Effect of a Dairy Cow’s Feeding System on the Flavor of Raw Milk: Indoor Feeding or Grazing
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk Sampling
2.2. HS-SPME/GC-MS
2.3. E-Nose
2.4. E-Tongue
2.5. Statistical Analysis
3. Results
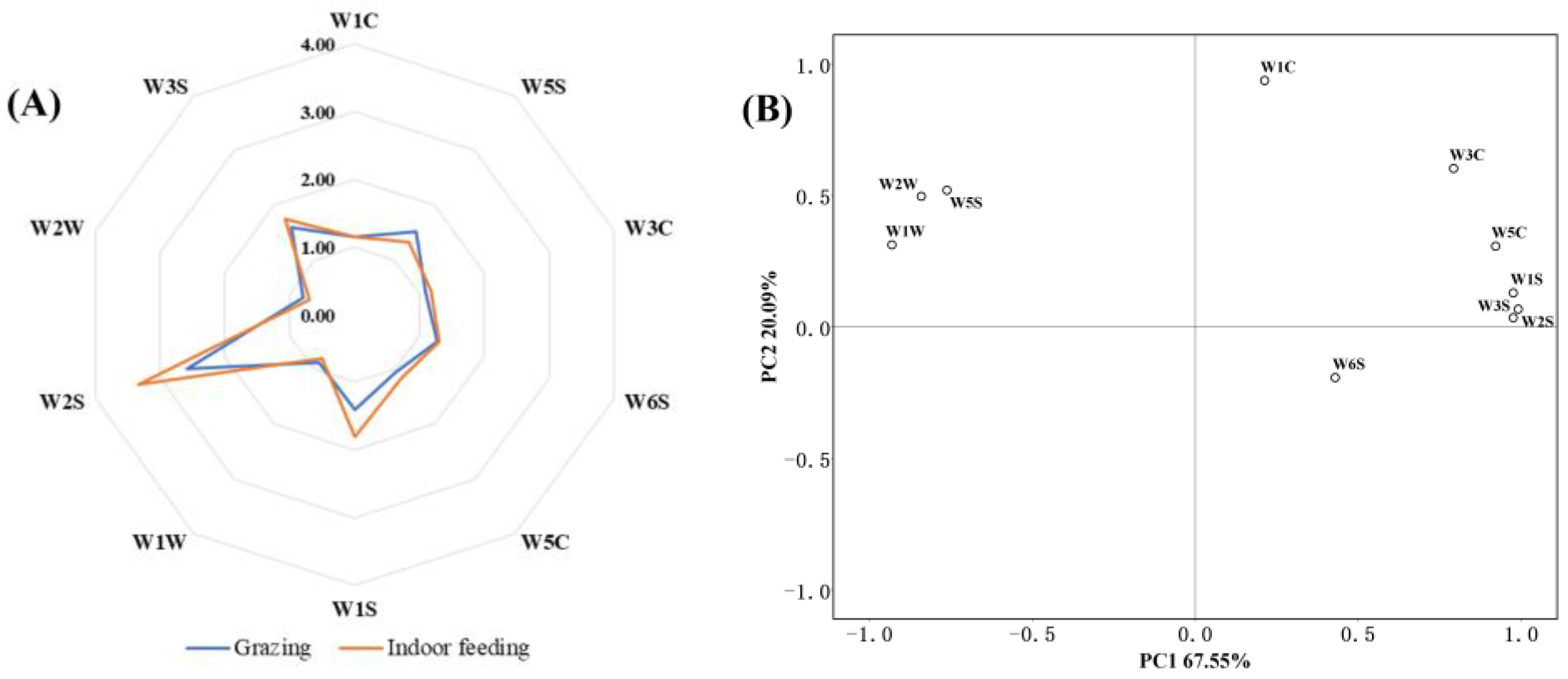
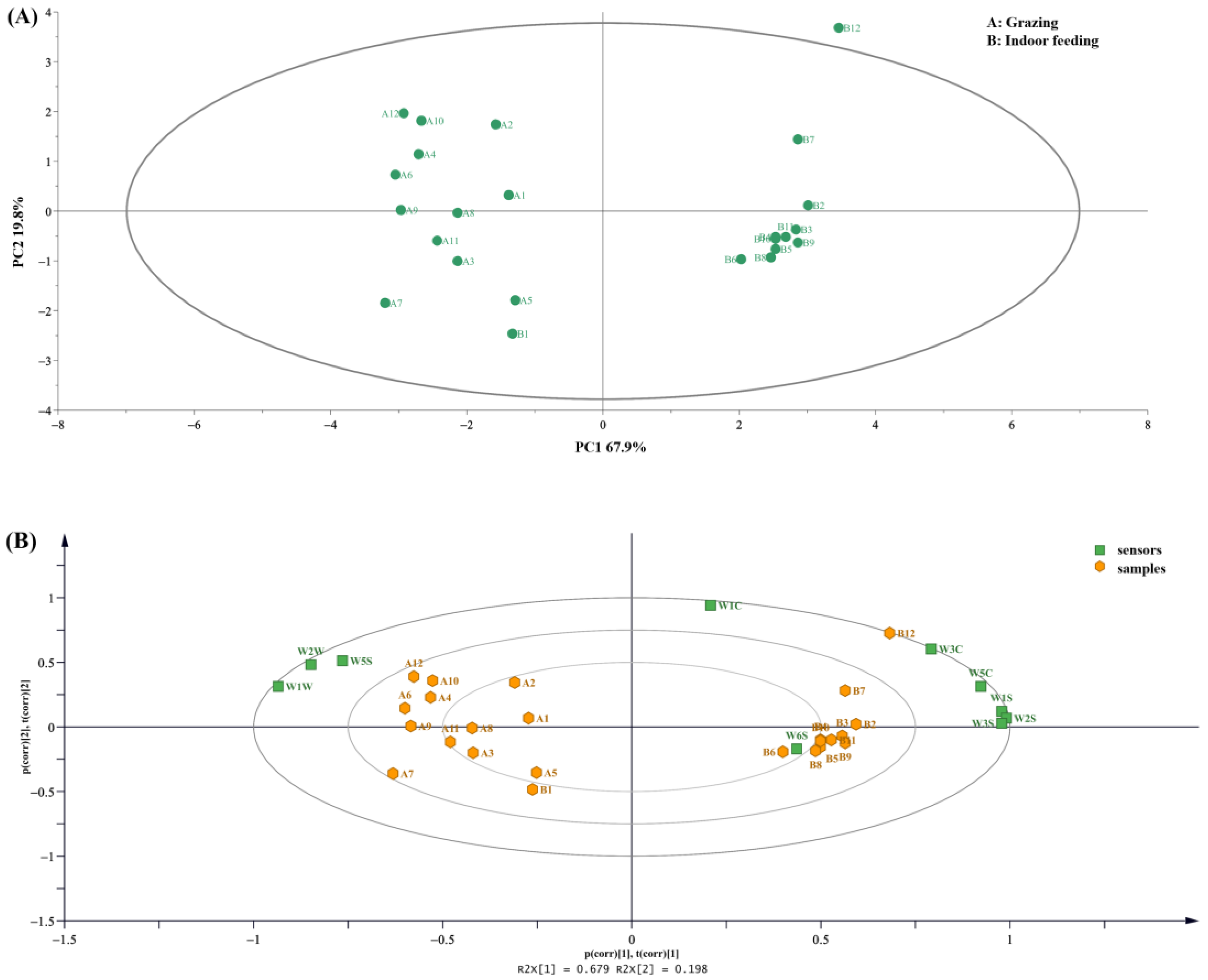
3.1. E-Nose Analysis
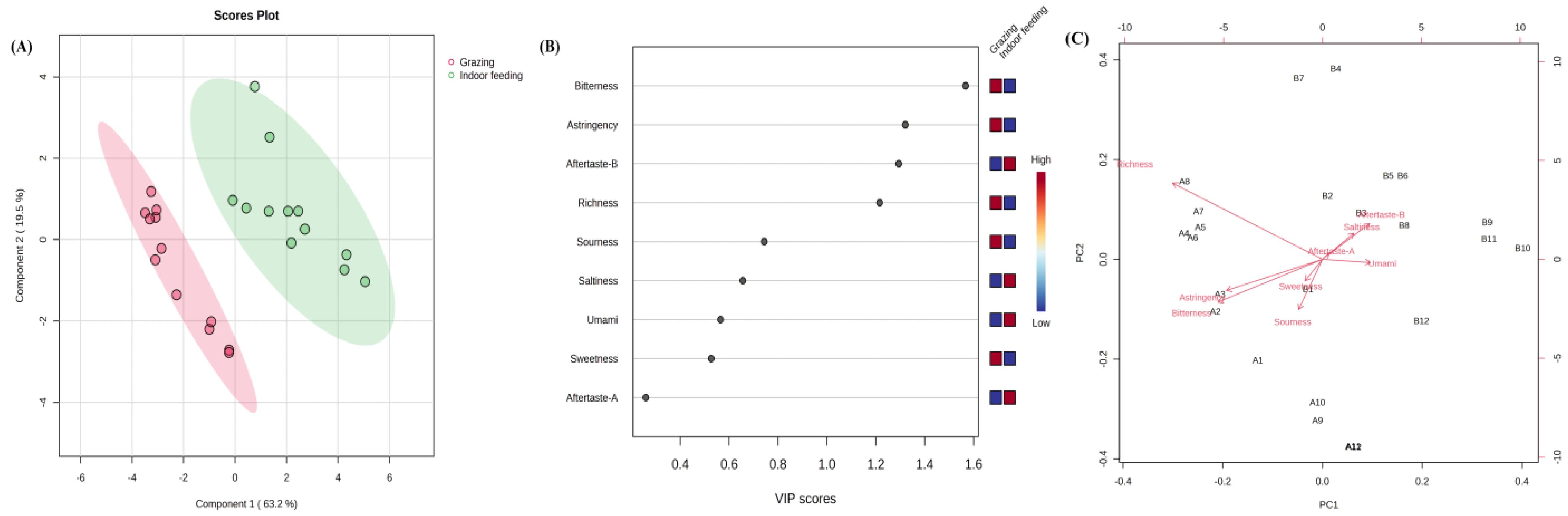
3.2. E-Tongue Analysis
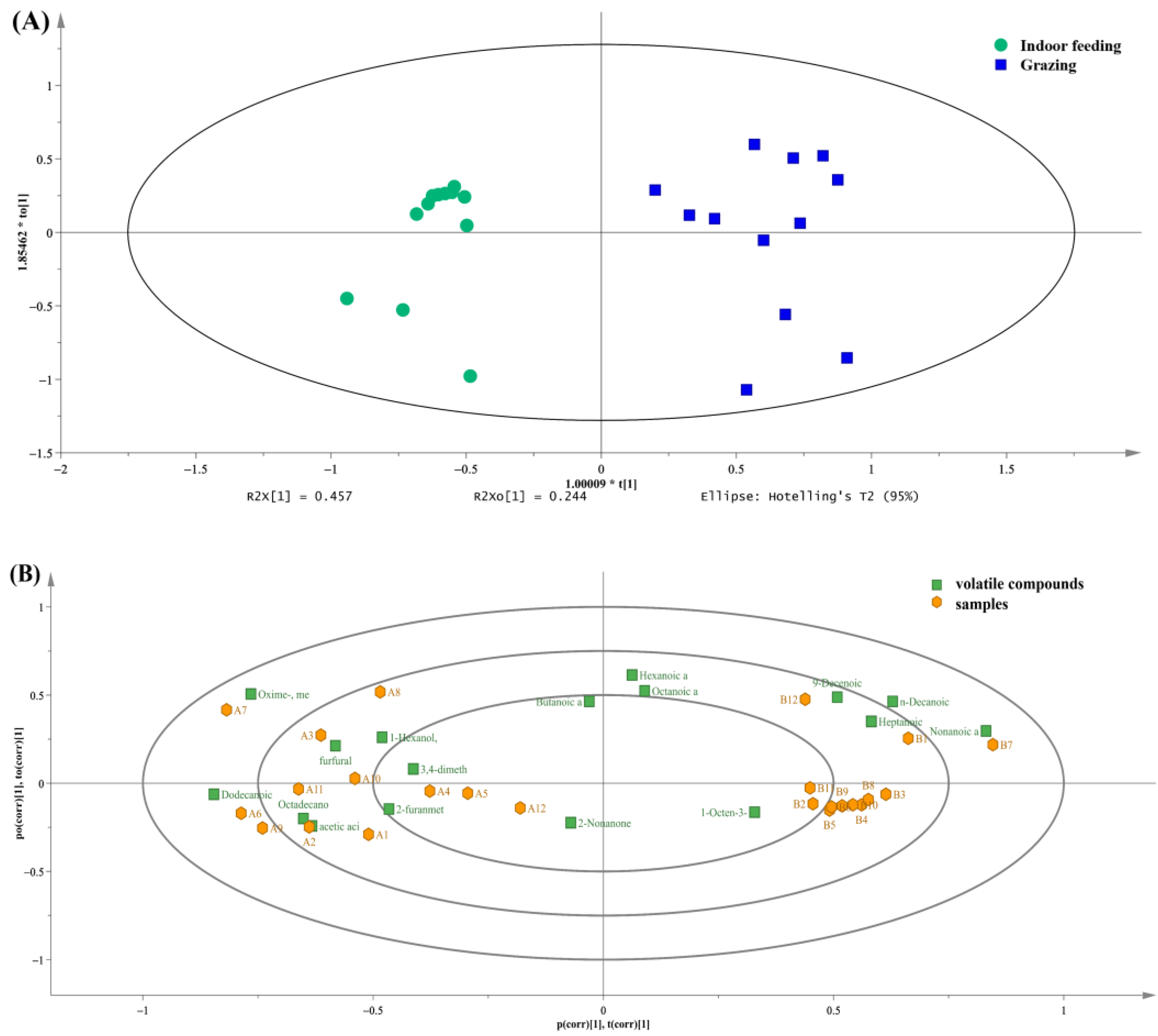
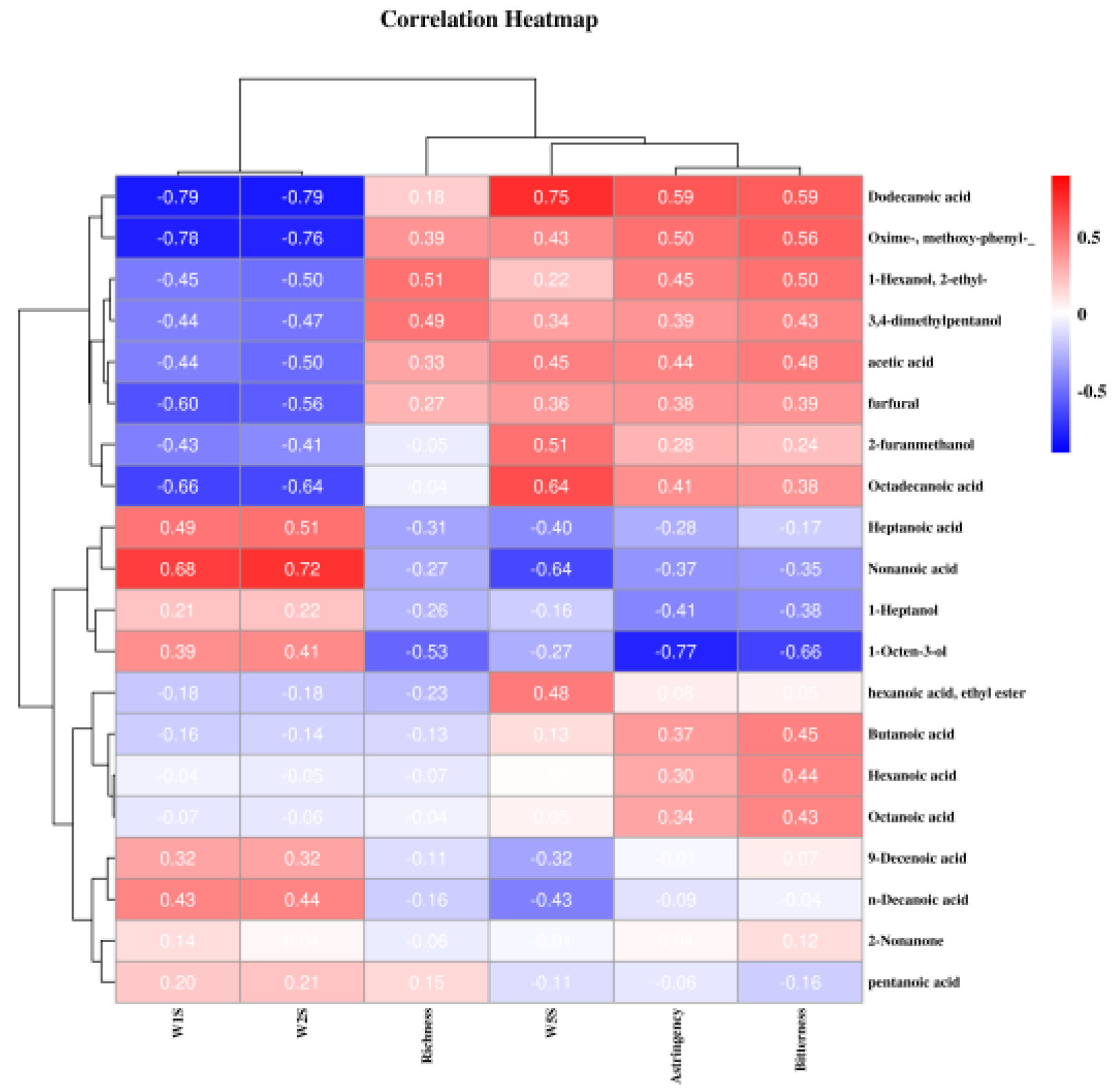
3.3. Volatile Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bendall, J.G. Aroma compounds of fresh milk from New Zealand cows fed different diets. J. Agric. Food Chem. 2001, 49, 4825–4832. [Google Scholar] [CrossRef]
- Adler, S.A.; Purup, S.; Hansen-Møller, J.; Thuen, E.; Gustavsson, A.M.; Steinshamn, H. Phyto-oestrogens and their metabolites in milk produced on two pastures with different botanical compositions. Livest. Sci. 2014, 163, 62–68. [Google Scholar] [CrossRef]
- Faulkner, H.; O’Callaghan, T.F.; McAuliffe, S.; Hennessy, D.; Stanton, C.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Effect of different forage types on the volatile and sensory properties of bovine milk. J. Dairy Sci. 2018, 101, 1034–1047. [Google Scholar] [CrossRef]
- Croissant, A.E.; Washburn, S.P.; Dean, L.L.; Drake, M.A. Chemical properties and consumer perception of fluid milk from conventional and pasture-based production systems. J. Dairy Sci. 2007, 90, 4942–4953. [Google Scholar] [CrossRef]
- Bravo-Lamas, L.; Aldai, N.; Kramer, J.K.G.; Barron, L.J.R. Case study using commercial dairy sheep flocks: Comparison of the fat nutritional quality of milk produced in mountain and valley farms. LWT 2018, 89, 374–380. [Google Scholar] [CrossRef]
