Visual Colorimetric Detection of Edible Oil Freshness for Peroxides Based on Nanocellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Preparation of CNFs
2.4. Preparation of CNF-AM Hydrogels
2.5. Preparation of Functional Hydrogels
2.6. Colorimetric Detection of Peroxide Values (PV) by Functional Hydrogels
2.7. Oxidation of Edible Oil
2.8. Application of Functional Hydrogel in Oil Spoilage
3. Results and Discussion
3.1. Characterization of Cellulose and CNFs
3.2. Cross-Linking of CNF-AM Hydrogels
3.3. Morphological Characterization of CNF-AM Hydrogels
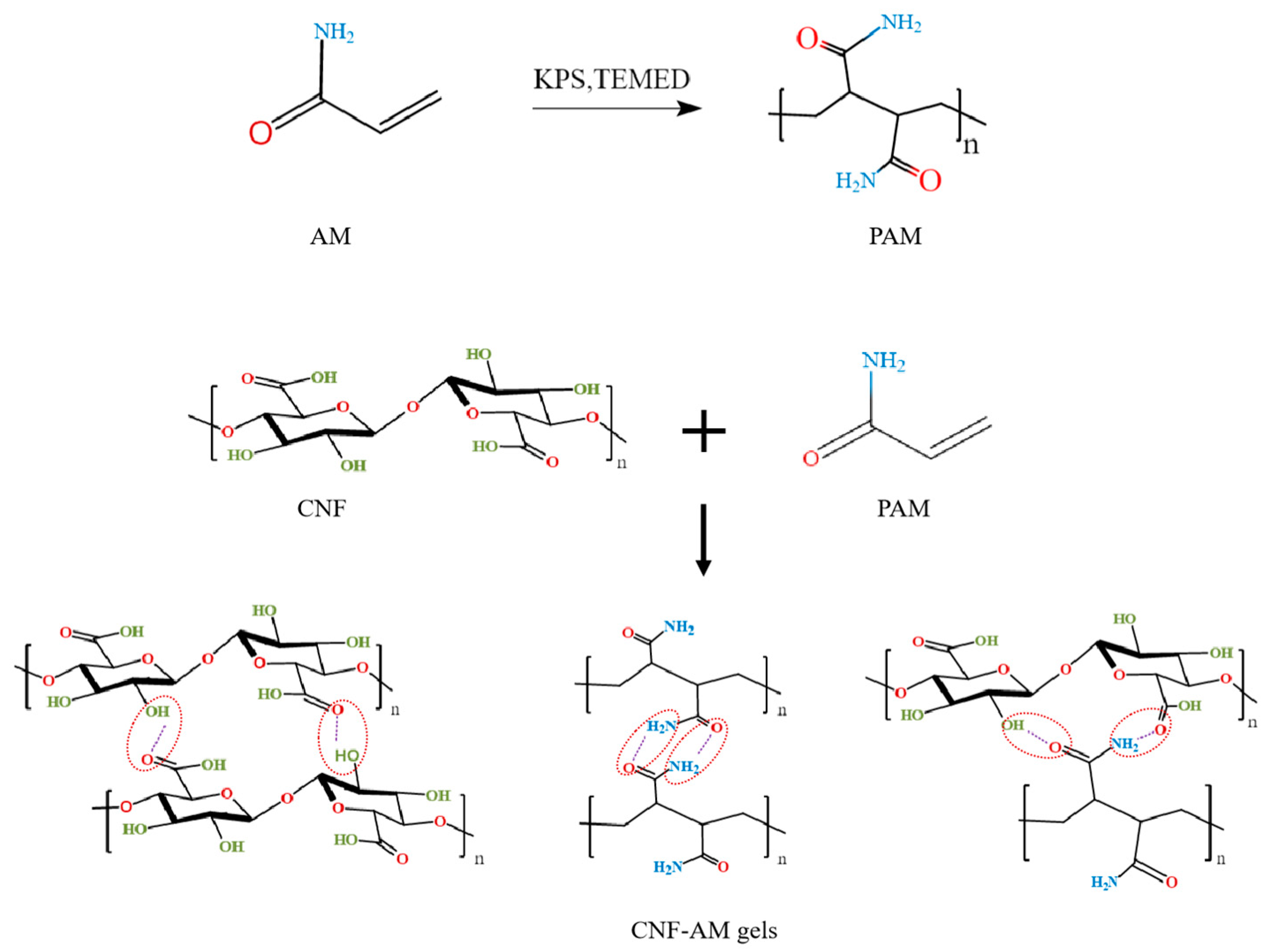
3.4. Formation Mechanism and Properties of CNF-AM Hydrogels
3.5. Response of Functional Hydrogels to Peroxide
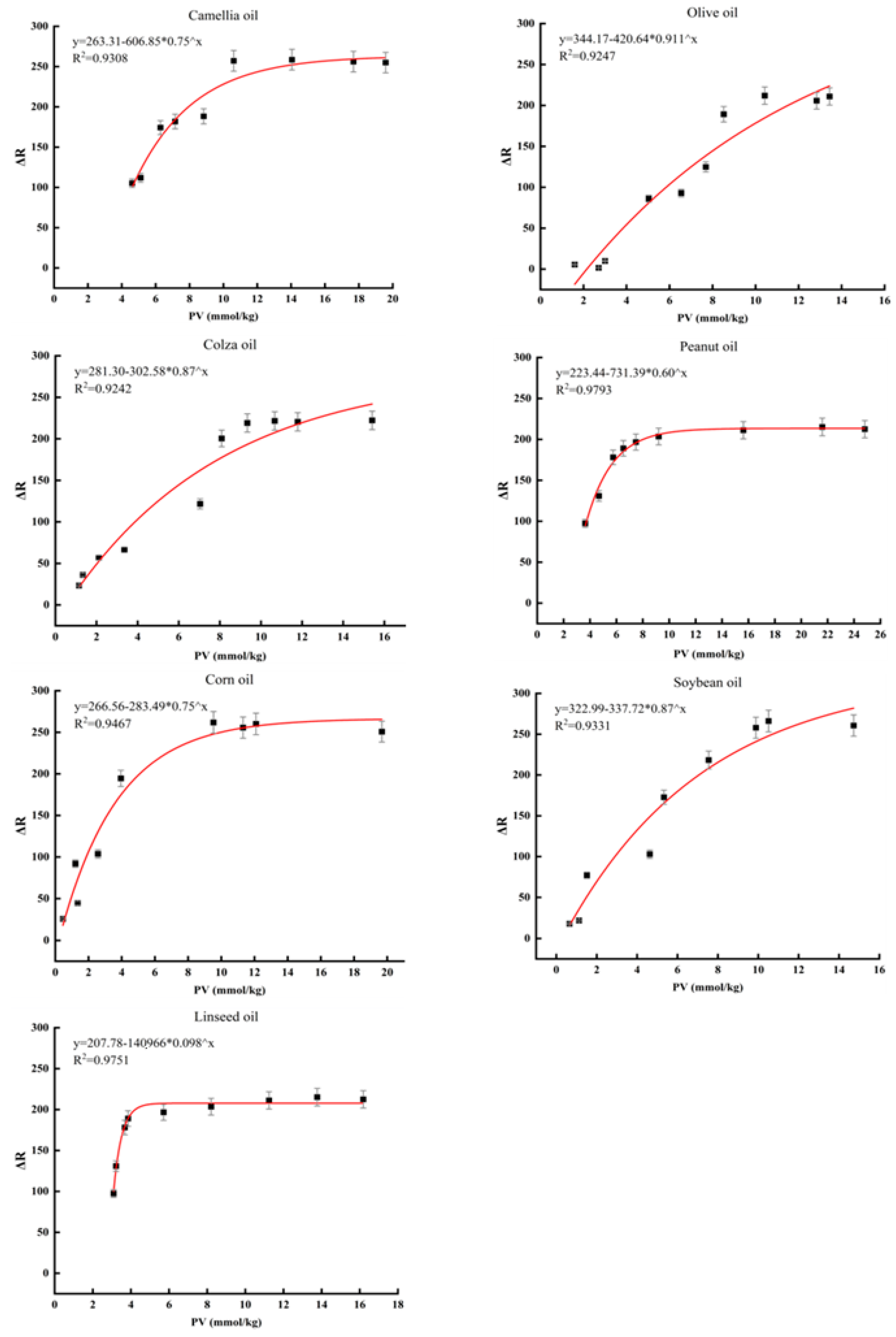
3.6. Application of Functional Hydrogel on the Edible Oil
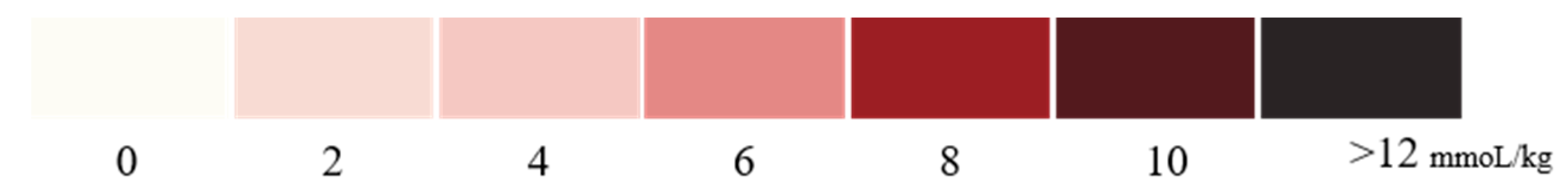
3.7. Accuracy of Standard Colorimetric Card
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.X.; Liu, Y.F. Analysis and Detection of Edible Oil Oxidation. Lipid Technol. 2016, 28, 8. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Jiang, J.; Wang, Y.; Zhang, X.; Xia, B.; Dong, W. Visual detection of edible oil oxidation by using chitin-based colorimetric sensor for aldehydes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127303. [Google Scholar] [CrossRef]
- Albert, B.B.; Cameron-Smith, D.; Hofman, P.L.; Cutfield, W.S. Oxidation of marine omega-3 supplements and human health. Biomed. Res. Int. 2013, 2013, 464921. [Google Scholar] [CrossRef] [PubMed]
- Eliot Patrick Botosoa, R.K. 3D front face fluorescence spectroscopy as a tool for monitoring the oxidation level of edible vegetable oil during storage at 60 °C. Light Weight. Tank 2022, 154, 112659. [Google Scholar]
- Cao, J.; Zou, X.; Deng, L.; Fan, Y.; Li, H.; Li, J.; Deng, Z. Analysis of nonpolar lipophilic aldehydes/ketones in oxidized edible oils using HPLC-QqQ-MS for the evaluation of their parent fatty acids. Food Res. Int. 2014, 64, 901–907. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, T.; Li, J. Rapid identification between edible oil and swill-cooked dirty oil by using a semi-supervised support vector machine based on graph and near-infrared spectroscopy. Chemom. Intell. Lab. Syst. 2015, 143, 1–6. [Google Scholar] [CrossRef]
