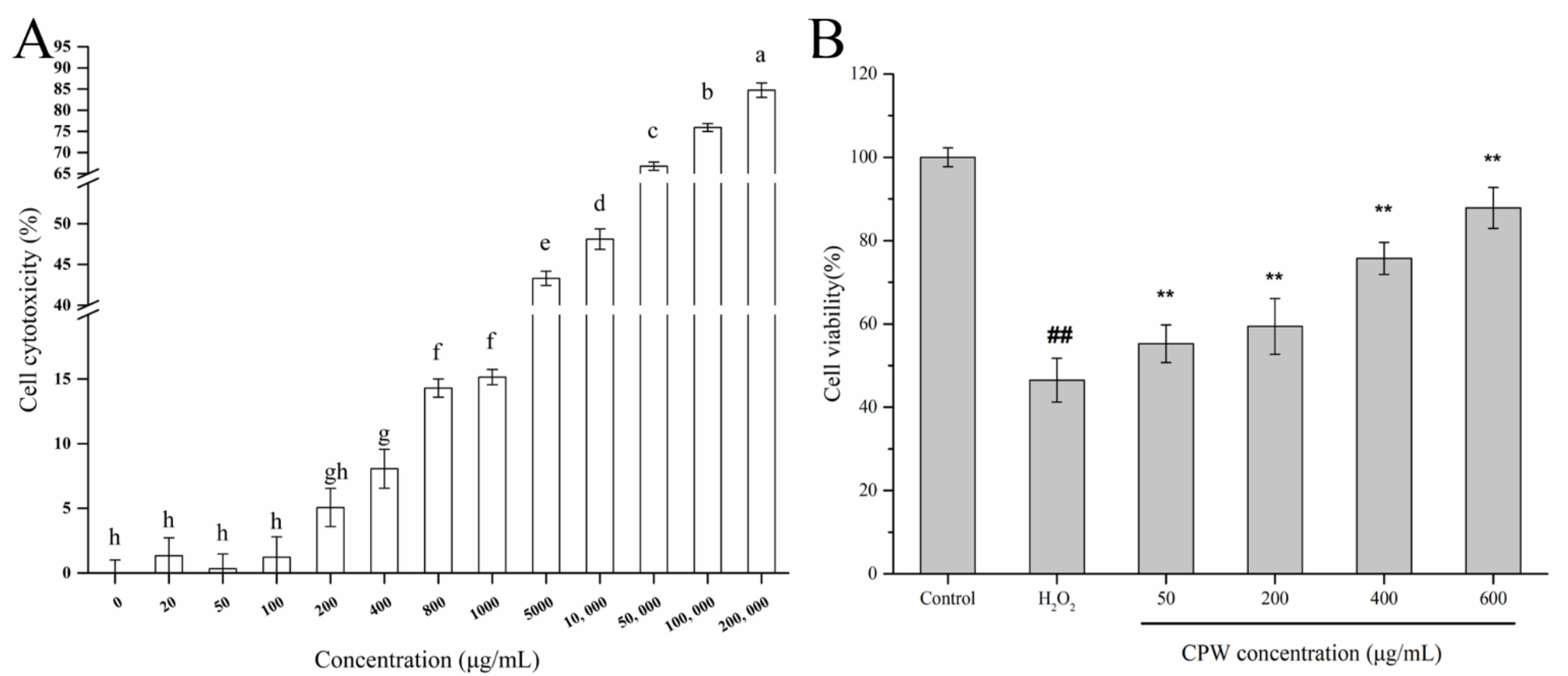
2.6. H2O2-Injured Cell Model (HI) and CPW Treatment
2.7. Cytotoxicity Assay
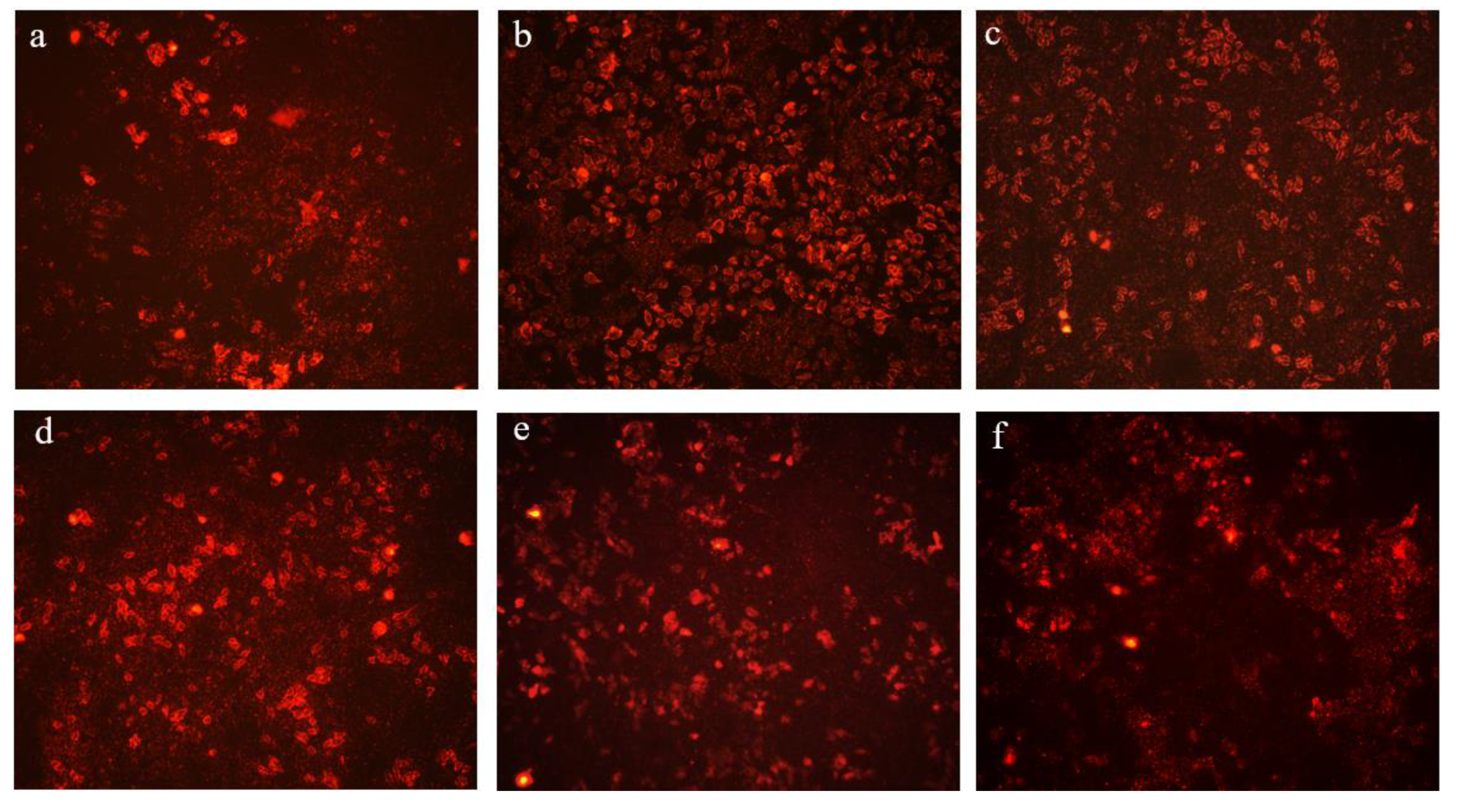
2.8. Intracellular ROS Determination
2.9. Cell Membrane Injury Determination
2.10. Intracellular Antioxidant Indexes Determination
2.11. RT-qPCR Analysis of mRNA Expression
2.12. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Antioxidant Activity
3.2. CPW Ameliorated the Cytotoxicity of H2O2-Induced HepG2 Cells
3.3. CPW Decreased ROS Production in H2O2-Induced HepG2 Cells
3.4. CPW Alleviated Cell Membrane Injury in H2O2-Induced HepG2 Cells
3.5. CPW Counteracted the Oxidative Stress and Reinforced Antioxidant Capacity in H2O2-Induced HepG2 Cells
3.6. CPW Regulated the Expression of Antioxidant Related Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.S.; Eweys, A.S.; Zhang, J.Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.B.; Xiao, X. Fermentation affects the antioxidant activity of plant-based food material through the release and production of bioactive components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lv, J.; Gu, Y.; He, Y.; Chen, J.; Ye, X.; Zhou, Z. Mixed fermentation of Chinese bayberry pomace using yeast, lactic acid bacteria and acetic acid bacteria: Effects on color, phenolics and antioxidant ingredients. LWT 2022, 163, 113503. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, J.; Gu, Y.; He, Y.; Chen, J.; Zhou, Z.; Ye, X. Polysaccharides of Chinese bayberry pomace wine: Structural characteristics, antioxidant activity and influence on the bayberry wine. Food Biosci. 2022, 50, 102025. [Google Scholar] [CrossRef]
- Xiang, J.; Li, W.; Victoria, U.; Trust, B. A comparative study of the phenolic compounds and in vitro antioxidant capacity of finger millets from different growing regions in Malawi. JCS 2019, 87, 143–149. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, J.; Wang, H.; Wang, M.; Zhao, J.; Wu, Z. Protective effect of apple phlorizin on hydrogen peroxide-induced cell damage in HepG2 cells. J. Food Biochem. 2019, 43, e13052. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Xie, H.; Ju, X.; Liu, R.H. Phytochemical profiles and antioxidant activity of Adlay Varieties. J. Agric. Food Chem. 2013, 61, 5103–5113. [Google Scholar] [CrossRef]