- Xiao, Y.; Yi, L.; Ming, L.; He, J.; Ji, R. Changes in milk components, amino acids, and fatty acids of Bactrian camels in different lactation periods. Int. Dairy J. 2022, 131, 105363. [Google Scholar] [CrossRef]
- Jo, Y.; Carter, B.G.; Barbano, D.M.; Drake, M.A. Identification of the source of volatile sulfur compounds produced in milk during thermal processing. J. Dairy Sci. 2019, 102, 8658–8669. [Google Scholar] [CrossRef]
- Chi, X.; Guo, H.; Zhang, Y.; Zheng, N.; Liu, H.; Wang, J. E-nose, E-tongue Combined with GC-IMS to Analyze the Influence of Key Additives during Processing on the Flavor of Infant Formula. Foods 2022, 11, 3708. [Google Scholar] [CrossRef]
- Coolbear, T.; Janin, N.; Traill, R.; Shingleton, R. Heat-induced changes in the sensory properties of milk. Int. Dairy J. 2022, 126, 105199. [Google Scholar] [CrossRef]
- Jo, Y.; Benoist, D.M.; Barbano, D.M.; Drake, M.A. Flavor and flavor chemistry differences among milks processed by high-temperature, short-time pasteurization or ultra-pasteurization. J. Dairy Sci. 2018, 101, 3812–3828. [Google Scholar] [CrossRef]
- Liu, C.; Yang, P.; Wang, H.; Song, H. Identification of odor compounds and odor-active compounds of yogurt using DHS, SPME, SAFE, and SBSE/GC-O-MS. LWT 2022, 154, 112689. [Google Scholar] [CrossRef]
- Simon, M.; Hansen, A.P.; Young, C.T. Effect of various dairy packaging materials on the headspace analysis of ultrapasteurized milk. J. Dairy Sci. 2001, 84, 774–783. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Jin, H.; Feng, X.; Tian, F.; Song, Y.; Ren, Y.; Man, C.; Zhang, W. Shelf-life prediction and chemical characteristics analysis of milk formula during storage. LWT 2021, 144, 111268. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef]