- Soni, A.; Yusuf, M.; Mishra, V.K.; Beg, M. An assessment of thermal impact on chemical characteristics of edible oils by using FTIR spectroscopy. Mater. Today Proc. 2022, 68, 710–716. [Google Scholar] [CrossRef]
- Yang, X.; Han, Z.; Xia, T.; Xu, Y.; Wu, Z. Monitoring the oxidation state evolution of unsaturated fatty acids in four microwave-treated edible oils by low-field nuclear magnetic resonance and 1H nuclear magnetic resonance. Light Weight. Tank 2021, 138, 110740. [Google Scholar] [CrossRef]
- Gibson, M.; Percival, B.C.; Edgar, M.; Grootveld, M. Low-Field Benchtop NMR Spectroscopy for Quantification of Aldehydic Lipid Oxidation Products in Culinary Oils during Shallow Frying Episodes. Foods 2023, 12, 1254. [Google Scholar] [CrossRef]
- Castro, R.C.; Ribeiro, S.M.; Santos, L.M.; Páscoa, N.M.J. The use of in-situ Raman spectroscopy to monitor at real time the quality of different types of edible oils under frying conditions. Food Control 2022, 136, 108879. [Google Scholar] [CrossRef]
- Xie, Y.; Dong, X.; Cai, N.; Yang, F.; Yao, W.; Huang, L. Application of a Novel Au@ZIF-8 Composite in the Detection of Bisphenol A by Surface-Enhanced Raman Spectroscopy. Foods 2023, 12, 813. [Google Scholar] [CrossRef]
- Kong, Y.L.; Li, T.; He, Y. Background-free room temperature phosphorescence and digital image colorimetry detection of melamine by carbon nitride quantum dots in cellulose matrix with smartphone-based portable device. Food Chem. 2022, 390, 133135. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Wang, S.; Chen, H.; Ren, L.; Liang, K.; Wei, L.; Long, W.; Yang, J.; Guo, L.; Han, X.; et al. Digital image colorimetry in combination with chemometrics for the detection of carbaryl based on the peroxidase-like activity of nanoporphyrins and the etching process of gold nanoparticles. Food Chem. 2022, 394, 133495. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wu, J.; Wang, H.; Feng, J.; Zheng, X.; Wang, X.; Wang, S. Bio-derived solvent-based dispersive liquid-liquid microextraction followed by smartphone digital image colorimetry for the detection of carbofuran in cereals. J. Food Compos. Anal. 2022, 114, 104782. [Google Scholar] [CrossRef]
- Ding, N.; Dong, S.; Zhang, Y.; Lu, D.; Lin, J.; Zhao, Q.; Shi, X. Portable silver-doped prussian blue nanoparticle hydrogels for colorimetric and photothermal monitoring of shrimp and fish freshness. Sens. Actuators B Chem. 2022, 363, 131811. [Google Scholar] [CrossRef]
