Potential Challenges of the Extraction of Carotenoids and Fatty Acids from Pequi (Caryocar brasiliense) Oil
Abstract
:1. Introduction
2. The Brazilian Cerrado Biome
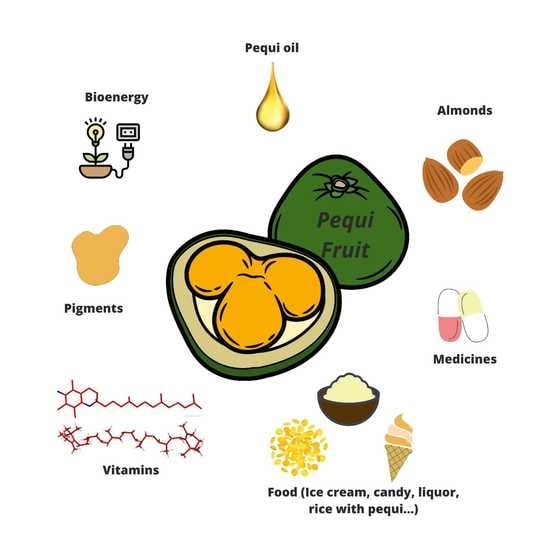
3. Pequi
4. Pequi Oil
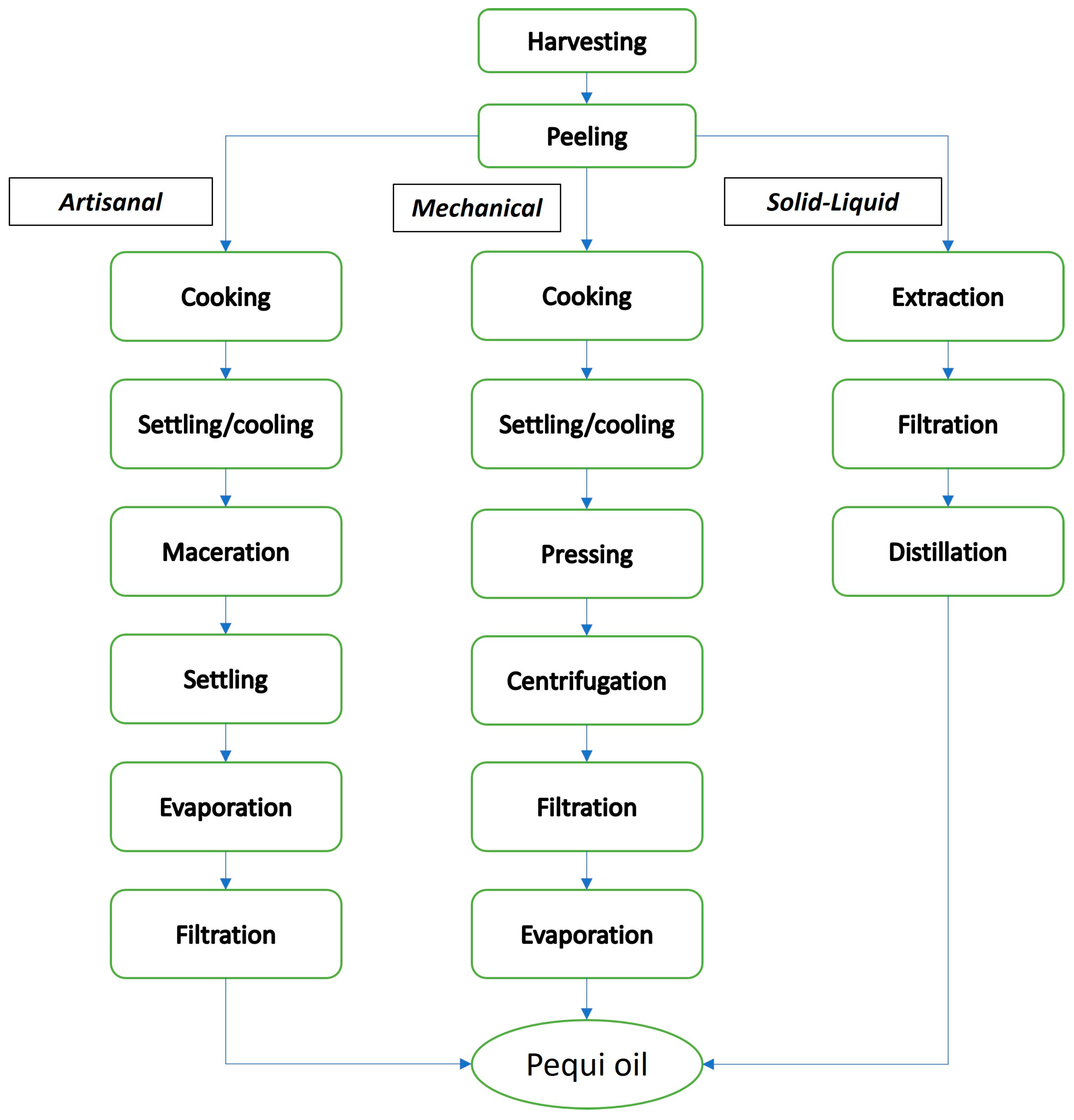
5. Pequi Oil Extraction Methods
5.1. Artisanal Extraction
5.2. Mechanical Extraction
5.3. Solid–Liquid Extraction
5.4. Supercritical Fluid Extraction
6. Bioactive Compounds of Pequi Oil
6.1. Carotenoids
6.2. Fatty Acids
7. Challenges in the Purification of Bioactives from Pequi-Based Products
8. Future Perspectives on the Valorization of Pequi and Its Derivatives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres, L.R.O.; Santana, F.C.; Shinagawa, F.B.; Mancini-Filho, J. Bioactive compounds and functional potential of pequi (Caryocar spp.), a native Brazilian fruit: A review. Grasas Aceites 2018, 69, 257. [Google Scholar] [CrossRef]
- Leão, D.P.; Franca, A.S.; Oliveira, L.S.; Bastos, R.; Coimbra, M.A. Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar Brasilense Camb.) fruit by-products. Food Chem. 2017, 225, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Vilela, A.L.; Pereira, L.C.S.; Gonçalves, C.A.; Grisolia, C.K. Pequi fruit (Caryocar Brasiliense Camb.) pulp oil reduces exercise-induced inflammatory markers and blood pressure of male and female runners. Nutr. Res. 2009, 29, 850–858. [Google Scholar] [CrossRef]
- Wang, J.; Hu, X.; Chen, J.; Wang, T.; Huang, X.; Chen, G. The extraction of β-carotene from microalgae for testing their health benefits. Foods 2022, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Salanță, L.C.; Uifălean, A.; Iuga, C.A.; Tofană, M.; Cropotova, J.; Pop, O.L.; González, C.V. Valuable food molecules with potential benefits for human health. In The Health Benefits of Foods—Current Knowledge and Further Development; IntechOpen: Rijeka, Croatia, 2020; pp. 1–45. [Google Scholar] [CrossRef]
- Hamulka, J.; Jeruszka-Bielak, M.; Górnicka, M.; Drywień, M.E.; Zielinska-Pukos, M.A. Dietary supplements during COVID-19 outbreak. Results of google trends analysis supported by plifeCOVID-19 online studies. Nutrients 2020, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Fiszman, S.; Puerta, P.; Chaya, C.; Tárrega, A. The impact of COVID-19 lockdown on food priorities. Results from a preliminary study using social media and an online survey with Spanish consumers. Food Qual. Pref. 2020, 86, 104028. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Tazeddinova, D.; Aljoumaa, K.; Kazhmukhanbetkyzy, Z.A.; Orazov, A.; Toshev, A.D. Carotenoids: Therapeutic strategy in the battle against viral emerging diseases, COVID-19: An overview. Prev. Nutr. Food Sci. 2021, 26, 241–261. [Google Scholar] [CrossRef]
- Yi, X.; Li, J.; Liao, D.; Peng, G.; Zheng, X.; Xu, H.; Zhang, T.; Ai, J. Carrot and carotene and multiple health outcomes: An umbrella review of the evidence. J. Sci. Food Agric. 2023, 103, 2251–2261. [Google Scholar] [CrossRef]
- Ferreira, I.; Gomes-Bispo, A.; Lourenço, H.; Matos, J.; Afonso, C.; Cardoso, C.; Castanheira, I.; Motta, C.; Prates, J.A.M.; Bandarra, N.M. The chemical composition and lipid profile of the chub cackerel (Scomber colias) show a strong seasonal dependence: Contribution to a nutritional evaluation. Biochimie 2020, 178, 181–189. [Google Scholar] [CrossRef]
- Almeida, E.S.; Damaceno, D.S.; Carvalho, L.; Victor, P.A.; Passos, R.M.; Almeida, P.P.V.; Cunha-Filho, M.; Sampaio, K.A.; Monteiro, S. Thermal and physical properties of crude palm oil with higher oleic content. Appl. Sci. 2021, 11, 7094. [Google Scholar] [CrossRef]
- Lisboa, M.C.; Wiltshire, F.M.S.; Fricks, A.T.; Dariva, C.; Carrière, F.; Lima, Á.S.; Soares, C.M.F. Oleochemistry poten-tial from Brazil northeastern exotic plants. Biochimie 2020, 178, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.C.; Fehrmann, L.; Soares, C.P.B.; Jacovine, L.A.G.; Kleinn, C.; Oliveira, G.R. Above and belowground biomass in a brazilian cerrado. For. Ecol. Manag. 2011, 262, 491–499. [Google Scholar] [CrossRef]