- Ren, X.; Yu, J.; Yin, X.; Tu, L.; Kong, Q. Isolation and purification of five phenolic compounds from the Xinjiang wine grape (Vitis Vinifera) and determination of their antioxidant mechanism at cellular level. Eur. Food Res. Technol. 2018, 244, 1569–1579. [Google Scholar]
- Juan, C.; Jose, M.; Francisco, J.; Eduardo, P. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Saniya, A.; Kumar, J.N.; Kumar, J.S.; Kumar, K.K.; Janne, R.; Shubhadeep, R.; Brijesh, R.; Dhruv, K. Oxidative stress in cancer cell metabolism. Antioxidants 2021, 10, 642. [Google Scholar]
- Deirdre, N.; Andrea, B.; Sruti, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar]
- Ma, Q.; Cai, S.; Liu, X.; Shi, J.; Yi, J. Characterization of phytochemical components and identification of main antioxidants in Crateva unilocalaris Buch. shoots by UHPLC-Q-Orbitrap-MS2 analysis. Food Res. Int. 2021, 143, 110264. [Google Scholar] [CrossRef] [PubMed]
- Utkualp, N.; Ozdemir, A. Oxidative stress and chronic diseases. Oxid. Commun. 2015, 38, 1690–1696. [Google Scholar]
- Oyeyinka, B.O.; Afolayan, A.J. Potentials of Musa species fruits against oxidative stress-induced and diet-linked chronic diseases: In vitro and in vivo implications of micronutritional factors and dietary secondary metabolite compounds. Molecules 2020, 25, 5036. [Google Scholar] [CrossRef]
- Popolo, A.; Autore, G.; Pinto, A.; Marzocco, S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013, 47, 346–356. [Google Scholar] [CrossRef]
- Kancheva, V.D.; Kasaikina, O.T. Bio-antioxidants—A chemical base of their antioxidant activity and beneficial effect on human health. Curr. Med. Chem. 2013, 20, 4784–4805. [Google Scholar] [CrossRef]
- Suchisnigdha, D.; Sukanya, G.; Anupam, B.; Dona, S. Flexion of Nrf2 by tea phytochemicals: A review on the chemopreventive and chemotherapeutic implications. Pharmacol. Res. 2022, 182, 106319. [Google Scholar]