- Sáez-Hernández, R.; Ruiz, P.; Mauri-Aucejo, A.R.; Yusa, V.; Cervera, M.L. Determination of acrylamide in toasts using digital image colorimetry by smartphone. Food Control 2022, 141, 109163. [Google Scholar] [CrossRef]
- Prasetyono, O.H.; Miranda, T.; Sari, M.; Noviana, H. Creating Benchmark of Color Skin Viability Based on Digital Photograph Using Mini Mobile Medical Photo Studio Prototype. J. Surg. Res. 2019, 235, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital image colorimetry on smartphone for chemical analysis: A review. Measurement 2021, 171, 108829. [Google Scholar] [CrossRef]
- Kaeun, S.B.; Dowan, K. A freshness indicator for monitoring chicken-breast spoilage using a Tyvek® sheet and RGB color analysis. Food Packag. Shelf Life 2019, 19, 6. [Google Scholar]
- Ezati, P.; Rhim, J.W. pH-responsive pectin-based multifunctional films incorporated with curcumin and sulfur nanoparticles. Carbohydr. Polym. 2020, 230, 115638. [Google Scholar] [CrossRef]
- Hong, J.I.; Chang, B.Y. Development of the smartphone-based colorimetry for multi-analyte sensing arrays. Lab Chip 2014, 14, 1725–1732. [Google Scholar] [CrossRef] [PubMed]
- Sutthasupa, S.; Padungkit, C.; Suriyong, S. Colorimetric ammonia (NH3) sensor based on an alginate-methylcellulose blend hydrogel and the potential opportunity for the development of a minced pork spoilage indicator. Food Chem. 2021, 362, 130151. [Google Scholar] [CrossRef] [PubMed]
- Luo, X. Application of inkjet-printing technology in developing indicators/sensors for intelligent packaging systems. Curr. Opin. Food Sci. 2022, 46, 100868. [Google Scholar] [CrossRef]
- Ghorbani, M.; Divsalar, E.; Molaei, R.; Ezati, P.; Moradi, M.; Tajik, H.; Abbaszadeh, M. A halochromic indicator based on polylactic acid and anthocyanins for visual freshness monitoring of minced meat, chicken fillet, shrimp, and fish roe. Innov. Food Sci. Emerg. Technol. 2021, 74, 102864. [Google Scholar] [CrossRef]
- Geng, W.; Haruna, S.A.; Li, H.; Kademi, H.; Chen, Q.A. Novel Colorimetric Sensor Array Coupled Multivariate Calibration Analysis for Predicting Freshness in Chicken Meat: A Comparison of Linear and Nonlinear Regression Algorithms. Foods 2023, 12, 720. [Google Scholar] [CrossRef] [PubMed]