- Berni, P.; Campoli, S.S.; Negri, T.C.; de Toledo, N.M.V.; Canniatti-Brazaca, S.G. Non-conventional tropical fruits: Characterization, antioxidant potential and carotenoid bioaccessibility. Plant. Foods Hum. Nutr. 2019, 74, 141–148. [Google Scholar] [CrossRef]
- Amaral, L.F.B.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Caryocar brasiliense supercritical co2 extract possesses antimicrobial and antioxidant properties useful for personal care products. BMC Complement. Altern. Med. 2014, 14, 73. [Google Scholar] [CrossRef]
- De Lima, A.; Silva, A.M.D.O.; Trindade, R.A.; Torres, R.P.; Mancini-Filho, J. Chemical composition and bioactive compounds present in pequi (Caryocar Brasiliense Camb.) pulp and almond. Rev. Bras. Frutic. 2007, 29, 695–698. [Google Scholar] [CrossRef]
- Pessoa, A.S.; Podestá, R.; Block, J.M.; Franceschi, E.; Dariva, C.; Lanza, M. Extraction of pequi (Caryocar coriaceum) pulp oil using subcritical propane: Determination of process yield and fatty acid profile. J. Supercrit. Fluids 2015, 101, 95–103. [Google Scholar] [CrossRef]
- Ribeiro, D.O. Physical, Chemical and Biochemical Properties of Pequi (Caryocar brasiliense Camb.) from Different Regions of the Cerrado. Master’s Thesis, University of Brasília, Brasília, Brazil, 2011. [Google Scholar]
- Sousa, A.G.O.; Fernandes, D.C.; Alves, A.M.; Freitas, J.B.; Naves, M.M.V. Nutritional quality and protein value of exotic almonds and nut from the Brazilian savanna compared to peanut. Food Res. Int. 2011, 44, 2319–2325. [Google Scholar] [CrossRef]
- Franco, G. Tabela de Composição Química de Alimentos, 9th ed.; Atheneu: Rio de Janeiro, Brazil, 1992; p. 307. [Google Scholar]
- Rodriguez-Amaya, D.B. Current knowledge on the health benefits of carotenoids: Focus on the scientific evidence. In Global Perspectives on Astaxanthin; Elsevier: Amsterdam, The Netherlands, 2021; pp. 693–717. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Esquivel, P.; Rodriguez-Amaya, D.B. Comprehensive review on carotenoid composition: Transformations during processing and storage of foods. Food Res. Int. 2023, 169, 112773. [Google Scholar] [CrossRef]
- Arruda, H.S.; Araújo, M.V.L.; Junior, M.R.M. Underexploited Brazilian cerrado fruits as sources of phenolic compounds for diseases management: A review. Food Chem. 2022, 5, 100148. [Google Scholar] [CrossRef]
- Magalhães, F.S.; Sá, M.S.M.; Cardoso, V.L.; Reis, M.H.M. Recovery of phenolic compounds from pequi (Caryocar brasiliense Camb.) fruit extract by membrane filtrations: Comparison of direct and sequential processes. J. Food Eng. 2019, 257, 26–33. [Google Scholar] [CrossRef]
- Cangussu, L.B.; Leão, D.P.; Oliveira, L.S.; Franca, A.S. Profile of bioactive compounds in pequi (Caryocar brasilense Camb.) peel flours. Food Chem. 2021, 350, 129221. [Google Scholar] [CrossRef] [PubMed]
- Boeira, C.P.; Flores, D.C.B.; Alves, J.S.; Moura, M.R.; Melo, P.T.S.; Rolim, C.M.B.; Nogueira-Librelotto, D.R.; Rosa, C.S. Effect of corn stigma extract on physical and antioxidant properties of biodegradable and edible gelatin and corn starch films. Int. J. Biol. Macromol. 2022, 208, 698–706. [Google Scholar] [CrossRef]
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of phenolic compounds with liposomal improvement in the cosmetic industry. Int. J. Pharm. 2021, 593, 120125. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Adhikari, B. Complexation between flaxseed protein isolate and phenolic compounds: Effects on interfacial, emulsifying and antioxidant properties of emulsions. Food Hydrocoll. 2019, 94, 20–29. [Google Scholar] [CrossRef]
- Yara-Varón, E.; Li, Y.; Balcells, M.; Canela-Garayoa, R.; Fabiano-Tixier, A.S.; Chemat, F. Vegetable oils as alternative solvents for green oleo-extraction, purification and formulation of food and natural products. Molecules 2017, 22, 1474. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.M.; Barcia, M.T.; Farias, C.A.A.; Zambiazi, R.C.; De Marchi, P.G.F.; Fujimori, M.; Honório-França, A.C.; França, E.L.; Pertuzatti, P.B. Bioactive compounds of pequi pulp and oil extracts modulate antioxidant activity and antiproliferative activity in cocultured blood mononuclear cells and breast cancer cells. J. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Chisté, R.C.; Mercadante, A.Z. Identification and quantification, by HPLC-DAD-MS/MS, of carotenoids and phenolic compounds from the amazonian fruit Caryocar villosum. J. Agric. Food Chem. 2012, 60, 5884–5892. [Google Scholar] [CrossRef]