- Zhao, Z.; Dong, R.; Cui, K.; You, Q.; Jiang, Z. An updated patent review of Nrf2 activators (2020–present). Expert Opin. Ther. Pat. 2023, 33, 29–49. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, J. The Keap1-Nrf2 system: A mediator between oxidative stress and aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Huang, D.; Yu, Q.; Yang, J.; Yao, J. Neuroligin-3 protects retinal cells from H2O2-induced cell death via activation of Nrf2 signaling. Biochem. Bioph. Res. Co. 2018, 502, 166–172. [Google Scholar] [CrossRef]
- Zhan, J.; Li, G.; Dang, Y.; Pan, D. Antioxidant stability of a novel peptide from porcine plasma hydrolysates by in vitro digestion/HepG-2 model. Eur. Food Res. Technol. 2022, 248, 797–806. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, J.; Yue, Y.; Feng, Z.; Chen, J.; Ye, X. Influence of mixed probiotics on the the bioactive composition, antioxidant activity and appearance of fermented red bayberry pomace. LWT 2020, 133, 110076. [Google Scholar] [CrossRef]
- Tao, J.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Bioactive peptides from cartilage protein hydrolysate of spotless smoothhound and their antioxidant activity in vitro. Mar Drugs 2018, 16, 100. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ma, Y.; Wang, Z.; Khan, A.; Zhou, W.; Zhao, T.; Cao, J.; Cheng, S.; Cai, S. Phenolic constituents, antioxidant and cytoprotective activities of crude extract and fractions from cultivated artichoke inflorescence. Ind. Crop. Prod. 2020, 143, 111433. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Lu, H.; Zong, Z.; Chen, Z.; Li, Y.; Luo, X.; Li, Y. Preservation of hydrogen peroxide-induced oxidative damage in HepG-2 cells by rice protein hydrolysates pretreated with electron beams. Sci. Rep. 2020, 10, 8415. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.; Johansen, C.L.; Honoré, A.H.; Jensen, H.M.; Jespersen, L.; Arneborg, N. Fluorescent labelling negatively affects the physiology of Lactococcus lactis. Int. Dairy J. 2015, 49, 130–138. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Peng, W.; Bai, Z.; Tian, Y.; Song, S. Study on the fermentation technology and antioxidant activity of Musella lasiocarpa. Food Ind. 2021, 42, 165–169. [Google Scholar]
- Zhang, X.; Huang, H.; Zhang, Q.; Fan, F.; Xu, C.; Sun, C.; Li, X.; Chen, K. Phytochemical characterization of Chinese Bayberry (Myrica rubra Sieb. et Zucc.) of 17 cultivars and their antioxidant properties. Int. J. Mol. Sci. 2015, 16, 12467–12481. [Google Scholar] [CrossRef]