- Almasi, H.; Forghani, S.; Moradi, M. Recent advances on intelligent food freshness indicators; an update on natural colorants and methods of preparation. Food Packag. Shelf Life 2022, 32, 100839. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Bring some colour to your package: Freshness indicators based on anthocyanin extracts. Trends Food Sci. Technol. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Min, W.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Pirayesh, H.; Park, B.D.; Khanjanzadeh, H.; Park, H.; Cho, Y. Nanocellulose-based ammonia sensitive smart colorimetric hydrogels integrated with anthocyanins to monitor pork freshness. Food Control 2023, 147, 109595. [Google Scholar] [CrossRef]
- Batista, R.A.; Espitia, J.P.; Quintans, S.S.; Freitas, M.M.; Cerqueira, M.Â.; Teixeira, J.A.; Cardoso, J.C. Hydrogel as an alternative structure for food packaging systems. Carbohydr. Polym. 2019, 205, 106–116. [Google Scholar] [CrossRef]
- Barajas-Ledesma, R.M.; Hossain, L.; Wong, N.L.; Patti, A.F.; Garnier, G. Effect of the counter-ion on nanocellulose hydrogels and their superabsorbent structure and properties. J. Colloid Interface Sci. 2021, 599, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Ji, Y.; Yu, S.; Liu, Q.; Zhou, Z.; Guo, C.; Lu, D.; Pei, D. Preparation and characterization of a dual cross-linking injectable hydrogel based on sodium alginate and chitosan quaternary ammonium salt. Carbohydr. Res. 2021, 507, 108389. [Google Scholar] [CrossRef] [PubMed]
- Bauli, C.R.; Lima, G.F.; Souza, A.G.; Ferreira, R.R.; Rosa, D.S. Eco-friendly carboxymethyl cellulose hydrogels filled with nanocellulose or nanoclays for agriculture applications as soil conditioning and nutrient carrier and their impact on cucumber growing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126771. [Google Scholar] [CrossRef]
- Li, S.; Gao, D.; Lin, J.; Wen, Z.; Zhang, K.; Xia, Z.; Wang, D. Preparation of double-network hydrogel consisting of chitosan, cellulose and polyacrylamide for enrichment of tetracyclines. Microchem. J. 2022, 182, 107931. [Google Scholar] [CrossRef]
- Huang, W.C.; Ying, R.; Wang, W.; Guo, Y.; He, Y.; Mo, X.; Xue, C.; Mao, X. A Macroporous Hydrogel Dressing with Enhanced Antibacterial and Anti-Inflammatory Capabilities for Accelerated Wound Healing. Adv. Funct. Mater. 2020, 30, 2000644. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Kopac, T.; Krajnc, M.; Rucigaj, A. A mathematical model for pH-responsive ionically crosslinked TEMPO nanocellulose hydrogel design in drug delivery systems. Int. J. Biol. Macromol. 2021, 168, 695–707. [Google Scholar] [CrossRef]