- De Oliveira, M.N.S.; Lopes, P.S.N.; Simões, M.O.M.; Pereira, E.G.; Ribeiro, L.M. Post-harvest quality of pequi (Caryocar brasiliense Camb.) collected from the plant or after naturally falling off and subjected to slow and quick freezing. Rev. Bras. Frutic 2017, 39. [Google Scholar] [CrossRef]
- Ramos, M.I.L.; Umaki, M.C.S.; Hiane, P.A.; Filho, M.M.R. Effect of conventional cooking on the provitamin “A” carotenoids from piqui pulp (Caryocar brasiliense Camb.). B. Ceppa 2001, 19, 23–32. [Google Scholar]
- Araújo, A.C.M.A.; Menezes, E.G.T.; Terra, A.W.C.; Dias, B.O.; Oliveira, É.R.D.; Queiroz, F. Bioactive compounds and chemical composition of Brazilian cerrado fruits’ wastes: Pequi almonds, murici, and sweet passionfruit seeds. Food Sci. Technol. 2018, 38, 203–214. [Google Scholar] [CrossRef]
- Miranda-Vilela, A.L.; Grisolia, C.K.; Longo, J.P.F.; Peixoto, R.C.A.; de Almeida, M.C.; Barbosa, L.C.P.; Roll, M.M.; Portilho, F.A.; Estevanato, L.L.C.; Bocca, A.L.; et al. Oil rich in carotenoids instead of vitamins c and e as a better option to reduce doxorubicin-induced damage to normal cells of ehrlich tumor-bearing mice: Hematological, toxicological and histopathological evaluations. J. Nutr. Biochem. 2014, 25, 1161–1176. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.R.; de Santana, F.C.; Torres-Leal, F.L.; de Melo, I.L.P.; Yoshime, L.T.; Matos-Neto, E.M.; Seelaender, M.C.L.; Araújo, C.M.M.; Cogliati, B.; Mancini-Filho, J. Pequi (Caryocar Brasiliense Camb.) almond oil attenuates carbon tetrachloride-induced acute hepatic injury in rats: Antioxidant and anti-inflammatory effects. Food Chem. Toxicol. 2016, 97, 205–216. [Google Scholar] [CrossRef]
- Ferreira, B.S.; De Almeida, C.G.; Faza, L.P.; De Almeida, A.; Diniz, C.G.; Da Silva, V.L.; Grazul, R.M.; Le Hyaric, M. Comparative properties of amazonian oils obtained by different extraction methods. Molecules 2011, 16, 5875–5885. [Google Scholar] [CrossRef] [PubMed]
- Bemfeito, C.M.; Carneiro, J.D.S.; Carvalho, E.E.N.; Coli, P.C.; Pereira, R.C.; Vilas Boas, E.V.B. Nutritional and functional potential of pumpkin (Cucurbita moschata) pulp and pequi (Caryocar brasiliense Camb.) peel flours. J. Food Sci. Technol. 2020, 57, 3920–3925. [Google Scholar] [CrossRef]
- Narita, I.M.P.; Filbido, G.S.; Ferreira, B.A.; Pinheiro, A.P.D.O.; Silva, D.D.C.; Nascimento, E.; Villa, D.L.; Oliveira, A.P.D. In vitro bioaccessibility of carotenoids and phenolic compounds and antioxidant capacity of pequi fruit flours (Caryocar brasiliense Camb.). Braz. J. Food Technol. 2022, 25, e2021068. [Google Scholar] [CrossRef]
- Nascimento-Silva, N.R.R.D.; Alves-Santos, A.M.; Oliveira, C.M.A.D.; Terezan, A.P.; Silva, A.P.G.D.; Naves, M.M.V. Energy and lipid contents, and polyphenols composition of pequi pulp according to the fruit native area. Ciênc. Rural 2022, 53. [Google Scholar] [CrossRef]
- Colombo, N.B.R.; Rangel, M.P.; Martins, V.; Hage, M.; Gelain, D.P.; Barbeiro, D.F.; Grisolia, C.K.; Parra, E.R.; Capelozzi, V.L. Caryocar Brasiliense Camb. protects against genomic and oxidative damage in urethane-induced lung carcinogenesis. Braz. J. Med. Biol. Res. 2015, 48, 852–862. [Google Scholar] [CrossRef]
- Batista, J.S.; Silva, A.E.; Rodrigues, C.M.F.; Costa, K.M.F.M.; Oliveira, A.F.; Paiva, E.S.; Nunes, F.V.A.; Olinda, R.G. Evaluation of the healing activity of pequi oil (Caryocar coriaceum Wittm.) in cutaneous wounds produced experimentally in rats. Arq. Inst. Biol. 2020, 77, 441–447. [Google Scholar] [CrossRef]
- Uitterhaegen, E.; Evon, P. Twin-screw extrusion technology for vegetable oil extraction: A review. J. Food Eng. 2017, 212, 190–200. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Vilas Boas, E.V.d.B.; Riul, T.R.; Pantoja, L.; Marinho, H.A.; Santos, A.S. Influence of the extraction method and storage time on the physicochemical properties and carotenoid levels of pequi (Caryocar Brasiliense Camb.) Oil. Food Sci. Technol. 2012, 32, 386–392. [Google Scholar] [CrossRef]