- O’Brien, D.; Shalloo, L.; Patton, J.; Buckley, F.; Grainger, C.; Wallace, M. A life cycle assessment of seasonal grass-based and confinement dairy farms. Agric. Syst. 2012, 107, 33–46. [Google Scholar] [CrossRef]
- Infascelli, L.; Tudisco, R.; Iommelli, P.; Capitanio, F. Milk Quality and Animal Welfare as a Possible Marketing Lever for the Economic Development of Rural Areas in Southern Italy. Animals 2021, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Rencricca, G.; Froldi, F.; Moschini, M.; Trevisan, M.; Ghnimi, S.; Lamastra, L. The environmental impact of permanent meadows-based farms: A comparison among different dairy farm management systems of an Italian cheese. Sustain. Prod. Consum. 2023, 37, 53–64. [Google Scholar] [CrossRef]
- Yakubu, H.G.; Kovacs, Z.; Toth, T.; Bazar, G. Trends in artificial aroma sensing by means of electronic nose technologies to advance dairy production—A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Carfora, V.; Cavallo, C.; Caso, D.; Del Giudice, T.; De Devitiis, B.; Viscecchia, R.; Nardone, G.; Cicia, G. Explaining consumer purchase behavior for organic milk: Including trust and green self-identity within the theory of planned behavior. Food Qual. Prefer. 2019, 76, 1–9. [Google Scholar] [CrossRef]
- de Graaf, S.; Van Loo, E.J.; Bijttebier, J.; Vanhonacker, F.; Lauwers, L.; Tuyttens, F.A.M.; Verbeke, W. Determinants of consumer intention to purchase animal-friendly milk. J. Dairy Sci. 2016, 99, 8304–8313. [Google Scholar] [CrossRef]
- Villeneuve, M.P.; Lebeuf, Y.; Gervais, R.; Tremblay, G.F.; Vuillemard, J.C.; Fortin, J.; Chouinard, P.Y. Milk volatile organic compounds and fatty acid profile in cows fed timothy as hay, pasture, or silage. J. Dairy Sci. 2013, 96, 7181–7194. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Dhiman, T.R.; Ure, A.L.; Brennand, C.P.; Boman, R.L.; McMahon, D.J. Consumer acceptability of conjugated linoleic acid-enriched milk and cheddar cheese from cows grazing on pasture. J. Dairy Sci. 2005, 88, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Khot, L.R.; Panigrahi, S. Biology and applications of olfactory sensing system: A review. Sens. Actuators B Chem. 2012, 171–172, 1–17. [Google Scholar] [CrossRef]
- Carrillo-Gómez, J.K.; Durán Acevedo, C.M.; García-Rico, R.O. Detection of the bacteria concentration level in pasteurized milk by using two different artificial multisensory methods. Sens. Bio-Sens. Res. 2021, 33, 100428. [Google Scholar] [CrossRef]
- Wang, B.; Xu, S.; Sun, D.-W. Application of the electronic nose to the identification of different milk flavorings. Food Res. Int. 2010, 43, 255–262. [Google Scholar] [CrossRef]
- Bougrini, M.; Tahri, K.; Haddi, Z.; El Bari, N.; Llobet, E.; Jaffrezic-Renault, N.; Bouchikhi, B. Aging time and brand determination of pasteurized milk using a multisensor e-nose combined with a voltammetric e-tongue. Mater Sci. Eng. C Mater Biol. Appl. 2014, 45, 348–358. [Google Scholar] [CrossRef]
- Chi, X.; Shao, Y.; Pan, M.; Yang, Q.; Yang, Y.; Zhang, X.; Ai, N.; Sun, B. Distinction of volatile flavor profiles in various skim milk products via HS-SPME–GC–MS and E-nose. Eur. Food Res. Technol. 2021, 247, 1539–1551. [Google Scholar] [CrossRef]
- Balivo, A.; Cipolletta, S.; Tudisco, R.; Iommelli, P.; Sacchi, R.; Genovese, A. Electronic Nose Analysis to Detect Milk Obtained from Pasture-Raised Goats. Appl. Sci. 2023, 13, 861. [Google Scholar] [CrossRef]
- Winquist, F.; Bjorklund, R.; Krantz-Rülcker, C.; Lundström, I.; Östergren, K.; Skoglund, T. An electronic tongue in the dairy industry. Sens. Actuators B Chem. 2005, 111–112, 299–304. [Google Scholar] [CrossRef]