- Barhoum, A.; Rastogi, V.K.; Mahur, B.K.; Rastogi, A.; Abdel-Haleem, F.M.; Samyn, P. Nanocelluloses as new generation materials: Natural resources, structure-related properties, engineering nanostructures, and technical challenges. Mater. Today Chem. 2022, 26, 101247. [Google Scholar] [CrossRef]
- Luo, Q.; Shen, H.; Zhou, G.; Xu, X. A mini-review on the dielectric properties of cellulose and nanocellulose-based materials as electronic components. Carbohydr. Polym. 2023, 303, 120449. [Google Scholar] [CrossRef]
- Mai, T.; Li, D.D.; Chen, L.; Ma, M. Collaboration of two-star nanomaterials: The applications of nanocellulose-based metal organic frameworks composites. Carbohydr. Polym. 2023, 302, 120359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shen, R.; Li, N.; Yang, X.; Lin, D. Nanocellulose: An amazing nanomaterial with diverse applications in food science. Carbohydr. Polym. 2023, 304, 120497. [Google Scholar] [CrossRef] [PubMed]
- Tsuguyuki, M.H.; Naoyuki, T. Individualization of Nano-Sized Plant Cellulose Fibrils by Direct Surface Carboxylation Using TEMPO Catalyst under Neutral Conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar]
- Ren, D.; Wang, Y.; Wang, H.; Xu, D.; Wu, X. Fabrication of nanocellulose fibril-based composite film from bamboo parenchyma cell for antimicrobial food packaging. Int. J. Biol. Macromol. 2022, 210, 152–160. [Google Scholar] [CrossRef]
- Su, J.; Zhang, L.; Wan, C.; Deng, Z.; Wei, S.; Yong, K.; Wu, Y. Dual-network self-healing hydrogels composed of graphene oxide@nanocellulose and poly(AAm-co-AAc). Carbohydr. Polym. 2022, 296, 119905. [Google Scholar] [CrossRef]
- Luo, Q.; Huang, Y.; Lei, Z.; Peng, J.; Xu, D.; Guo, X.; Wu, Y. Wood-derived nanocellulose hydrogel incorporating gold nanoclusters using in situ multistep reactions for efficient sorption and sensitive detection of mercury ion. Ind. Crops Prod. 2021, 173, 114142. [Google Scholar] [CrossRef]
- Ling, Z.; He, H.; Can, J.; Ma, L.; Yu, P. Cellulose Nanocrystals from Cotton Stalk for Reinforcement of Poly(Vinyl Alcohol) Composites. Cellul. Chem. Technol. 2017, 51, 109–119. [Google Scholar]
- Geng, L.; Naderi, A.; Mao, Y.; Zhan, C.; Sharma, P.; Peng, X.; Hsiao, B.S. Rheological Properties of Jute-Based Cellulose Nanofibers under Different Ionic Conditions. ACS Symp. Ser. 2017, 1251, 113–132. [Google Scholar]