- Johner, J.C.F.; Hatami, T.; Meireles, M.A.A. Developing a supercritical fluid extraction method assisted by cold pressing for extraction of pequi (Caryocar Brasiliense). J. Supercrit. Fluids 2018, 137, 34–39. [Google Scholar] [CrossRef]
- Cavalcanti, M.C.B.T.; Ramos, M.A.; Araújo, E.L.; Albuquerque, U.P. Implications from the use of non-timber forest products on the consumption of wood as a fuel source in human-dominated semiarid landscapes. Environ. Manag. 2015, 56, 389–401. [Google Scholar] [CrossRef]
- Aquino, L.P.; Borges, S.V.; Queiroz, F.; Antoniassi, R.; Cirillo, M.A. Extraction of oil from pequi fruit (Caryocar brasiliense Camb.) using several solvents and their mixtures. Grasas Aceites 2011, 62, 245–252. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.L.; Vorobiev, E. Mechanical continuous oil expression from oilseeds: A review. Food Bioproc. Tech. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Castanheira, L.S. Pequi Pulp and Oil Extraction Using Mechanical Press. Bachelor’s Thesis, Pontifical Catholic University, Goiânia, Brazil, 2005. [Google Scholar]
- Cunha, L.M.S.; Pires, R.F.; Santos, K.G.d.; Dantas, S.C. Comparison of yield by different methods of oil extraction from pequi pulp. Res. Soc. Dev. 2020, 9, e342973876. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (Uae) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Pinto, M.R.M.R.; Paula, D.d.A.; Alves, A.I.; Rodrigues, M.Z.; Vieira, É.N.R.; Fontes, E.A.F.; Ramos, A.M. Encapsulation of carotenoid extracts from pequi (Caryocar brasiliense Camb.) by emulsification (o/w) and foam-mat drying. Powder Technol. 2018, 339, 939–946. [Google Scholar] [CrossRef]
- Aquino, L.P.; Ferrua, F.Q.; Borges, S.V.; Antoniassi, R.; Correa, J.L.G.; Cirillo, M.A. Influence of drying pequi (Caryocar Brasiliense Camb.) on the quality of extracted oil. Food Sci. Technol. 2009, 29, 354–357. [Google Scholar] [CrossRef]
- Ketenoglu, O.; Kiralan, M.; Ramadan, M.F. Cold pressed pequi (Caryocar brasiliense Camb.) almond oil. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Ramandan, M.F., Ed.; Academic Press: London, UK, 2020; pp. 365–372. [Google Scholar] [CrossRef]
- Gasparetto, H.; Castilhos, F.; Salau, N.P.G. Screening, experimental data, and robust kinetic modeling of vegetable oil extraction using p-cymene as a neoteric solvent for n-hexane replacement. J. Clean. Prod. 2023, 392, 136336. [Google Scholar] [CrossRef]
- Menezes, E.G.O.; Silva, A.P.S.; Sousa, K.R.P.; Azevedo, F.F.M.; Morais, R.M.; Júnior, R.N.C. Development of an innovative strategy capable of describing the large-scale extraction of tucumã-of-Pará oil (Astrocaryum vulgare Mart.) using supercritical CO2 as solvent. J. Supercrit. Fluids 2023, 193, 105825. [Google Scholar] [CrossRef]
- Moreno, L.G.; Evangelista-Silva, P.H.; Santos, E.C.; Prates, R.P.; Lima, A.C.; Mendes, M.F.; Ottone, V.O.; Ottoni, M.H.F.; Pereira, W.F.; Melo, G.E.B.A.; et al. Pequi oil, a MUFA/carotenoid-rich oil, exhibited protective effects against dss-induced ulcerative colitis in mice. Eur. J. Lipid. Sci. Technol. 2021, 123, 2000332. [Google Scholar] [CrossRef]
- Vidoca, L.; Almeida, E.; Cardoso, M.; Otavio, L.; Valadares, L.; Monteiro, S. Extraction of carotene from crude hybrid palm oil using polymeric resin. J. Food Eng. 2020, 278, 109944. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef]
- Jiménez-González, O.; Guerrero-Beltrán, J.Á. Extraction, microencapsulation, color properties, and experimental design of natural pigments obtained by spray drying. Food Eng. Rev. 2021, 13, 769–811. [Google Scholar] [CrossRef]
- Alves, A.I.; Rodrigues, M.Z.; Pinto, M.R.M.R.; Vanzela, E.S.L.; Stringheta, P.C.; Perrone, Í.T.; Ramos, A.M. Morphological characterization of pequi extract microencapsulated through spray drying. Int. J. Food Prop. 2017, 20, 1298–1305. [Google Scholar] [CrossRef]