- Naka, K.; Muraguchi, T.; Hoshii, T.; Hirao, A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid. Redox Signal. 2008, 10, 1883–1894. [Google Scholar] [CrossRef]
- Gutterer, J.M.; Dringen, R.; Hirrlinger, J.; Hamprecht, B. Purification of glutathione reductase from bovine brain, generation of an antiserum, and immunocytochemical localization of the enzyme in neural cells. J. Neurochem. 1999, 73, 1422–1430. [Google Scholar] [CrossRef]
- Willmore, W.G.; Storey, K.B. Purification and properties of glutathione reductase from liver of the anoxia-tolerant turtle, Trachemys scripta elegans. Mol. Cell Biochem. 2007, 297, 139–149. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, B.; Li, L.; Li, X.; Zhang, J.; Chen, G. Fermented noni (Morinda citrifolia L.) fruit juice improved oxidative stress and insulin resistance under the synergistic effect of Nrf2/ARE pathway and gut flora in db/db mice and HepG2 cells. Food Funct. 2022, 13, 8254–8273. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ren, J.; Yao, S.; Wang, J.; Huang, L.; Zhou, P.; Yun, D.; Xu, Q.; Wu, S.; Wang, Z.; et al. Novel antioxidants’ synthesis and their anti-oxidative activity through activating Nrf2 signaling pathway. Bioorg. Med. Chem. Lett. 2017, 27, 1616–1619. [Google Scholar] [CrossRef]
- Chun, K.S.; Kim, D.H.; Surh, Y.J. Role of reductive versus oxidative stress in tumor progression and anticancer drug resistance. Cells 2021, 10, 758. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Fujikawa, N.; Komatsu, M.; Ishii, T.; Unno, M.; Akaike, T.; Motohashi, H.; Yamamoto, M. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 13561–13566. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Dong, S.; Bai, W.; Di, Y.; Gu, R.; Liu, F.; Zhao, B.; Wang, Y.; Liu, X. Methylated metabolites of chicoric acid ameliorate hydrogen peroxide (H2O2)-induced oxidative stress in HepG2 Cells. J. Agric. Food Chem. 2021, 69, 2179–2189. [Google Scholar] [CrossRef]