- Lungu, A.; Cernencu, A.I.; Dinescu, S.; Balahura, R.; Mereuta, P.; Costache, M.; Syverud, K.; Stancu, L.C.; Lovu, H. Nanocellulose-enriched hydrocolloid-based hydrogels designed using a Ca2+ free strategy based on citric acid. Mater. Des. 2021, 197, 109200. [Google Scholar] [CrossRef]
- Wang, F.; Huang, K.; Xu, Z.; Shi, F.; Chen, C. Self-healable nanocellulose composite hydrogels combining multiple dynamic bonds for drug delivery. Int. J. Biol. Macromol. 2022, 203, 143–152. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, R.; Wu, J.; Penkova, A.; Qi, W.; He, Z.; Su, R. Injectable self-healing nanocellulose hydrogels crosslinked by aluminum: Cellulose nanocrystals vs. cellulose nanofibrils. Chin. J. Chem. Eng. 2022, 50, 389–397. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Huang, L.; Chen, H.; Xu, K.; Tan, Y.; Wang, P.; Zheng, J. Fracture of the Physically Cross-Linked First Network in Hybrid Double Network Hydrogels. Macromolecules 2014, 47, 2140–2148. [Google Scholar] [CrossRef]
- Dong, B.; Qu, H.; Yan, L.; Liu, C.; Mao, Y.; Zheng, L. Colorimetric detection of 2-tert-butyl-1,4-benzoquinone in edible oils based on a chromogenic reaction with commercial chemicals. Food Chem. 2023, 400, 134037. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Dong, X.; Yu, Y.; Cui, L. A novel method for detection of lipid oxidation in edible oil. Light Weight. Tank—Food Sci. Technol. 2020, 123, 109068. [Google Scholar] [CrossRef]
- Baek, S.; Kim, D.; Jeon, S.L.; Seo, J. Preparation and characterization of pH-responsive poly(N,N-dimethyl acrylamide-co-methacryloyl sulfadimethoxine) hydrogels for application as food freshness indicators. React. Funct. Polym. 2017, 120, 57–65. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, Y.; Zhou, Y.; Liu, S.; Gao, W.; Shi, X. Portable functional hydrogels based on silver metallization for visual monitoring of fish freshness. Food Control 2021, 123, 107824. [Google Scholar] [CrossRef]




| Materials | Indicators | Detected Substances | Mechanism | Application | References |
|---|---|---|---|---|---|
| Chitin nanowhiskers film | Congo red and hydroxylamine sulfate | Aldehydes | Hydroxylamine sulfate reacts with aldehydes, releasing sulfuric acid and leading to a decrease in pH | Sunflower oil | [2] |