- Machado, M.T.C.; Mello, B.C.B.S.; Hubinger, M.D. Study of alcoholic and aqueous extraction of pequi (Caryocar brasiliense Camb.) natural antioxidants and extracts concentration by nanofiltration. J. Food Eng. 2013, 117, 450–457. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments—A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Asker, M.M.S.; Tadros, M. Antiradical and antimicrobial properties of cold-pressed black cumin and cumin oils. Eur. Food Res. Technol. 2012, 234, 833–844. [Google Scholar] [CrossRef]
- Deus, T.N. Extraction and Characterization of Pequi Oil (Caryocar brasiliensis Camb.) for Sustainable use in Oil/Water (o/w) Cosmetic Formulations. Master’s Thesis, Pontifical Catholic University, Goiânia, Brazil, 2008. [Google Scholar]
- Cornelio-Santiago, H.P.; Bodini, R.B.; Mazalli, M.R.; Gonçalves, C.B.; Rodrigues, C.E.C.; Oliveira, A.L. Oil extraction from pequi (Caryocar brasiliensis Camb.) and sacha inchi (Plukenetia huayllabambana sp. nov.) almonds by pressurized liquid with intermittent purge: The effects of variables on oil yield and composition. J. Supercrit. Fluids 2022, 182, 105527. [Google Scholar] [CrossRef]
- Traesel, G.K.; Menegati, S.E.L.T.; Dos Santos, A.C.; Souza, R.I.C.; Villas Boas, G.R.; Justi, P.N.; Kassuya, C.A.L.; Argandoña, E.J.S.; Oesterreich, S.A. Oral acute and subchronic toxicity studies of the oil extracted from pequi (Caryocar brasiliense Camb.) pulp in rats. Food Chem. Toxicol. 2016, 97, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Silva, M.P.; Moraes, I.C.F.; Favaro-Trindade, C.S. Reducing carotenoid loss during storage by co-encapsulation of pequi and buriti oils in oil-in-water emulsions followed by freeze-drying: Use of heated and unheated whey protein isolates as emulsifiers. Food Res. Int. 2020, 130, 108901. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, S.B.; Duarte, C.; Bourbon, A.I.; Pinheiro, A.C.; Serra, A.T.; Martins, M.M.; Januário, M.I.N.; Vicente, A.A.; Delgadillo, I.; Duarte, C.; et al. Effect of the matrix system in the delivery and in vitro bioactivity of microencapsulated oregano essential oil. J. Food Eng. 2012, 110, 190–199. [Google Scholar] [CrossRef]
- de Freitas Santos, P.D.; Rubio, F.T.V.; Da Silva, M.P.; Pinho, L.S.; Favaro-Trindade, C.S. Microencapsulation of carotenoid-rich materials: A review. Food Res. Int. 2021, 147, 110571. [Google Scholar] [CrossRef]
- Su, Q.; Zhao, X.; Zhang, X.; Wang, Y.; Zeng, Z.; Cui, H.; Wang, C. Nano functional food: Opportunities, development, and future perspectives. Int. J. Mol. Sci. 2022, 24, 234. [Google Scholar] [CrossRef]
- Cedran, M.F.; Rodrigues, F.J.; Sato, H.H.; Bicas, J.L. Optimization of a water-in-oil emulsion containing Limosilactobacillus reuteri: Applicability of pequi oil as a continuous phase. Curr. Res. Biotechnol. 2022, 4, 318–325. [Google Scholar] [CrossRef]
- Guerra, I.C.; Sousa, T.L.; Farias, P.M.; Cappato, L.P.; Freitas, B.S.M.; Romani, V.P.; Plácido, G.R. Films and coatings from pequi mesocarp incorporated with nano-ZnO: Properties and capacity to increase mango shelf life. Ind. Crop. Prod. 2023, 195, 116414. [Google Scholar] [CrossRef]
- Silva, L.C.; Castelo, R.M.; Magalhães, H.C.R.; Furtado, R.F.; Cheng, H.N.; Biswas, A.; Alves, C.R. Characterization and controlled release of pequi oil microcapsules for yogurt application. LWT 2022, 157, 113105. [Google Scholar] [CrossRef]
- Ombredane, A.S.; Silva, L.R.A.; Araújo, V.H.S.; Costa, P.L.; Silva, L.C.; Sampaio, M.C.; Lima, M.C.F.; Junior, V.F.V.; Vieira, I.J.C.; Azevedo, R.B.; et al. Pequi oil (Caryocar brasilense Cambess.) nanoemulsion alters cell proliferation and damages key organelles in triple-negative breast cancer cells in vitro. Biomed. Pharmacother. 2022, 153, 113348. [Google Scholar] [CrossRef]
- Ghesti, G.F.; Silveira, E.A.; Guimarães, M.G.; Evaristo, R.B.W.; Costa, M. Towards a sustainable waste-to-energy pathway to pequi biomass residues: Biochar, syngas, and biodiesel analysis. Waste Manag. 2022, 143, 144–156. [Google Scholar] [CrossRef]
- Martins, J.P.G.; Setter, C.; Ataíde, C.H.; Oliveira, T.J.P.; Magriotis, Z.M. Study of pequi peel pyrolysis: Thermal decomposition analysis and product characterization. Biomass Bioenergy 2021, 149, 106095. [Google Scholar] [CrossRef]


| Pulp | Almond | Refs. | |||||
|---|---|---|---|---|---|---|---|