- Kilcawley, K.; Faulkner, H.; Clarke, H.; O’Sullivan, M.; Kerry, J. Factors Influencing the Flavour of Bovine Milk and Cheese from Grass Based versus Non-Grass Based Milk Production Systems. Foods 2018, 7, 37. [Google Scholar] [CrossRef]
- Dias, L.A.; Peres, A.M.; Veloso, A.C.A.; Reis, F.S.; Vilas-Boas, M.; Machado, A.A.S.C. An electronic tongue taste evaluation: Identification of goat milk adulteration with bovine milk. Sens. Actuators B Chem. 2009, 136, 209–217. [Google Scholar] [CrossRef]
- Han, H.; Wang, Z.; Li, C.; Ma, Z.; Yang, Z.; Ma, X. Purity detection of goat milk based on electronic tongue and improved artificial fish swarm optimized extreme learning machine. IFAC-PapersOnLine 2019, 52, 391–396. [Google Scholar] [CrossRef]
- Pan, M.; Tong, L.; Chi, X.; Ai, N.; Cao, Y.; Sun, B. Comparison of Sensory and Electronic Tongue Analysis Combined with HS-SPME-GC-MS in the Evaluation of Skim Milk Processed with Different Preheating Treatments. Molecules 2019, 24, 1650. [Google Scholar] [CrossRef]
- Söderström, C.; Winquist, F.; Krantz-Rülcker, C. Recognition of six microbial species with an electronic tongue. Sens. Actuators B Chem. 2003, 89, 248–255. [Google Scholar] [CrossRef]
- Guo, Q.; Adelina, N.M.; Hu, J.; Zhang, L.; Zhao, Y. Comparative analysis of volatile profiles in four pine-mushrooms using HS-SPME/GC-MS and E-nose. Food Control 2022, 134, 108711. [Google Scholar] [CrossRef]
- Song, J.; Chen, Q.; Bi, J.; Meng, X.; Wu, X.; Qiao, Y.; Lyu, Y. GC/MS coupled with MOS e-nose and flash GC e-nose for volatile characterization of Chinese jujubes as affected by different drying methods. Food Chem. 2020, 331, 127201. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Rojo-Poveda, O.; Ferrocino, I.; Giordano, M.; Zeppa, G. Assessment of volatile fingerprint by HS-SPME/GC-qMS and E-nose for the classification of cocoa bean shells using chemometrics. Food Res. Int. 2019, 123, 684–696. [Google Scholar] [CrossRef]