- Chu, Q.; Jia, R.; Chen, C.; Wang, Y.; Yu, X.; Zhang, Y.; Wu, T.; Zheng, X. Apios americana Medik leaf extracts attenuate H2O2-induced hepatotoxicity. Food Biosci. 2021, 41, 100996. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Lv, F.; Chen, S.; Chen, J.; Liu, D.; Ye, X. Domestic cooking methods affect the phytochemical composition and antioxidant activity of purple-fleshed potatoes. Food Chem. 2016, 197, 1264–1270. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Fan, L. Evaluation of the composition of Chinese bayberry wine and its effects on the color changes during storage. Food Chem. 2019, 276, 451–457. [Google Scholar] [CrossRef]




| Gene | Forward Primer Sequences (5′-3′) | Reverse Primer Sequences (5′-3′) |
|---|---|---|
| GAPDH | CGACCACTTTGTCAAGCTCA | AGGGGTCTACATGGCAACTG |
| SOD | TGGAGATAATACAGCAGGCT | AGTCACATTGCCCAAGTCTC |
| CAT | CCATTATAAGACTGACCAGGGC | AGTCCAGGAGGGGTACTTTCC |
| Nrf2 | AGTGTGGAGAGGTATGAGCC | CGTTCCTCTCTGGGTAGTAA |
| Keap1 | AGAGCGGGATGAGTGGCA | GCTGAATTAAGGCGGTTTGTC |
| NQO1 | GAAAGGATGGGAGGTGGTGG | CTGGAGTGTGCCCAATGCTA |
| HO-1 | ATCGCTACTTCCCTGCCTTTG | GAGGGAAGCTGGAAATAAGGCTA |
| GCLC | TGGGCAATTGCTGTCTCCAG | TCGCTCCTCCCGAGTTCTA |
| GPx | AGAAGTGCGAGGTGAACGGT | CCCACCAGGAACTTCTCAAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Zhu, Y.; Wang, M.; Chen, J. Protective Effect of Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Pomace Wine on Oxidative Stress of Hydrogen Peroxide by Regulating Keap1/Nrf2 Pathway in HepG2 Cells. Foods 2023, 12, 1863. https://doi.org/10.3390/foods12091863
Jiang J, Zhu Y, Wang M, Chen J. Protective Effect of Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Pomace Wine on Oxidative Stress of Hydrogen Peroxide by Regulating Keap1/Nrf2 Pathway in HepG2 Cells. Foods. 2023; 12(9):1863. https://doi.org/10.3390/foods12091863
Chicago/Turabian StyleJiang, Jing, Yanyun Zhu, Mengting Wang, and Jianchu Chen. 2023. "Protective Effect of Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Pomace Wine on Oxidative Stress of Hydrogen Peroxide by Regulating Keap1/Nrf2 Pathway in HepG2 Cells" Foods 12, no. 9: 1863. https://doi.org/10.3390/foods12091863
APA StyleJiang, J., Zhu, Y., Wang, M., & Chen, J. (2023). Protective Effect of Chinese Bayberry (Myrica rubra Sieb. et Zucc.) Pomace Wine on Oxidative Stress of Hydrogen Peroxide by Regulating Keap1/Nrf2 Pathway in HepG2 Cells. Foods, 12(9), 1863. https://doi.org/10.3390/foods12091863