| Minas Gerais | Tocantins | Goiás | Mato Grosso | Piauí | |||
| Proteins (g) | 2.9 ± 0.49 | 1.74 ± 0.01 | 2.03 ± 0.25 | 2.63 ± 0.28 | 3.00 ± 0.13 | 1.74 ± 0.01 | [17,19] |
| Ashes (g) | 0.67 ± 0.05 | 0.57 ± 0.02 | 0.66 ± 0.02 | 0.62 ± 0.01 | 0.63 ± 0.01 | 0.57 ± 0.02 | |
| Lipids (g) | 27.13 ± 0.89 | 19.03 ± 1.61 | 18.95 ± 0.65 | 32.57 ± 0.78 | 33.4 ± 3.76 | 19.03 ± 1.61 | |
| Carbohydrates (g) | 7.02 | 6.78 | 6.4 | 3.6 | 11.45 | 6.78 | |
| Moisture (%) | 52.37 ± 1.34 | 61.56 ± 1.32 | 61.54 ± 1.22 | 53.2 ± 0.38 | 41.5 ± 2.00 | 61.56 ± 1.32 | |
| Dietary fibers (total, g) | 9.91 ± 0.17 | 10.32 ± 0.11 | 10.42 ± 0.18 | 7.43 ± 0.15 | 10.2 ± 0.2 | 10.32 ± 0.11 | |
| Energetic value (kcal) | 283.85 | 205.35 | 204.27 | 317.85 | 358.4 | 205.35 | |
| Phenolics (total, mg/100 g) | 215.87 ± 0.17 | 207.8 ± 0.09 | 221.2 ± 0.17 | 3.3 ± 0.14 | 209.00 ± 0.05 | 207.80 ± 0.09 | |
| Carotenoids (total, mg/100 g) | 18.7 ± 1.24 | 13.35 ± 0.47 | 11.1 ± 0.72 | 0.54 ± 0.01 | 7.25 ± 0.6 | 13.35 ± 0.47 | |
| Mineral composition (mg/100 g) | |||||||
| Pulp | Almond | ||||||
| Calcium | 50–60 | 90.12 ± 0.71 | [3,20] | ||||
| Copper | 240–400 | --- a | |||||
| Iron | 830–1600 | 2.28 ± 0.13 | |||||
| Phosphor | 1.7–2.1 | 2214.46 ± 1.85 | |||||
| Magnesium | 130 | 452.11 ± 62.1 | |||||
| Potassium | 134 | 835.7 ± 15.46 | |||||
| Sodium | 210 | --- a | |||||
| Selenium (μg/100 g) | --- a | 1.4 ± 0.01 | |||||
| Vitamin composition (μg/100 g) | |||||||
| Pulp | Almond | ||||||
| Vitamin A | 20,000 | 650 | [21] | ||||
| Vitamin B1 | 30 | 10 | |||||
| Vitamin B2 | 463 | 360 | |||||
| Pulp | Almond | |||||
|---|---|---|---|---|---|---|
| Fatty Acids | Goiás | Mato Grosso | Minas Gerais | Tocantins | Piauí | |
| Saturated | ||||||
| Butyric (C4:0) | 0.15 | 0.02 | ||||
| Caprylic (C8:0) | 0.02 | 0.03 | ||||
| Capric (C10:0) | 0.01 | 0.03 | ||||
| Lauric (C12:0) | 0.09 | 0.02 | 0.05 | 0.04 | ||
| Myristic (C14:0) | 0.18 | 0.07 | 0.1 | 0.10 | 0.27 | 0.46 ± 0.01 |
| Palmitic (C16:0) | 28.05 ± 0.8 | 40.37 ± 0.11 | 36.78 ± 0.56 | 28.14 ± 0.28 | 35.17 ± 0.27 | 43.76 ± 0.04 |
| Margaric (C17:0) | 0.13 | 0.09 | 0.07 | 0.06 | ||
| Stearic (C18:0) | 1.73 ± 0.02 | 2.3 | 2.07 | 1.39 | 2.25 ± 0.04 | 2.54 ± 0.06 |
| Arachnid (C20:0) | 0.2 | 0.14 | 0.23 ± 0.01 | 0.14 ± 0.01 | 0.23 | 0.02 |
| Behenic (C22:0) | 0.06 | 0.02 | 0.04 | |||
| Lignoceric (C24:0) | 0.04 | 0.04 | 0.02 | 0.06 | ||
| Monounsaturated | ||||||
| Palmitoleic (C16:1) | 0.42 ± 0.01 | 0.96 | 1.18 | 1.03 | 1.23 ± 0.03 | |
| Heptadecenoic (C17:1) | 0.15 | 0.08 | ||||
| Oleic (C18:1) cis | 60.4 ± 0.89 | 51.56 ± 0.15 | 50.72 ± 1.49 | 63.54 ± 0.35 | 55.87 ± 0.3 | 43.59 ± 0.16 |
| Elaidic (C18:1) trans | 0.03 | |||||
| cis-vaccenic (C18:1) n7 b | 1.9 ± 0.08 | 1.38 ± 0.01 | ||||
| Eicosamonoenoic (C20:1) | 0.26 | 0.14 | 0.46 ± 0.01 | 0.34 | ||
| Nervonic (C24:1) | 0.14 | 0.08 | ||||
| Polyunsaturated | ||||||
| Linoleic (C18:2) | 3.67 ± 0.04 | 1.01 | 1.13 | 1.61 | ||
| Docosadienoic (C22:2) | 0.02 | 0.02 | ||||
| Eicosatrienoic (C20:3) n3 b | 0.11 | 0.07 | ||||
| Eicosapentaenoic (C20:3) n6 b | 0.04 | |||||
| Arachidonic (C20:4) | 0.03 | |||||
| Eicosapentaenoic (C20:5) | 0.38 ± 0.01 | 0.27 | ||||
| Docosahexaenoic (C22:6) | ---- | |||||
| Total saturated | 30.64 | 43.07 | 39.44 | 29.89 | 37.97 | 46.78 |
| Total unsaturated | 65.58 | 53.67 | 54.08 | 65.49 | 58.8 | 46.20 |
| Carotenoids | |||
|---|---|---|---|
| Fruit Part | Compound | Concentration (mg/100 g) | Refs. |
| Pulp | β-carotene | 6.26–11.4 | [33] |
| Lycopene | 1.12–2.08 | ||
| Total carotenoids | 6.75–11.34 | ||
| β-Carotene | 13.76 ± 0.01 | [31] | |
| β-Carotene | 0.07 ± 0.00 | [32] | |
| All-trans-neoxanthin | 0.23 ± 0.06 | ||
| 9-Cis-neoxanthin | 0.08 ± 0.02 | ||
| All-trans-violaxanthin | 0.11 ± 0.02 | ||
| 9-Cis-violaxanthin | 0.04 ± 0.01 | ||
| Lutein | 0.17 ± 0.04 | ||
| All-trans-lutein | 0.09 ± 0.02 | ||
| 9-Cis-antheraxanthin | 0.06 ± 0.02 | ||
| Antheroxanthin | 0.34 ± 0.08 | ||
| Zeaxanthin | 0.29 ± 0.03 | ||
| Mutatoxanthin | 0.06 ± 0.05 | ||
| Total carotenoids | 1.73 ± 0.24 | ||
| Antheroxanthin | 9.24 | [34] | |
| Zeaxanthin | 7.91 | ||
| Cryptoflavin | 1.78 | ||