- Yue, J.; Zheng, Y.; Liu, Z.; Deng, Y.; Jing, Y.; Luo, Y.; Yu, W.; Zhao, Y. Characterization of Volatile Compounds in Microfiltered Pasteurized Milk Using Solid-Phase Microextraction and GC×GC-TOFMS. Int. J. Food Prop. 2015, 18, 2193–2212. [Google Scholar] [CrossRef]
- Lemos, A.T.; Casal, S.; Barba, F.J.; Phimolsiripol, Y.; Delgadillo, I.; Saraiva, J.A. Preservation of high pressure pasteurised milk by hyperbaric storage at room temperature versus refrigeration on inoculated microorganisms, fatty acids, volatile compounds and lipid oxidation. Food Chem. 2022, 387, 132887. [Google Scholar] [CrossRef]
- Mitani, T.; Kobayashi, K.; Ueda, K.; Kondo, S. Discrimination of “grazing milk” using milk fatty acid profile in the grassland dairy area in Hokkaido. Anim. Sci. J. 2016, 87, 233–241. [Google Scholar] [CrossRef]
- Cadwallader, D.C.; Gerard, P.D.; Drake, M.A. The role of packaging on the flavor of fluid milk. J. Dairy Sci. 2023, 106, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Wang, L.; Song, H.; Li, Z. Detection of odor difference between human milk and infant formula by sensory-directed analysis. Food Chem. 2022, 382, 132348. [Google Scholar] [CrossRef]
- Larsen, M.K.; Kidmose, U.; Kristensen, T.; Beaumont, P.; Mortensen, G. Chemical composition and sensory quality of bovine milk as affected by type of forage and proportion of concentrate in the feed ration. J. Sci. Food Agric. 2013, 93, 93–99. [Google Scholar] [CrossRef]
- Boltar, I.; Čanžek Majhenič, A.; Jarni, K.; Jug, T.; Bavcon Kralj, M. Volatile compounds in Nanos cheese: Their formation during ripening and sesonal variation. J. Food Sci. Technol. 2014, 52, 608–623. [Google Scholar] [CrossRef]