| Polyethyleneimine (PEI) Solution | PEI | 2-tert-butyl-1,4-benzoquinone (TBBQ) | PEI link with TBBQ through Michael addition to form colored adducts | TBBQ-spiked soybean oil and peanut oil | [53] |
| polyvinyl alcohol film | Schiff’s reagen | Aldehydes | Schiff’s reagent and aldehydes form colored compounds | Sunflower oil | [54] |
| Agarose hydrogel | Silver-doped Prussian blue nanoparticles | Volatile amines | Decomposition of silver-doped Prussian blue nanoparticles caused by volatile aldehydes | Shrimp and fish | [15] |
| Sugarcane bagasse nanocellulose hydrogel | Bromothymol blue and methyl red | CO2 | CO2 levels increase with the spoilage of chicken, leading to a decrease in pH | Chicken | [28] |
| N,N-dimethyl acrylamide-co-methacryloyl sulfadimethoxine hydrogel | N,N-dimethyl acrylamide, methacryloyl sulfadimethoxine | - | Changes in pH lead to changes in the transparency of the hydrogel | - | [55] |
| Agarose hydrogel | β-D-glucose pentaacetate (β-D-GP) and silver ions | Biogenic amines | Biogenic amines hydrolyze β-D-GP to β-D-glucose, and β-D-glucose reduces silver ions to silver nanoparticles with a color change. | Fish | [56] |
| Nanocellulose hydrogel | Anthocyanins | Total volatile basic nitrogen (TVB-N) | TVB-N causes a change in pH with a color change | Pork | [29] |
| Alginate-methylcellulose blend hydrogel | Bromothymol blue | TVB-N | TVB-N causes a change in pH with a color change | A minced pork | [22] |
| CNF-AM hydrogel | Fe(II), SA, and AA | Peroxides | Peroxides oxidize Fe(II) to Fe(Ⅲ) with a color change | Seven types of edible oils | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Cheng, J.; Yang, F.; Hu, Z.; Zheng, Z.; Deng, Y.; Cao, B.; Xie, Y. Visual Colorimetric Detection of Edible Oil Freshness for Peroxides Based on Nanocellulose. Foods 2023, 12, 1896. https://doi.org/10.3390/foods12091896
Jiang X, Cheng J, Yang F, Hu Z, Zheng Z, Deng Y, Cao B, Xie Y. Visual Colorimetric Detection of Edible Oil Freshness for Peroxides Based on Nanocellulose. Foods. 2023; 12(9):1896. https://doi.org/10.3390/foods12091896
Chicago/Turabian StyleJiang, Xiongli, Jun Cheng, Fangwei Yang, Zhenyang Hu, Zhen Zheng, Yu Deng, Buyuan Cao, and Yunfei Xie. 2023. "Visual Colorimetric Detection of Edible Oil Freshness for Peroxides Based on Nanocellulose" Foods 12, no. 9: 1896. https://doi.org/10.3390/foods12091896