| β-Carotene | 1.47 | ||
| ζ-Carotene | 0.93 | ||
| β-Cryptoxanthin | 1.21 | ||
| Mutatoxanthin | 0.43 | ||
| Total carotenoids | 23.11 | ||
| Almond | Total carotenoids | 0.03 ± 0.01 | [35] |
| Pulp oil | β-carotene | 54.98 ± 0.01 | [31] |
| Total carotenoids | 49.99 ± 0.01 | [36] | |
| Almond oil | Total carotenoids | 0.26 ± 0.00 | [37] |
| Total carotenoids | 27.49 ± 0.34 | [38] | |
| Peel flour | β-Carotene | 1.49 ± 0.18 | [26] |
| β-Cyptoxanthin | 0.11 ± 0.01 | ||
| Lutein | 1.36 ± 0.16 | ||
| Total carotenoids | 3.49 ± 0.03 | [2] | |
| Total carotenoids | 3.38 ± 0.00 | [39] | |
| α-Carotene | 1.74 ± 0.27 | [40] | |
| β-Carotene | 1.85 ± 0.18 | ||
| Lycopene | 1.19 ± 0.12 | ||
| Pulp flour | α-Carotene | 6.38 ± 0.11 | |
| β-Carotene | 5.98 ± 0.18 | ||
| Lycopene | 4.07 ± 0.09 | ||
| Almond flour | α-Carotene | 1.47 ± 0.28 | |
| β-Carotene | 0.94 ± 0.13 | ||
| Lycopene | 1.36 ± 0.09 | ||
| Phenolic compounds | |||
| Pulp | Gallic acid | 78.30 ± 1.08 | [41] |
| Monogalloyl hexoside | 2.11 ± 0.14 | [32] | |
| Gallic acid | 18.24 ± 1.7 | ||
| Hexahydroxydiphenoyl-hexoside | 2.04 ± 0.24 | ||
| Coumaroyl-galloyl hexoside | 0.45 ± 0.06 | ||
| Coumaroyl quinic acid | 0.27 ± 0.06 | ||
| HHDP-dihexoside | 1.74 ± 0.24 | ||
| Digalloyl hexoside | 1.84 ± 0.57 | ||
| Galloyl-HHDP hexoside | 2.06 ± 0.48 | ||
| Ellagic acid hexoside | 1.69 ± 0.2 | ||
| Ellagic acid pentoside | 0.93 ± 0.18 | ||
| Ellagic acid deoxyhexoside | 10.7 ± 0.94 | ||
| Ellagic acid | 10.4 ± 1.05 | ||
| Methyl ellagic acid pentoside | 0.82 ± 0.22 | ||
| Methyl ellagic acid deoxyhexoside | 1.05 ± 0.21 | ||
| Methyl quercetin dihexoside | 0.63 ± 0.11 | ||
| Dimethyl ellagic acid pentoside | 1.03 ± 0.16 | ||
| Almond | Gallic acid | 211 ± 6 | [35] |
| Pulp oil | Chlorogenic Acid | 1.27 ± 0.12 | [31] |
| Almond Oil | Gallic acid | 0.39 ± 0.00 | [37] |
| Gallic acid | 22,910,000 ± 165,000 | [38] | |
| Pulp Extract | Gallic acid | 18.97 ± 1.93 | [25] |
| Ellagic acid | 7.14 ± 0.07 | ||
| p–Coumaric acid | 0.31 ± 0.03 | ||
| Peel Flour | Gallic Acid | 11.52–418.67 | [26] |
| Ellagic Acid | 509.47–1630.66 | ||
| Gallic Acid | 20,893.73 ± 1462.14 | ||
| Ethyl Gallate | 2026.75–5205.90 | ||
| Gallic Acid | 9475.69 ± 12.74 | [40] | |
| Pulp Flour | Gallic Acid | 402.21 ± 35.75 | |
| Almond Flour | Gallic Acid | 210.50 ± 34.95 | |
| Method | Operating Condition | Extraction Yield (%) | Refs. |
|---|---|---|---|
| Artisanal | Solvent: water; cooking: 100 °C; 1 h | 19.37 | [45] |
| Mechanical | Pre-drying: 60 °C; 24 h; pressing: 28 °C | 22.4 | [45] |
| Solid–Liquid | Solvent: ethyl ether; extraction time: 4 h | 58.47 | [45] |
| Solvent: acetone; extraction time: 4 h | 60.5 | ||
| Supercritical | Solvent: CO2; 40 MPa; 333.15 K | 49 | [46] |
| Solvent | Operating Conditions | Extracted Oil (%) | Refs. | |
|---|---|---|---|---|
| Pulp | Hexane | 69 °C | 59.96 | [51] |
| 50 °C; W = 10.37–1.36 | 42.5–59.4 | [54] | ||
| NR a | 49.6 | [18] | ||
| T = 50 °C, 22 Hz, 16 h, S-L = 1/10 | 60.2 | [48] | ||
| Ethanol | T = 78.37 °C | 34.8 | [51] | |
| NR a | 52.8 | [18] | ||
| T = 50 °C, 22 Hz, 16 h, S-L = 1/10 | 39.8 | [48] | ||
| Acetone | 50 °C, 22 Hz, 16 h, S-L = 1/10 | 61.07 | [48] | |
| Almond | Hexane | 55 °C | 98.4 | [35] |
| Ethanol | 55 °C | 76.1 | ||
| Acetone | 55 °C | 86.0 | ||
| Isopropanol | 55 °C | 87.9 |
| Solvents | Operating Conditions | Extracted Oil (%) | Refs. |
|---|---|---|---|
| CO2 | GT: 30 s, 333.15 K, 40 MPa, SR: 2.93 × 10−4 kg/s | 49.0 | [46] |
| CO2 | GT: 50 s, 333.15 K, 40 MPa, SR: 2.93 × 10−4 kg/s | 47.0 | [46] |
| Propane | 333.15 K, 15 MPa | 43.7 | [18] |
| Extraction Method | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Artisanal | No sophisticated equipment. No generation of toxic waste. Oil with good quality. Oxidative enzyme inactivation via fruit cooking. Financial income for communities living off of fruit extraction. | Many hours of work. Low oil yield. High cooking temperatures can degrade nutraceutical compounds. | [45,51,55] |