- Panseri, S.; Giani, I.; Mentasti, T.; Bellagamba, F.; Caprino, F.; Moretti, V.M. Determination of flavour compounds in a mountain cheese by headspace sorptive extraction-thermal desorption-capillary gas chromatography-mass spectrometry. LWT-Food Sci. Technol. 2008, 41, 185–192. [Google Scholar] [CrossRef]
- Clarke, H.J.; Griffin, C.; Rai, D.K.; O’Callaghan, T.F.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Dietary Compounds Influencing the Sensorial, Volatile and Phytochemical Properties of Bovine Milk. Molecules 2019, 25, 26. [Google Scholar] [CrossRef] [PubMed]
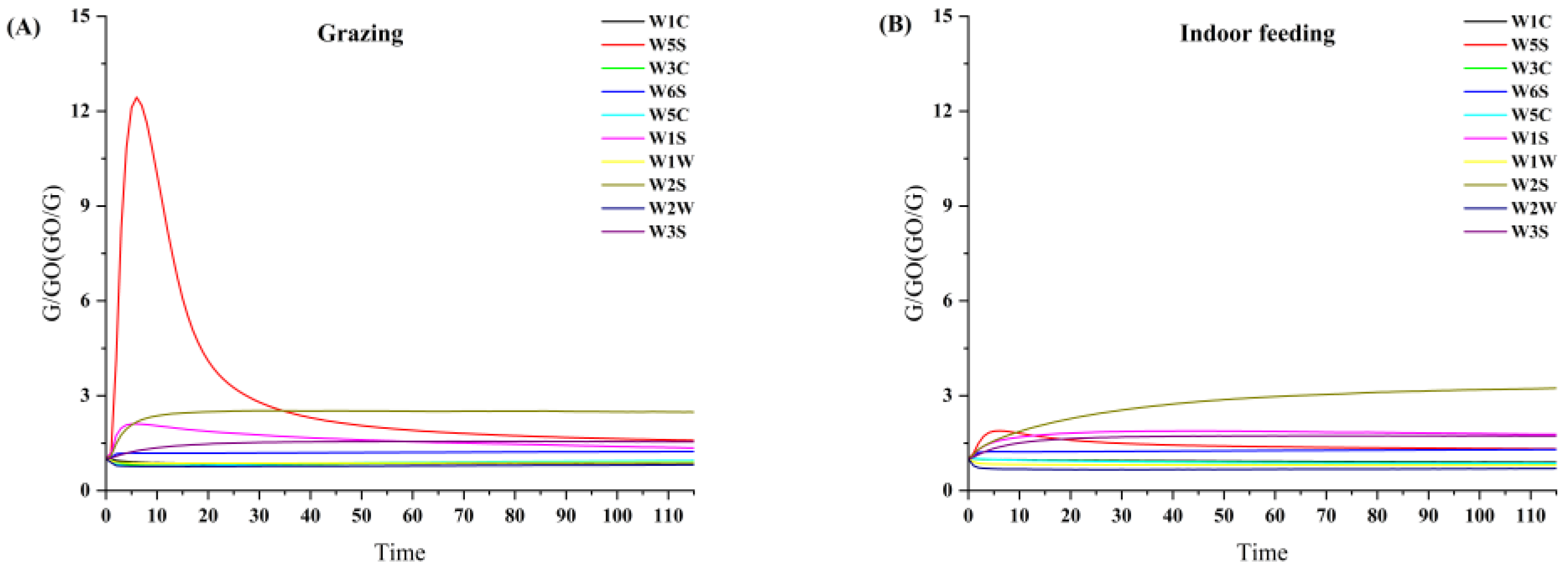
| NO. | Sensor | Performance Description (Sensitivity to) |
|---|---|---|
| 1 | W5S | nitrogen oxides |
| 2 | W1S | methyl |
| 3 | W2S | alcohols, ketones, and aldehydes |
| 4 | W3C | ammonia |
| 5 | W1C | benzene |
| 6 | W5C | short-chain aromatic compounds and olefin |
| 7 | W1W | sulfur compounds |
| 8 | W2W | organic sulfides |
| 9 | W6S | hydrogen |
| 10 | W3S | long-chain alkanes |
| Group | Sourness | Bitterness | Astringency | Aftertaste-B | Aftertaste-A | Umami | Richness | Saltiness | Sweetness |
|---|---|---|---|---|---|---|---|---|---|
| Grazing | −39.76 ± 0.57 | 2.38 ± 0.52 | −2.41 ± 0.342 | −0.20 ± 0.17 | 0.13 ± 0.051 | 8.76 ± 0.60 | 12.87 ± 2.21 | 11.88 ± 0.28 | 12.57 ± 0.13 |
| Indoor feeding | −40.86 ± 0.50 | 0.042 ± 1.332 | −4.38 ± 1.30 | 1.73 ± 1.154 | 0.51 ± 0.35 | 9.60 ± 0.55 | 11.07 ± 1.80 | 12.85 ± 0.53 | 11.79 ± 0.39 |
| p value | 0.37 | 0.02 | 0.01 | 0.00 | 0.00 | 0.77 | 0.24 | 0.04 | 0.01 |
| No. | Compound | Retention Time | CAS# | Formula |
|---|---|---|---|---|
| 1 | Ethyl hexanoate | 11.82 | 123-66-0 | C8H16O2 |
| 2 | 3,4-Dimethylpentanol | 13.78 | 64502-86-9 | C7H16O |
| 3 | 2-Nonanone | 14.24 | 821-55-6 | C9H18O |