| Mechanical | Low investment in energy and equipment. Preservation of the bioactive compound integrity. Simple operating methodology. No toxic waste generation. | Low yield. Considerable oil loss in the residual cake. Oil extract may contain fibers and/or impurities. Direct contact between fruit and metallic press parts can produce oil with free fatty acids. | [45,46,55] |
| Solid–Liquid | High efficiency. Minimal energy demand at low temperatures. Preservation of thermosensitive compounds. | Environmental and operational risks due to solvent toxicity. Possibility of bioactive thermal degradation. Higher operating costs. Requirement for oil separation from solvent. | [45,56] |
| Supercritical | High efficiency. High-purity oil extract free of residues. Shorter extraction time. Low temperatures. Bioactive compound preservation. | Expensive equipment. | [46,57] |
| Operating Conditions | Carotene | Acidity | PV | SV | Refs. | |
|---|---|---|---|---|---|---|
| Almonds | ||||||
| Artisanal | Cooking: 100 °C, water, Almond/water: 1/3 m/v | 0.1 | 0.55 ± 0.07 | 2.91 ± 1.44 | --- | [37] |
| Mechanical | Hydraulic pressing: 25 °C, 1 h, 9 ton-force | 0.1 | 1.09 ± 0.68 | 0.85 ± 0.88 | --- | [37] |
| Solid–Liquid | Hexane, 90 °C, 6 h | --- | 4.94 ± 0.08 | 28.23 ± 0.05 | 206.10 ± 0.93 | [66] |
| Pulp | ||||||
| Artisanal | Cooking: 100 °C, 1 h | 25.0 | 1.44 | 0.760 | 214.36 | [45] |
| Mechanical | Pre-drying: 60 °C, 24 h, Continuous pressing: 28 °C | 26.9 | 5.44 | 1.07 | 225.1 | [45] |
| Solid–Liquid (S-L:1:10, 16 h, 22 Hz) | Ethyl ether, 4 h | 42.7 | 3.24 | 0.752 | 218.66 | [45,48] |
| Acetone, 4 h | 38.9 | 2.71 | 0.725 | 217.3 | ||
| Ethanol | 29.9 | --- | 197.23 | 195–198 | ||
| Hexane | 20.3 | --- | 197.23 | 195–198 |
| Pulp | |||||
|---|---|---|---|---|---|
| Solid–Liquid [35] | Supercritical [18] | Mechanical [68] | |||
| Fatty Acid | Content (%) | ||||
| Oleic (C18:1) | 60.39 | 58.73 | 56.5 | ||
| Palmitic (C16:0) | 33.87 | 34.95 | 38.11 | ||
| Stearic (C18:0) | 1.71 | 1.74 | 2.61 | ||
| Palmitoleic (C16:1) | 0.73 | 0.63 | 1.01 | ||
| Linoleic (C18:2) | 2.22 | 2.84 | 0.96 | ||
| Linolenic (C18:3) | 0.44 | 0.35 | 0.17 | ||
| Gadoleic (C20:1) | --- | --- | 0.29 | ||
| Myristic (C14:0) | 0.1 | 0.07 | 0.07 | ||
| Arachidonic (C20:0) | 0.19 | 0.26 | 0.15 | ||
| Almonds | |||||
| Solid–Liquid | Artisanal | Mechanical | |||
| [66] | [1] | [37] | [1] | [37] | |
| Oleic (C18:1) | 50.2 | 54.97 ± 0.17 | 56.34 ± 0.15 | 54.39 ± 0.28 | 59.99 ± 0.03 |
| Palmitic (C16:0) | 42.3 | 34.92 ± 0.11 | 33.76 ± 0.03 | 34.78 ± 0.39 | 29.48 ± 0.00 |
| Stearic (C18:0) | 1.5 | 2.50 ± 0.04 | 2.62 ± 0.01 | 2.37 ± 0.07 | 2.44 ± 0.00 |
| Palmitoleic (C16:1) | 1.0 | 0.53 ± 0.01 | 0.59 ± 0.01 | 0.66 ± 0.05 | 0.66 ± 0.01 |
| Linoleic (C18:2) | 0.6 | 6.11 ± 0.11 | 5.74 ± 0.03 | 6.73 ± 0.13 | 6.48 ± 0.00 |
| Linolenic (C18:3) | 0.5 | --- | --- | --- | --- |
| Myristic (C14:0) | 0.2 | 0.35 ± 0.00 | 0.35 ± 0.00 | 0.37 ± 0.00 | 0.36 ± 0.00 |
| Arachidonic (C20:0) | 0.3 | --- | --- | --- | |
| Erucic (C22:1) | --- | --- | 0.60 ± 0.01 | 0.63 ± 0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, C.R.; Alhaji, A.M.; da Silva, C.A.S.; de Sousa, R.d.C.S.; Monteiro, S.; Coimbra, J.S.d.R. Potential Challenges of the Extraction of Carotenoids and Fatty Acids from Pequi (Caryocar brasiliense) Oil. Foods 2023, 12, 1907. https://doi.org/10.3390/foods12091907
Carneiro CR, Alhaji AM, da Silva CAS, de Sousa RdCS, Monteiro S, Coimbra JSdR. Potential Challenges of the Extraction of Carotenoids and Fatty Acids from Pequi (Caryocar brasiliense) Oil. Foods. 2023; 12(9):1907. https://doi.org/10.3390/foods12091907
Chicago/Turabian StyleCarneiro, Camila Rodrigues, Adamu Muhammad Alhaji, César Augusto Sodré da Silva, Rita de Cássia Superbi de Sousa, Simone Monteiro, and Jane Sélia dos Reis Coimbra. 2023. "Potential Challenges of the Extraction of Carotenoids and Fatty Acids from Pequi (Caryocar brasiliense) Oil" Foods 12, no. 9: 1907. https://doi.org/10.3390/foods12091907
APA StyleCarneiro, C. R., Alhaji, A. M., da Silva, C. A. S., de Sousa, R. d. C. S., Monteiro, S., & Coimbra, J. S. d. R. (2023). Potential Challenges of the Extraction of Carotenoids and Fatty Acids from Pequi (Caryocar brasiliense) Oil. Foods, 12(9), 1907. https://doi.org/10.3390/foods12091907