| 4 | Acetic acid | 15.09 | 68475-71-8 | C2H4O2 |
| 5 | 1-Octen-3-ol | 15.12 | 3191-86-4 | C8H16O |
| 6 | Furfural | 15.19 | 98-01-1 | C5H4O2 |
| 7 | 1-Hexanol, 2-ethyl- | 15.6 | 104-76-7 | C8H18O |
| 8 | 1-Heptanol | 15.2 | 111-70-6 | C7H16O |
| 9 | Butanoic acid | 17.32 | 1977-33-9 | C10H19NO5 |
| 10 | Pentanoic acid | 17.31 | 109-52-4 | C5H10O2 |
| 11 | 2-Furanmethanol | 17.83 | 98-00-0 | C5H6O2 |
| 12 | Oxime-, methoxy-phenyl- | 18.81 | 1775-61-7 | C8H9NO2 |
| 13 | Hexanoic acid | 19.79 | 142-62-1 | C6H12O2 |
| 14 | Heptanoic acid | 20.92 | 1173022-17-7 | C7H14O2 |
| 15 | Octanoic acid | 22 | 124-07-2 | C8H16O2 |
| 16 | Dodecanoic acid | 24.01 | 143-07-7 | C12H24O2 |
| 17 | Octadecanoic acid | 27.57 | 57-11-4 | C18H36O2 |
| 18 | Nonanoic acid | 23.01 | 112-05-0 | C9H18O2 |
| 19 | n-Decanoic acid | 24 | 334-48-5 | C10H20O2 |
| 20 | 9-Decenoic acid | 24.53 | 14436-32-9 | C10H18O2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, X.; Yuan, N.; Zhang, Y.; Zheng, N.; Liu, H. Effect of a Dairy Cow’s Feeding System on the Flavor of Raw Milk: Indoor Feeding or Grazing. Foods 2023, 12, 1868. https://doi.org/10.3390/foods12091868
Chi X, Yuan N, Zhang Y, Zheng N, Liu H. Effect of a Dairy Cow’s Feeding System on the Flavor of Raw Milk: Indoor Feeding or Grazing. Foods. 2023; 12(9):1868. https://doi.org/10.3390/foods12091868
Chicago/Turabian StyleChi, Xuelu, Ning Yuan, Yangdong Zhang, Nan Zheng, and Huimin Liu. 2023. "Effect of a Dairy Cow’s Feeding System on the Flavor of Raw Milk: Indoor Feeding or Grazing" Foods 12, no. 9: 1868. https://doi.org/10.3390/foods12091868
APA StyleChi, X., Yuan, N., Zhang, Y., Zheng, N., & Liu, H. (2023). Effect of a Dairy Cow’s Feeding System on the Flavor of Raw Milk: Indoor Feeding or Grazing. Foods, 12(9), 1868. https://doi.org/10.3390/foods12091868






